Abstract
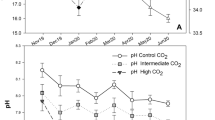
Acidification of the World’s oceans may directly impact reproduction, performance and shell formation of marine calcifying organisms. In addition, since shell production is costly and stress in general draws on an organism’s energy budget, shell growth and stability of bivalves should indirectly be affected by environmental stress. The aim of this study was to investigate whether a combination of warming and acidification leads to increased physiological stress (lipofuscin accumulation and mortality) and affects the performance [shell growth, shell breaking force, condition index (Ci)] of young Mytilus edulis and Arctica islandica from the Baltic Sea. We cultured the bivalves in a fully-crossed 2-factorial experimental setup (seawater (sw) pCO2 levels “low”, “medium” and “high” for both species, temperature levels 7.5, 10, 16, 20 and 25 °C for M. edulis and 7.5, 10 and 16 °C for A. islandica) for 13 weeks in summer. Mytilus edulis and A. islandica appeared to tolerate wide ranges of sw temperature and pCO2. Lipofuscin accumulation of M. edulis increased with temperature while the Ci decreased, but shell growth of the mussels only sharply decreased while its mortality increased between 20 and 25 °C. In A. islandica, lipofuscin accumulation increased with temperature, whereas the Ci, shell growth and shell breaking force decreased. The pCO2 treatment had only marginal effects on the measured parameters of both bivalve species. Shell growth of both bivalve species was not impaired by under-saturation of the sea water with respect to aragonite and calcite. Furthermore, independently of water temperatures shell breaking force of both species and shell growth of A. islandica remained unaffected by the applied elevated sw pCO2 for several months. Only at the highest temperature (25 °C), growth arrest of M. edulis was recorded at the high sw pCO2 treatment and the Ci of M. edulis was slightly higher at the medium sw pCO2 treatment than at the low and high sw pCO2 treatments. The only effect of elevated sw pCO2 on A. islandica was an increase in lipofuscin accumulation at the high sw pCO2 treatment compared to the medium sw pCO2 treatment. Our results show that, despite this robustness, growth of both M. edulis and A. islandica can be reduced if sw temperatures remain high for several weeks in summer. As large body size constitutes an escape from crab and sea star predation, this can make bivalves presumably more vulnerable to predation—with possible negative consequences on population growth. In M. edulis, but not in A. islandica, this effect is amplified by elevated sw pCO2. We follow that combined effects of elevated sw pCO2 and ocean warming might cause shifts in future Western Baltic Sea community structures and ecosystem services; however, only if predators or other interacting species do not suffer as strong from these stressors.






Similar content being viewed by others
References
Aarab N, Pampanin DM, Naevdal A, Oysaed KB, Gastaldi L, Bechmann RK (2008) Histopathology alterations and histochemistry measurements in mussel, Mytilus edulis collected offshore from an aluminium smelter industry (Norway). Mar Pollut Bull 57(6–12):569–574
Abele D, Puntarulo S (2004) Formation of reactive species and induction of antioxidant defence systems in polar and temperate marine invertebrates and fish. Comp Biochem Physiol A Mol Integr Physiol 138(4):405–415
Abele D, Burlando B, Viarengo A, Portner HO (1998) Exposure to elevated temperatures and hydrogen peroxide elicits oxidative stress and antioxidant response in the Antarctic intertidal limpet Nacella concinna. Comp Biochem Physiol B Biochem Mol Biol 120(2):425–435
