Abstract
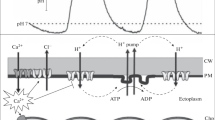
Local irradiation of the alga Vaucheria sessilis (Vauch.) D.C. with blue light, which stimulates cortical fiber reticulation and chloroplast aggregation (M.R. Blatt and W.R. Briggs, 1980, Planta 147, 355–362), also induces an outward-directed current from the irradiated region of the cell. This current appears in conjunction with cortical fiber reticulation and precedes chloroplast aggregation. The current is not photosynthetic in origin, as indicated by experiments with 3(3,4-dichlorophenyl)-1,1-dimethyl urea and carbonyl-cyanide-m-chlorophenylhydrazone (CCCP). It shows a wavelength-dependence similar to that of chloroplast aggregation and reaches a maximum of 500 nA cm-2 with saturating light intensities. The current is not dependent upon the presence of Na+, K+, or Cl- in a test medium containing only Na+, K+, Ca2+, and Cl-, but is inhibited, apparently nonspecifically, in the absence of external calcium. Both the light-induced current and chloroplast aggregation are stimulated by increases in the external KCl concentration and are inhibited by sub-micromolar concentrations of CCCP or by external pHs below approximately 5.5. We suggest that blue light stimulates the local extrusion of cations, possibly of protons, at the plasma membrane, an event which may act to destabilize the cortical fibers in Vaucheria, disrupt cytoplasmic streaming, and eventually lead to organelle aggregation in the light.
Similar content being viewed by others
References
Begg, D., Rebhun, L. (1979) pH regulates the polymerization of actin in the sea urchin egg cortex. J. Cell Biol. 83, 241–248
Bentrup, F. (1974) Lichtabhängige Membranpotentiale bei Pflanzen. Ber. dtsch. Bot. Ges. 87, 515–528
Bentrup, F. (1979) Reception and transduction of electrical and mechanical stimuli. In: Encyclopedia of Plant Physiology, N.S., Vol. 7, pp. 42–70, Haupt, W., Feinleib, M., eds. Springer, Berlin, Heidelberg, New York
Blatt, M. (1980) A study of events associated with light-dependent chloroplast aggregation in Vaucheria sessilis. Ph. D. thesis, Stanford University, Stanford, Cal., USA
Blatt, M., Briggs, W. (1977) A recording microphotometer for measurement of chloroplast orientation movements in single algal filaments. Carnegie Instn. of Wash. Yr. Bk. 76, 278–281
Blatt, M., Briggs, W. (1980) Blue-light-induced cortical fiber reticulation concomitant with chloroplast aggregation in the alga Vaucheria sessilis. Planta 147, 355–362
Blatt, M., Wessells, N., Brigg, W. (1980) Actin and cortical fiber reticulation in the siphonaceous alga Vaucheria sessilis. Planta 147, 363–375
Clarke, M., Spudich, J. (1977) Non-muscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu. Rev. Biochem. 46, 797–822
Condeelis, J., Taylor, D. (1977) The contractile basis of amoeboid movement. V. The control of gelation solation and contraction in extracts of Dictyostelium discoideum. J. Cell Biol. 74, 901–927
Davis, R. (1974) Photoinduced changes in electrical potentials and H+ activities of the chloroplast, cytoplasm, and vacuole of Phaeoceros laevis. In: Membrane transport in plants, pp. 197–201, Zimmermann, U., Dainty, J., eds. Springer, Berlin, Heidelberg, New York
Dreyer, E., Weisenseel, M. (1979) Phytochrome-mediated uptake of calcium in Mougeotia cells. Planta 146, 31–39
Felle, H., Bentrup, F. (1977) A study of the primary effect of the uncoupler carbonyl-cyanide-m-chlorophenylhydrazone on membrane potential and conductance in Riccia fluitans. Biochim. Biophys. Acta 464, 179–187
Fischer-Arnold, G. (1963) Untersuchungen über die Chloroplastenbewegung bei Vaucheria sessilis. Protoplasma 56, 496–520
Heiple, J., Taylor, D. (1980) Intracellular pH in single motile cells. J. Cell Biol. 86, 885–890
Hellewell, S., Taylor, D. (1979) The contractile basis of ameboid movement. VI. The solation-contraction coupling hypothesis. J. Cell Biol. 83, 633–648
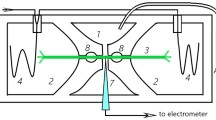
Jaffe, L.F., Nucitelli, R. (1974) An ultrasensitive vibrating probe for measuring steady extracellular currents. J. Cell Biol. 63, 614–628
Kiefer, D., Spanswick, R. (1978) Activity of the electrogenic pump in Chara corallina as inferred from measurements of the membrane potential, conductance and potassium permeability. Plant Physiol. 62, 653–661
Kiefer, D., Spanswick, R. (1979) Correlation of adenosine triphosphate levels in Chara corallina with the activity of the electrogenic pump. Plant Physiol. 64, 165–168
Kitasato, H. (1968) The influence of H+ on the membrane potential and ion fluxes of Nitella. J. Gen. Physiol. 52, 60–87
