Abstract
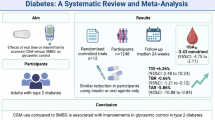
Aims/hypothesis
Characterisation of genetic variation that influences the response to glucose-lowering medications is instrumental to precision medicine for treatment of type 2 diabetes. The Study to Understand the Genetics of the Acute Response to Metformin and Glipizide in Humans (SUGAR-MGH) examined the acute response to metformin and glipizide in order to identify new pharmacogenetic associations for the response to common glucose-lowering medications in individuals at risk of type 2 diabetes.
Methods
One thousand participants at risk for type 2 diabetes from diverse ancestries underwent sequential glipizide and metformin challenges. A genome-wide association study was performed using the Illumina Multi-Ethnic Genotyping Array. Imputation was performed with the TOPMed reference panel. Multiple linear regression using an additive model tested for association between genetic variants and primary endpoints of drug response. In a more focused analysis, we evaluated the influence of 804 unique type 2 diabetes- and glycaemic trait-associated variants on SUGAR-MGH outcomes and performed colocalisation analyses to identify shared genetic signals.
Results
Five genome-wide significant variants were associated with metformin or glipizide response. The strongest association was between an African ancestry-specific variant (minor allele frequency [MAFAfr]=0.0283) at rs149403252 and lower fasting glucose at Visit 2 following metformin (p=1.9×10−9); carriers were found to have a 0.94 mmol/l larger decrease in fasting glucose. rs111770298, another African ancestry-specific variant (MAFAfr=0.0536), was associated with a reduced response to metformin (p=2.4×10−8), where carriers had a 0.29 mmol/l increase in fasting glucose compared with non-carriers, who experienced a 0.15 mmol/l decrease. This finding was validated in the Diabetes Prevention Program, where rs111770298 was associated with a worse glycaemic response to metformin: heterozygous carriers had an increase in HbA1c of 0.08% and non-carriers had an HbA1c increase of 0.01% after 1 year of treatment (p=3.3×10−3). We also identified associations between type 2 diabetes-associated variants and glycaemic response, including the type 2 diabetes-protective C allele of rs703972 near ZMIZ1 and increased levels of active glucagon-like peptide 1 (GLP-1) (p=1.6×10−5), supporting the role of alterations in incretin levels in type 2 diabetes pathophysiology.
Conclusions/interpretation
We present a well-phenotyped, densely genotyped, multi-ancestry resource to study gene–drug interactions, uncover novel variation associated with response to common glucose-lowering medications and provide insight into mechanisms of action of type 2 diabetes-related variation.
Data availability
The complete summary statistics from this study are available at the Common Metabolic Diseases Knowledge Portal (https://hugeamp.org) and the GWAS Catalog (www.ebi.ac.uk/gwas/, accession IDs: GCST90269867 to GCST90269899).
Graphical Abstract




Similar content being viewed by others
Abbreviations
- AOC:
-
Area over the curve
- DPP:
-
Diabetes Prevention Program
- EAF:
-
Effect allele frequency
- gePS:
-
Global extended polygenic score
- GLP-1:
-
Glucagon-like peptide 1
- GWAS:
-
Genome-wide association study
- LD:
-
Linkage disequilibrium
- MAF:
-
Minor allele frequency
- PC:
-
Principal component
- PP:
-
Posterior probability
- pPS:
-
Process-specific polygenic score
- SUGAR-MGH:
-
Study to Understand the Genetics of the Acute Response to Metformin and Glipizide in Humans
- V1:
-
Visit 1
- V2:
-
Visit 2
References
Davies MJ, D’Alessio DA, Fradkin J et al (2018) Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 41(12):2669–2701. https://doi.org/10.2337/dci18-0033
Chung WK, Erion K, Florez JC et al (2020) Precision medicine in diabetes: a consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 43(7):1617–1635. https://doi.org/10.2337/dci20-0022
Zhou K, Yee SW, Seiser EL et al (2016) Variation in the glucose transporter gene SLC2A2 is associated with glycemic response to metformin. Nat Genet 48(9):1055–1059. https://doi.org/10.1038/ng.3632
Zhou K, Bellenguez C, Spencer CC et al (2011) Common variants near ATM are associated with glycemic response to metformin in type 2 diabetes. Nat Genet 43(2):117–120. https://doi.org/10.1038/ng.735
Srinivasan S, Kaur V, Chamarthi B et al (2018) TCF7L2 Genetic variation augments incretin resistance and influences response to a sulfonylurea and metformin: the study to understand the genetics of the acute response to Metformin and Glipizide in Humans (SUGAR-MGH). Diabetes Care 41(3):554–561. https://doi.org/10.2337/dc17-1386
Zhou K, Donnelly L, Burch L et al (2010) Loss-of-function CYP2C9 variants improve therapeutic response to sulfonylureas in type 2 diabetes: a Go-DARTS study. Clin Pharmacol Ther 87(1):52–56. https://doi.org/10.1038/clpt.2009.176