Abele D, Heise K, Portner HO, Puntarulo S (2002) Temperature dependence of mitochondrial function and production of reactive oxygen species in the intertidal mud clam Mya arenaria. J Exp Biol 205(13):1831–1841
Almada-Villela PC, Davenport J, Gruffydd LD (1982) The effects of temperature on the shell growth of young Mytilus edulis L. J Exp Mar Biol Ecol 59(2–3):275–288
Appelhans YS, Thomsen J, Pansch C, Melzner F, Wahl M (2012) Sour times: seawater acidification effects on growth, feeding behaviour and acid–base status of Asterias rubens and Carcinus maenas. Mar Ecol Prog Ser 459:85–97
Bamber RN (1990) The effects of acidic seawater on 3 species of lamellibranch mollusk. J Exp Mar Biol Ecol 143(3):181–191
Bayne BL, Worrall CM (1980) Growth and production of mussels Mytilus edulis from 2 populations. Mar Ecol-Prog Ser 3(4):317–328
Begum S, Basova L, Heilmayer O, Philipp EER, Abele D, Brey T (2010) Growth and energy budget models of the bivalve Arctica islandica at six different sites in the northeast atlantic realm. J Shellfish Res 29(1):107–115
Berge JA, Bjerkeng B, Pettersen O, Schaanning MT, Oxnevad S (2006) Effects of increased sea water concentrations of CO2 on growth of the bivalve Mytilus edulis L. Chemosphere 62(4):681–687
Bleich M, Melzner F, Hiebenthal C, Mempel H, Schulz K, Riebesell U, Wahl M, Sommer F, Sommer U, Form A, Piatkowski U, Hanel R, Piepenburg D, Spindler M, Eisenhauer A, Franke A, Möller V, Baumert G, Clemmesen-Bockelmann C (2008) Poster: Kiel CO2 manipulation experimental facility (KICO2). Paper presented at the second symposium on the ocean in a high-CO2 World, Monaco, 06–09 Oct 2008
Boulding E (1984) Crab-resistant features of shell-burrowing bivalves: decreasing vulnerability by increasing handling time. J Exp Mar Biol Ecol 76:201–223
Brey T, Arntz WE, Pauly D, Rumohr H (1990) Arctica (Cyprina) islandica in Kiel Bay (Western Baltic): growth, production and ecological significance. J Exp Mar Biol Ecol 136:217–235
Burnett LE (1997) The challenges of living in hypoxic and hypercapnic aquatic environments. Am Zool 37(6):633–640
Byrne M (2011) Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanogr Mar Biol Annu Rev 49(49):1–42
Byrne M, Ho M, Wong E, Soars NA, Selvakumaraswamy P, Shepard-Brennand H, Dworjanyn SA, Davis AR (2011) Unshelled abalone and corrupted urchins: development of marine calcifiers in a changing ocean. Proc Royal Soc B Biol Sci 278(1716):2376–2383
Caldeira K, Wickett ME (2003) Anthropogenic carbon and ocean pH. Nature 425(6956):365
Cargnelli LM, Griesbach SJ, Packer DB, Weissberger E (1999) Ocean quahog Arctica islandica, life history and habitat characteristics. NOAA Tech Memo NMFS-NE-148
Childress JJ, Lee RW, Sanders NK, Felbeck H, Oros DR, Toulmond A, Desbruyeres D, Kennicutt MC, Brooks J (1993) Inorganic carbon uptake in hydrothermal vent tubeworms facilitated by high environmental pCO2. Nature 362(6416):147–149
Christmas J, Jordan S (1987) Biological monitoring of selected oyster bars in the lower Choptank. In: MacKiernan G (ed) Dissolved oxygen in the Chesapeake Bay. Maryland Sea Grant, College park, pp 125–128
Clarke A (2003) Costs and consequences of evolutionary temperature adaptation. Trends Ecol Evol 18(11):573–581
Climate Change in the Baltic Sea Area. Baltic Sea Environment Proceedings No. 111 (2007) Helsinki Commission. http://www.helcom.fi/groups/monas/CombineManual/en_GB/main/. Accessed 09 Nov 2010
Cochran RE, Burnett LE (1996) Respiratory responses of the salt marsh animals, Fundulus heteroclitus, Leiostomus xanthurus, and Palaemonetes pugio to environmental hypoxia and hypercapnia and to the organophosphate pesticide, azinphosmethyl. J Exp Mar Biol Ecol 195(1):125–144