Klein, K., Wagner, G., Blatt, M. (1980) Heavy-meromyosin-decoration of microfilaments from Mougeotia protoplasts. Planta 150, 354–356
MacRobbie, E. (1975) Ion transport in plant cells. In: Current Topics in Membranes and Transport, vol. 7, pp. 1–48, Bronner, F., Kleinzeller, A., eds. Academic Press, New York
Nultsch, W., Häder, D.-P. (1979) Photomovement of motile microorganisms. Photochem. Photobiol. 29, 423–437
Raschke, K. (1975) Stomatal action. Ann. Rev. Plant Physiol. 26, 309–340
Raschke, K. (1979) Movements of stomata. In: Encyclopedia of Plant Physiology, N.S., Vol. 7, pp. 383–441, Haupt, W., Feinleib, M., eds. Springer, Berlin, Heidelberg, New York
Raven, J. (1975) Transport in algal cells. In: Encyclopedia of Plant Physiology, N.S., Vol. 2B, pp. 129–188, Lüttge, U., Pittman, M., eds. Springer, Berlin, Heidelberg, New York
Richards, J., Hope, A. (1974) The role of protons in determining membrane electrical characteristics in Chara corallina. J. Membr. Biol. 16, 121–144
Robinson, K., Jaffe, L.F. (1973) Ion movements in developing fucoid eggs. Dev. Biol. 35, 349–361
Sanders, D. (1980) The mechanism of Cl- transport at the plasma membrane of Chara corallina. I. Cotransport with H+. J. Membr. Biol. 53, 129–141
Satter, R., Marinoff, P., Galston, A. (1970) Phytochrome controlled nyctinasty in Albizzia julibrissin. II. Potassium fluxes as a basis for leaf movement. Am. J. Bot. 57, 916–926
Satter, R., Geballe, G., Applewhite, P., Galston, A. (1974) Potassium flux and leaf movement II. Phytochrome-controlled movement. J. Gen. Physiol. 64, 431–442
Smith, F., Lucas, W. (1973) The role of H+ and OH- fluxes in the ionic relations of characean cells. In: Ion transport in plants, pp. 223–231, Anderson, W., ed. Academic Press, New York
Spanswick, R., Williams, E. (1965) Calcium fluxes and membrane potentials in Nitella translucens. J. Exp. Biol. 16, 463–473
Taylor, D. (1977) Dynamics of cytoplasmic structure and contractility. In: International Cell Biology, '77, pp. 367–377, Brinkley, B., Porter, K., eds. Rockefeller University Press, Boston
Taylor, D., Wang, Y., Heiple, J. (1980a) The contractile basis of amoeboid movement. VII. Distribution of fluorescently labelled actin in living amoebae. J. Cell Biol. 86, 590–598
Taylor, D., Blinks, J., Reynolds, G. (1980b) The contractile basis of amoeboid movement. VIII. Aequorin luminescence during amoeboid movement, endocytosis, and capping. J. Cell Biol. 86, 599–607
Tilney, L. (1977) Actin: its association with membranes and the regulation of its polymerization. In: International Cell Biology, '77, pp. 388–402, Brinkley, B., Porter, K., eds. Rockefeller University Press, Boston
Tilney, L., Kiehart, D., Sardet, C., Tilney, M. (1978) Polymerization of actin. IV. Role of Ca++ and H+ in the assembly of actin and in membrane fusion in the acrosomal reaction of echinoderm sperm. J. Cell Biol. 77, 536–550
Wagner, G. (1979) Actomyosin as a basic mechanism of movement in animals and plants. In: Encyclopedia of Plant Physiology, N.S., Vol. 7, pp. 114–126, Haupt, W., Feinleib, M., eds. Springer, Berlin, Heidelberg, New York
Wagner, G., Haupt, W., Laux, A. (1972) Reversible inhibition of chloroplast movement by cytochalasin B in the green alga Mougeotia. Science 176, 808–809
Wagner, G., Klein, K. (1978) Differential effect of calcium on chloroplast movement in Mougeotia. Photochem. Photobiol. 27, 137–140
Walker, E., Yoon, M., Song, P.-S. (1981) The pH dependence of photosensory responses in Stentor coeruleus and model system. Biochim. Biophys. Acta 634, 289–308
Walker, N., Hope, A. (1969) Membrane fluxes and electrical conductance in characean cells. Austr. J. Biol. Sci. 22, 1179–1195
Walker, N., Smith, F. (1975) Intracellular pH in Chara corallina measured by DMO distribution. Plant Sci. Lett. 4, 125–132
Weisenseel, M., Dorn, A., Jaffe, L.F. (1979) Natural H+ currents traverse growing roots and root hairs of Barley. Plant Physiol. 64, 512–518
Weisenseel, M., Jaffe, L.F. (1976) The major growth current through lily pollen tubes enters as K+ and leaves as H+. Planta 133, 1–7
Author information
Authors and Affiliations
Additional information
C.I.W.-D.P.B. Publication No. 712
Rights and permissions
About this article
Cite this article
Blatt, M.R., Weisenseel, M.H. & Haupt, W. A light-dependent current associated with chloroplast aggregation in the alga Vaucheria sessilis . Planta 152, 513–526 (1981). https://doi.org/10.1007/BF00380822
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00380822