Dujic T, Zhou K, Donnelly LA, Leese G, Palmer CNA, Pearson ER (2018) Interaction between variants in the CYP2C9 and POR genes and the risk of sulfonylurea-induced hypoglycaemia: A GoDARTS Study. Diabetes Obes Metab 20(1):211–214. https://doi.org/10.1111/dom.13046
Chen L, Li JH, Kaur V et al (2020) The presence of two reduced function variants in CYP2C9 influences the acute response to glipizide. Diabet Med 37(12):2124–2130. https://doi.org/10.1111/dme.14176
Dawed AY, Yee SW, Zhou K et al (2021) Genome-wide meta-analysis identifies genetic variants associated with glycemic response to sulfonylureas. Diabetes Care 44(12):2673–2682. https://doi.org/10.2337/dc21-1152
Xue A, Wu Y, Zhu Z et al (2018) Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat Commun 9(1):2941. https://doi.org/10.1038/s41467-018-04951-w
Florez JC, Jablonski KA, Bayley N et al (2006) TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med 355(3):241–250. https://doi.org/10.1056/NEJMoa062418
Lyssenko V, Lupi R, Marchetti P et al (2007) Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest 117(8):2155–2163. https://doi.org/10.1172/JCI30706
Villareal DT, Robertson H, Bell GI et al (2010) TCF7L2 variant rs7903146 affects the risk of type 2 diabetes by modulating incretin action. Diabetes 59(2):479–485. https://doi.org/10.2337/db09-1169
Walford GA, Colomo N, Todd JN et al (2015) The study to understand the genetics of the acute response to metformin and glipizide in humans (SUGAR-MGH): design of a pharmacogenetic resource for type 2 diabetes. PloS One 10(3):e0121553. https://doi.org/10.1371/journal.pone.0121553
Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ (2015) Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4:7. https://doi.org/10.1186/s13742-015-0047-8
Delaneau O, Zagury J-F, Marchini J (2013) Improved whole-chromosome phasing for disease and population genetic studies. Nat Methods 10(1):5–6. https://doi.org/10.1038/nmeth.2307
Taliun D, Harris DN, Kessler MD et al (2021) Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature 590(7845):290–299. https://doi.org/10.1038/s41586-021-03205-y
R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria
Pruim RJ, Welch RP, Sanna S et al (2010) LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics (Oxford, England) 26(18):2336–2337. https://doi.org/10.1093/bioinformatics/btq419
Vujkovic M, Keaton JM, Lynch JA et al (2020) Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet 52(7):680–691. https://doi.org/10.1038/s41588-020-0637-y
Mahajan A, Spracklen CN, Zhang W et al (2022) Multi-ancestry genetic study of type 2 diabetes highlights the power of diverse populations for discovery and translation. Nat Genet 54(5):560–572. https://doi.org/10.1038/s41588-022-01058-3
Chen J, Spracklen CN, Marenne G et al (2021) The trans-ancestral genomic architecture of glycemic traits. Nat Genet 53(6):840–860. https://doi.org/10.1038/s41588-021-00852-9
Machiela MJ, Chanock SJ (2015) LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics (Oxford, England) 31(21):3555–3557. https://doi.org/10.1093/bioinformatics/btv402
Giambartolomei C, Zhenli Liu J, Zhang W et al (2018) A Bayesian framework for multiple trait colocalization from summary association statistics. Bioinformatics (Oxford, England) 34(15):2538–2545. https://doi.org/10.1093/bioinformatics/bty147
Ge T, Chen C-Y, Ni Y, Feng Y-CA, Smoller JW (2019) Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat Commun 10(1):1776. https://doi.org/10.1038/s41467-019-09718-5
Udler MS, Kim J, von Grotthuss M et al (2018) Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: A soft clustering analysis. PLoS Med 15(9):e1002654. https://doi.org/10.1371/journal.pmed.1002654
The Diabetes Prevention Program Research Group (2000) The Diabetes Prevention Program: baseline characteristics of the randomized cohort. Diabetes Care 23(11):1619–1629. https://doi.org/10.2337/diacare.23.11.1619
The Diabetes Prevention Program (1999) Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 22(4):623–634. https://doi.org/10.2337/diacare.22.4.623
Li JH, Perry JA, Jablonski KA et al (2022) Identification of genetic variation influencing metformin response in a multi-ancestry genome-wide association study in the Diabetes Prevention Program (DPP). Diabetes. https://doi.org/10.2337/db22-0702
Sung K-C, Reaven GM, Kim SH (2009) Utility of homeostasis model assessment of β-cell function in predicting diabetes in 12,924 healthy Koreans. Diabetes Care 33(1):200–202. https://doi.org/10.2337/dc09-1070
Song Y, Manson JE, Tinker L et al (2007) Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: the Women’s Health Initiative Observational Study. Diabetes Care 30(7):1747–1752. https://doi.org/10.2337/dc07-0358
Li JH, Szczerbinski L, Dawed AY et al (2021) A polygenic score for type 2 diabetes risk is associated with both the acute and sustained response to sulfonylureas. Diabetes 70(1):293–300. https://doi.org/10.2337/db20-0530
Ohara-Imaizumi M, Aoyagi K, Ohtsuka T (2019) Role of the active zone protein, ELKS, in insulin secretion from pancreatic β-cells. Mol Metab 27(Suppl):S81–S91. https://doi.org/10.1016/j.molmet.2019.06.017