Crenshaw M (1972) The inorganic composition of molluscan extrapallial fluid. Biol Bull 143:506–512
Crenshaw M, Neff J (1969) Decalcification at the mantle-shell interface in mollusks. Am Zool 9:881–889
Davenport J, Chen Z (1987) A comparison of methods for the assessment of condition in the mussel (Mytilus edulis L.). J Moll Stud 53:293–297
Denman KL, Brasseur G, Chidthaisong A, Ciais P, Cox PM, Dickinson RE, Hauglustaine D, Heinze C, Holland E, Jacob D, Lohmann U, Ramachandran S, da Silva Dias PL, Wofsy SC, Zhang X (2007) Couplings between changes in the climate system and biogeochemistry. climate change 2007: the physical science basis. Contribution of working group i to the fourth assessment report of the intergovernmental panel on climate change (IPCC), vol 4. Cambridge University Press, UK
DePaolo DJ (2004) Calcium isotopic variations produced by biological, kinetic, radiogenic and nucleosynthetic processes. Geochem Non-Tradit Stable Isot 55:255–288
Dickson AG, Afghan JD, Anderson GC (2003) Reference materials for oceanic CO2 analysis: a method for the certification of total alkalinity. Mar Chem 80(2–3):185–197
Dickson A, Sabine C, Christian J (2007) Guide to best practices for ocean CO2 measurements. PICES Special Publication 3, IOCCP Report No. 8
Elner RW, Hughes RN (1978) Energy maximization in diet of shore crab, Carcinus maenas. J Anim Ecol 47(1):103–116
Enderlein P, Wahl M (2004) Dominance of blue mussels versus consumer-mediated enhancement of benthic diversity. J Sea Res 51(2):145–155
Enderlein P, Moorthi S, Rohrscheidt H, Wahl M (2003) Optimal foraging versus shared doom effects: interactive influence of mussel size and epibiosis on predator preference. J Exp Mar Biol Ecol 292(2):231–242
Fabry VJ, Seibel BA, Feely RA, Orr JC (2008) Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci 65(3):414–432. doi:10.1093/icesjms/fsn048
Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry VJ, Millero FJ (2004) Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305(5682):362–366
Frankignoulle M, Bourge I, Wollast R (1996) Atmospheric CO2 fluxes in a highly polluted estuary (the Scheldt). Limnol Oceanogr 41(2):365–369
Gazeau F, Quiblier C, Jansen JM, Gattuso JP, Middelburg JJ, Heip CHR (2007) Impact of elevated CO2 on shellfish calcification. Geophys Res Lett 34(7):L07603
Gazeau F, Gattuso JP, Dawber C, Pronker AE, Peene F, Peene J, Heip CHR, Middelburg JJ (2010) Effect of ocean acidification on the early life stages of the blue mussel Mytilus edulis. Biogeosciences 7(7):2051–2060
Gazeau F, Gattuso JP, Greaves M, Elderfield H, Peene J, Heip CHR, Middelburg JJ (2011) Effect of carbonate chemistry alteration on the early embryonic development of the Pacific oyster (Crassostrea gigas). PLoS ONE 6(8):e23010
Glasby TM (1998) Estimating spatial variability in developing assemblages of epibiota on subtidal hard substrata. Mar Freshw Res 49(5):429–437
Gran G (1952) Determination of the equivalence point in potentiometric titrations of seawater with hydrochloric acid. Oceanologia Acta 5:209–218
Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM, Rowley SJ, Tedesco D, Buia MC (2008) Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454(7200):96–99
Hansen HP, Giesenhagen HC, Behrends G (1999) Seasonal and long-term control of bottom-water oxygen deficiency in a stratified shallow-water coastal system. ICES J Mar Sci 56:65–71
Hansson D, Gustafsson E (2011) Salinity and hypoxia in the Baltic Sea since AD 1500. J Geophys Res-Oceans 116:C03027
Hiebenthal C, Philipp EER, Eisenhauer A, Wahl M (2012) Interactive effects of temperature and salinity on shell increment, condition and cellular stress of two bivalve populations, Mytilus edulis (L.) and Arctica islandica (L.), from the Western Baltic Sea. Aquat Biol 14:289–298
Hill KT, Womersley CZ (1993) Interactive effects of some environmental and physiological variables on fluorescent age pigment accumulation in brain and heart tissues of an aquatic poikilotherm. Environ Biol Fishes 37(4):397–405
Hofmann M, Schellnhuber HJ (2010) Ocean acidification: a millennial challenge. Energy Environ Sci 3(12):1883–1896
Hofmann GE, Smith JE, Johnson KS, Send U, Levin LA, Micheli F, Paytan A, Price NN, Peterson B, Takeshita Y, Matson PG, Crook ED, Kroeker KJ, Gambi MC, Rivest EB, Frieder CA, Yu PC, Martz TR (2011) High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS ONE 6(12):e28983
Irie T, Iwasa Y (2005) Optimal growth pattern of defensive organs: the diversity of shell growth among mollusks. Am Nat 165(6):727
Jansson A-M, Kautsky N (1977) Quantitative survey of hard bottom communities in a Baltic archipelago. In: Keegan BF, O’Ceidigh P, Boaden PJS (eds) Biology of benthic organisms. 11th European symposium marine biology, Galway, Perg. Press, Oxford, pp 359–366, Oct. 1976
Kagley AN, Snider RG, Krishnakumar PK, Casillas E (2003) Assessment of seasonal variability of cytochemical responses to contaminant exposure in the blue mussel Mytilus edulis (complex). Arch Environ Contam Toxicol 44(1):43–52
Kautsky N (1982) Growth and size structure in a Baltic Mytilus edulis population. Mar Biol 68:117–133
Kautsky N, Johannesson K, Tedengren M (1990) Genotypic and phenotypic differences between Baltic and North Sea populations of Mytilus edulis evaluated through reciprocal transplantations. I. Growth and morphology. Mar Ecol Prog Ser 59:203–210
Keeling RF, Kortzinger A, Gruber N (2010) Ocean deoxygenation in a warming world. Ann Rev Mar Sci 2:199–229
Knoll AH, Bambach RK, Canfield DE, Grotzinger JP (1996) Comparative earth history and late permian mass extinction. Science 273(5274):452–457
Kossak U (2006) How climate change translates into ecological change: impacts of warming and desalination on prey properties and predator-prey interactions in the Baltic Sea. Ph.D. thesis, Chriatian-Albrechts-Universität, Kiel, Germany
Krishnakumar PK, Casillas E, Varanasi U (1997) Cytochemical responses in the digestive tissue of Mytilus edulis complex exposed to microencapsulated PAHs or PCBs. Comp Biochem Phys C Pharmacol Toxicol Endocrinol 118(1):11–18
Lehmann A, Getzlaff K, Harlass J (2011) Detailed assessment of climate variability in the Baltic Sea area for the period 1958 to 2009. Clim Res 46(2):185–196
Lesser MP, Bailey MA, Merselis DG, Morrison JR (2010) Physiological response of the blue mussel Mytilus edulis to differences in food and temperature in the Gulf of Maine. Comp Biochem Physiol A: Mol Integr Physiol 156(4):541–551
Lewis E, Wallace D (1998) Program developed for CO2 system calculations. ORNL/CDIAC-105. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, Tennessee
Loosanoff VL (1953) Reproductive Cycle in Cyprine islandica. Biol Bull 104(2):146–155
Martin S, Rodolfo-Metalpa R, Ransome E, Rowley S, Buia MC, Gattuso JP, Hall-Spencer J (2008) Effects of naturally acidified seawater on seagrass calcareous epibionts. Biol Lett 4(6):689–692
Mascaro M, Seed R (2001) Choice of prey size and species in Carcinus maenas (L.) feeding on four bivalves of contrasting shell morphology. Hydrobiologia 449(1–3):159–170