Omar-Hmeadi M, Idevall-Hagren O (2021) Insulin granule biogenesis and exocytosis. Cell Mol Life Sci 78(5):1957–1970. https://doi.org/10.1007/s00018-020-03688-4
Wrann CD, Eguchi J, Bozec A et al (2012) FOSL2 promotes leptin gene expression in human and mouse adipocytes. J Clin Invest 122(3):1010–1021. https://doi.org/10.1172/jci58431
Accelerating Medicines Partnership. T2D Knowledge Portal. Available from https://t2d.hugeamp.org/region.html?chr=2&end=28690184&phenotype=TG&start=28565315. Accessed 21 Sep 2022
Thomsen SK, Ceroni A, van de Bunt M et al (2016) Systematic functional characterization of candidate causal genes for type 2 diabetes risk variants. Diabetes 65(12):3805–3811. https://doi.org/10.2337/db16-0361
Ruetten H, Gebauer M, Raymond RH et al (2018) Mixed meal and intravenous L-arginine tests both stimulate incretin release across glucose tolerance in man: lack of correlation with β cell function. Metab Syndr Relat Disord 16(8):406–415. https://doi.org/10.1089/met.2018.0022
Dennis JM, Shields BM, Henley WE, Jones AG, Hattersley AT (2019) Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: an analysis using clinical trial data. Lancet Diabetes Endocrinol 7(6):442–451. https://doi.org/10.1016/s2213-8587(19)30087-7
The GTEx Consortium (2020) The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science (New York, NY) 369(6509):1318–1330. https://doi.org/10.1126/science.aaz1776
Shu Y, Brown C, Castro RA et al (2008) Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther 83(2):273–280. https://doi.org/10.1038/sj.clpt.6100275
Zhou K, Donnelly LA, Kimber CH et al (2009) Reduced-function SLC22A1 polymorphisms encoding organic cation transporter 1 and glycemic response to metformin: a GoDARTS study. Diabetes 58(6):1434–1439. https://doi.org/10.2337/db08-0896
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Acknowledgements
We would like to thank J. Del Rio (freelance graphic designer, Cambridge, MA, USA) for his support with Fig. 1 generation. Portions of this study were previously presented as an oral presentation at the 81st Virtual Scientific Sessions of the American Diabetes Association, 25–29 June 2021.
Data availability
The complete summary statistics from this study will be deposited and made available at the Common Metabolic Diseases Knowledge Portal (https://hugeamp.org) and the GWAS Catalog (www.ebi.ac.uk/gwas/, accession IDs: GCST90269867 to GCST90269899) following article publication. Related study documents, including the original study protocol and informed consent forms, are available [14]. Additional data requests should be sent by email to the corresponding author.
Funding
This work was conducted with support from National Institutes of Health/NIDDK awards R01 GM117163, R01 DK088214, R03 DK077675 and P30 DK036836; from the Joslin Clinical Research Center from its philanthropic donors; and from the Harvard Catalyst: the Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH Awards M01-RR-01066, 1 UL1 RR025758-04 and 8UL1TR000170-05, and financial contributions from Harvard University and its affiliated academic healthcare centres). JHL received individual support from NIH T32DK007028 and NIDDK K23DK131345. LNB is supported by NIDDK K23DK125839. MSU is supported by NIDDK K23DK114551. AL is supported by grant 2020096 from the Doris Duke Charitable Foundation and the American Diabetes Association grant 7-22-ICTSPM-23. JMM is supported by American Diabetes Association Innovative and Clinical Translational Award 1-19-ICTS-068, American Diabetes Association grant #11-22-ICTSPM-16, and by NHGRI U01HG011723. JCF is supported by NHLBI K24HL157960.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
All authors took part in designing the experiments presented in this manuscript. VK, LNB, MSU, AL and JCF recruited participants in SUGAR-MGH. VK supervised participant recruitment, data collection, and IRB review and approval, and performed DNA extractions and managed GWAS genotyping. Quality control, imputation of the genetic data and GWAS analyses were performed by JMM. JHL, LNB, VK, KF, PS, AH-C and JMM performed follow-up of GWAS data analysis. JHL, LNB, VK, JMM and JCF contributed to the interpretation of the results. JHL, LNB, VK and JMM wrote and prepared the manuscript. All authors revised and approved the final manuscript. JMM and JCF jointly supervised this study. JCF is the guarantor of this work.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Josep M. Mercader and Jose C. Florez jointly directed this work.
Members of the MAGIC Consortium and the DPP Research Group are included as collaborators and listed in the electronic supplementary material (ESM) text.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J.H., Brenner, L.N., Kaur, V. et al. Genome-wide association analysis identifies ancestry-specific genetic variation associated with acute response to metformin and glipizide in SUGAR-MGH. Diabetologia 66, 1260–1272 (2023). https://doi.org/10.1007/s00125-023-05922-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-023-05922-7