Melzner F, Stange P, Trubenbach K, Thomsen J, Casties I, Panknin U, Gorb SN, Gutowska MA (2011) Food supply and seawater pCO2 impact calcification and internal shell dissolution in the blue mussel Mytilus edulis. PLoS ONE 6(9):e24223
Melzner F, Thomsen J, Koeve W, Oschlies A, Gutowska MA, Bange HW, Hansen HP, Körtzinger A (2012) Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar Biol Published online: 29 May 2012. doi:10.1007/s00227-012-1954-1
Michaelidis B, Ouzounis C, Paleras A, Pörtner HO (2005) Effects of long-term moderate hypercapnia on acid-base balance and growth rate in marine mussels Mytilus galloprovincialis. Mar Ecol-Prog Ser 293:109–118
Morris S, Taylor A (1983) Diurnal and seasonal variation in physico-chemical conditions within intertital rock pools. Estuar Coast Shelf Sci 17:339–355
Moy A, Howard W, Bray S, Trull T (2009) Reduced calcification in modern Southern Ocean planktonic foraminifera. Nat Geosci 2:276–280
Nagarajan R, Lea SEG, Goss-Custard JD (2006) Seasonal variations in mussel, Mytilus edulis L. shell thickness and strength and their ecological implications. J Exp Mar Biol Ecol 339(2):241–250
Palmer AR (1992) Calcification in marine mollusks: how costly is it? Proc Nat Acad Sci USA 89(4):1379–1382
Parker LM, Ross PM, O’Connor WA (2010) Comparing the effect of elevated pCO(2) and temperature on the fertilization and early development of two species of oysters. Mar Biol 157(11):2435–2452
Philipp E, Brey T, Pörtner HO, Abele D (2005) Chronological and physiological ageing in a polar and a temperate mud clam. Mech Ageing Dev 126(5):598–609
Philippart CJM, Anadon R, Danovaro R, Dippner JW, Drinkwater KF, Hawkins SJ, Oguz T, O’Sullivan G, Reid PC (2011) Impacts of climate change on European marine ecosystems: observations, expectations and indicators. J Exp Mar Biol Ecol 400(1–2):52–69
Pörtner HO (2008) Ecosystem effects of ocean acidification in times of ocean warming: a physiologist’s view. Mar Ecol Prog Ser 373:203–217. doi:10.3354/meps07768
Remane A, Schlieper C (1971) Biology of brackish waters. Die Binnengewässer, 2nd edn. Wiley Interscience Division, Wiley, New York
Reusch TBH, Chapman ARO (1997) Persistence and space occupancy by subtidal blue mussel patches. Ecol Monogr 67(1):65–87
Reuter P (2004) Einfluss klimarelevanter Faktoren auf das Wachstum der Miesmuschel (Mytilus edulis). University of Kiel, Kiel, Diploma thesis
Sabine CL, Feely RA, Gruber N, Key RM, Lee K, Bullister JL, Wanninkhof R, Wong CS, Wallace DWR, Tilbrook B, Millero FJ, Peng TH, Kozyr A, Ono T, Rios AF (2004) The oceanic sink for anthropogenic CO2. Science 305(5682):367–371
Sareyka J, Kraufvelin P, Lenz M, Lindström M, Tollrian R, Wahl M (2011) Differences in stress tolerance and brood size between a non-indigenous and an indigenous gammarid in the northern Baltic Sea. Mar Biol. doi:10.1007/s00227-011-1708-5
Schalkhausser B, Bock C, Stemmer K, Brey T, Pörtner H-O, Lannig G (2012) Impact of ocean acidification on escape performance of the king scallop, Pecten maximus, from Norway. Mar Biol. doi:10.1007/s00227-012-2057-8
Stumpp M, Trubenbach K, Brennecke D, Hu MY, Melzner F (2012) Resource allocation and extracellular acid-base status in the sea urchin Strongylocentrotus droebachiensis in response to CO2 induced seawater acidification. Aquat Toxicol 110:194–207
Suchanek TH (1985) Mussels and their role in structuring rocky shore communities. In: Moore PG, Seed R (eds) The ecology of rocky coasts. Hodder & Stoughton, London, pp 70–96
Sukhotin AA, Abele D, Pörtner HO (2002) Growth, metabolism and lipid peroxidation in Mytilus edulis: age and size effects. Mar Ecol-Prog Ser 226:223–234
Sunday JM, Crim RN, Harley CDG, Hart MW (2011) Quantifying rates of evolutionary adaptation in response to ocean acidification. PLoS ONE 6(8):e22881
Talmage SC, Gobler CJ (2011) Effects of elevated temperature and carbon dioxide on the growth and survival of larvae and juveniles of three species of Northwest Atlantic Bivalves. PLoS ONE 6(10):e26941
Taylor A (1976) The cardiac responses to shell opening and closure in the bivalve Arctica islandica (L.). J Exp Biol 64:751–759
Thomsen J, Gutowska MA, Saphorster J, Heinemann A, Trubenbach K, Fietzke J, Hiebenthal C, Eisenhauer A, Kortzinger A, Wahl M, Melzner F (2010) Calcifying invertebrates succeed in a naturally CO2-rich coastal habitat but are threatened by high levels of future acidification. Biogeosciences 7(11):3879–3891
Truchot JP, Duhameljouve A (1980) Oxygen and carbon-dioxide in the marine inter-tidal environment - diurnal and tidal changes in rockpools. Respir Physiol 39(3):241–254
Tunnicliffe V, Davies KTA, Butterfield DA, Embley RW, Rose JM, Chadwick WW (2009) Survival of mussels in extremely acidic waters on a submarine volcano. Nat Geosci 2(5):344–348
Vernet M, Hunter JR, Vetter RD (1988) Accumulation of age pigments (lipofuscin) in 2 cold-water fishes. Fish Bull 86(2):401–407
Wahl M, Jormalainen V, Eriksson BK, Coyer JA, Molis M, Schubert H, Dethier M, Ehlers A, Karez R, Kruse I, Lenz M, Pearson G, Rohde S, Wikström SA, Olsen JL (2011) Stress ecology in FUCUS: abiotic, biotic and genetic interactions. Adv Mar Biol 59:37–105
Waldbusser GG (2011) The causes of acidification in Chesapeake Bay and consequences to oyster shell growth and dissolution. J Shellfish Res 30(2):559–560
Weigelt M, Rumohr H (1986) Effects of wide-range oxygen depletion on benthic fauna and demersal fish in Kiel Bay 1981–1983. Meeresforsch 31:124–136
Welladsen HM, Southgate PC, Heimann K (2010) The effects of exposure to near-future levels of ocean acidification on shell characteristics of Pinctada fucata (Bivalvia: Pteriidae). Moll Res 30(3):125–130
Witbaard R, Bergman MJN (2003) The distribution and population structure of the bivalve Arctica islandica L. in the North Sea: what possible factors are involved? J Sea Res 50(1):11–25
Yu PC, Matson PG, Martz TR, Hofmann GE (2011) The ocean acidification seascape and its relationship to the performance of calcifying marine invertebrates: laboratory experiments on the development of urchin larvae framed by environmentally-relevant pCO(2)/pH. J Exp Mar Biol Ecol 400(1–2):288–295
Zettler ML, Bönsch R, Gosselck F (2001) Distribution, abundance and some population characteristics of the ocean quahog, Arctica islandica (Linnaeus, 1767), in the Mecklenburg Bight (Baltic Sea). J Shellfish Res 20:161–169
Acknowledgments
We thank Mandy Kierspel for carrying out lipofuscin measurements and Titiana Gelmi for supporting us with Ci data from free-living Kiel Fjord mussels. The authors also thank two anonymous reviewers for their comments and suggestions that helped to improve the quality of this paper. This study was conducted within the framework of the European Scientific Foundation (ESF)-Project CASIOPEIA and funded by the German Research Foundation (Deutsche Forschungsgemeinschaft) DFG, Project-No. Ei272/20-1/-2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Dupont.
Rights and permissions
About this article
Cite this article
Hiebenthal, C., Philipp, E.E.R., Eisenhauer, A. et al. Effects of seawater pCO2 and temperature on shell growth, shell stability, condition and cellular stress of Western Baltic Sea Mytilus edulis (L.) and Arctica islandica (L.). Mar Biol 160, 2073–2087 (2013). https://doi.org/10.1007/s00227-012-2080-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-2080-9