Abstract
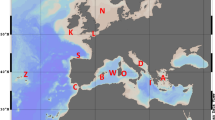
Horizontal starch gel electrophoresis was employed to investigate levels of genetic differentiation between 13 samples of the neritic squid species Loligo forbesi Steenstrup obtained from throughout the majority of its known geographical range. Six enzyme loci identified in a preliminary study as being polymorphic were screened for variation between samples. No significant differences in allele distribution were detected between any of the samples obtained from the Faroe Bank in the north to Lisbon in the south, suggesting that squid throughout this range in the vicinity of the continental shelf are able to maintain panmixia, and effectively belong to a single population sharing a common gene pool. No clinal variation in allele distribution was detected throughout this range, a result which complements the findings of a detailed morphological companion study of the same individuals. Comparison of this homogenous European continental shelf population with squid from the Azores revealed highly significant (P<0.01) differences in allele distribution at five of the six polymorphic enzyme loci studied. A genetic identity value (I) equivalent to 0.93 over 33 loci was obtained. Analysis of F-statistics suggested migration rates between sites to be as low as one individual per five generations, a rate deemed insufficient under most models to prevent divergence by random genetic drift. The large distance and oceanic depths separating the Azores from continental Europe seem to present an effective barrier to gene flow to L. forbesi, a squid belonging to a family considered to be confined in distribution to relatively shallow, near coastal waters. The two populations of squid in the Azores and along the European continental shelf currently both ascribed to L. forbesi should therefore probably best be regarded as relative subspecies.
Similar content being viewed by others
References
Amaratunga T (1987) Population biology. In: Boyle PR (ed) Cephalopod life cycles. Vol. 2. Comparative reviews. Academic Press, London, pp 239–252
Augustyn CJ, Grant WS (1988) Biochemical and morphological systematics of Loligo vulgaris vulgaris Lamarck and Loligo vulgaris reynaudi D'Orbigny nov. comb. (Cephalopoda: Myopsida). Malacologia 29:215–233
Avise JC (1974) Systematic value ofelectrophoretic data. Syst Zool 23:465–481
Ayala FJ (1983) Enzymes as taxanomic characters. In: Oxford GS, Rollinson D, (eds) Protein polymorphism: adaptive and taxonomic significance. Academic Press, London, pp 3–26
Boyle PR, Ngoile, MAK (1993) Population variation and growth in Loligo forbesi (Cephalopoda: Loliginidae) from Scottish water. In: Okutani T, O'Dor RK, Kubodera T (eds) Recent advances in cephalopod fishery biology. Tokai University Press, Tokoy, Japan, pp 49–59
Brierley AS (1992) Aspects of genetic diversity and population structure in squid. Unpublished thesis. University of Liverpool, Port Erin, Isle of Man
Brierley AS, Thorpe JP (1994) Biochemical gentic evidence supporting the taxonomic separation of Loligo gahi from the genus Loligo. Antarctic Sci 6:143–148
Brierley AS, Thorpe JP, Clarke MR, Martins HR (1993a). A preliminary biochemical genetic investigation of the population structure of Loligo forbesi Steenstrup, 1856 from the U.K. and the Azores. In: Okutani T, O'Dor RK, Kubodera T (eds) Recent advances in cephalopod fishery biology. Tokai University Press, Tokyo, Japan, pp 61–69
Brierley AS, Rodhouse PG, Thorpe JP, Clarke MR (1993b). Genetic evidence of population heterogeneity and cryptic speciation in the ommastrephid squid Martialia hyadesi from the Patagonian Shelf and Antarctic Polar Frontal Zone. Mar Biol 116:593–602
Burton RS, Feldman MW (1983) Physiological effects of an allozyme polymorphism: glutamate pyruvate transaminase and response to hyperosmotic stress in the copoepod Tigriopus californicus. Biochem Genet 21:239–251
Carvalho GR, Loney KH (1989) Biochemical genetic studies on the Patagonian squid Loligo gahi d'Orbigny. I. Electrophoretic study of genetic variability. J exp mar Biol Ecol 126:231–241
Carvalho GR, Pitcher TJ (1989) Biochemical genetic studies on the Patagonian squid Loligo gahi d'Orbigny. II. Population structures in Falkland waters using morphometrics and life history data. J exp mar Biol Ecol 126:243–258
Carvalho GR, Thompson A, Stoner AL (1992) Genetic diversity and population differentiation in the shortfin squid (Illex argentinus) in the south-west Atlantic. J exp mar Biol Ecol 158:105–121
Christoffersen JP, Foss A, Lambert WE, Welge B (1978). An electrophoretic study of select proteins fom the market squid, Loligo opalescens, along the California coast. Calif Fish Game Fish Bull 169:123–133
Coelho ML (1985) Review of the influence of oceanographic factors on cephalopod distribution and life cycles. N Atlant Fish Orgn (NAFO) scient Coun Stud 9:47–57
Coyne JA (1992) Genetics and speciation. Nature, Lond 355:511–515
Dickson WT, Brown MB, Engelman L, Jennrich RI (1990). BMDP statistical software manual. 2 Vols. University of California Press, Berkeley
Dooley HD (1984) Aspects of oceanographic variability on Scottish fishing grounds. Unpublished thesis. University of Aberdeen
Fisher RA (1958) Statistical methods for research workers. 13th edn. Hafner, New York
Gaard E (1987) An investigation of the squid Loligo forbesi Steenstrup on Faroe Bank. International Council for the Exploration of the Sea, Copenhagen (Comm Meet Pap Rep K 18)
Garthwaite RL, Berg CJ Jr., Harrigan J (1989). Population genetics of the common squid Loligo pealei Le Seur, 1821, from Cape Cod to Cape Hatteras. Biol Bull mar biol Lab, Woods Hole 177:277–294
Hanlon RT, Hixon RF, Turk PE, Lee PG, Yang WT (1985) Behaviour, feeding and growth of young Loligo forbesi (Cephalopoda: Myopsida) reared in the laboratory. Vie Milieu 35:p 247
Hansen B, Ellet D, Meldrum D (1986) Evidence for an anticy clonic circulation on Faroe Bank. International Council for the Exploration of the Sea, Copenhagen (Comm Meet Pap Rep C:15
Harris H, Hopkinson DA (1977) Handbook of enzyme electrophoresis in human genetics. North Holland Publishing Co, Amsterdam
Hartl DL, Clark AG (1989) Principles of population genetics. 2nd edn. Sinauer Ass. Inc., Sunderland, Massachusetts
Hillis DM, Moritz C (eds) (1990) Molecular systematics. Sinauer Ass. Inc., Sunderland, Massachusetts
Holme NA (1974) The biology of Loligo forbesi Steenstrup (Mollusca: Cephalopoda) in the Plymouth area. J mar biol Ass UK 54:481–503
Howard FG, Ngoile MA, Mason J (1987) Loligo forbesi: its present status in Scottish fisheries. International Council for the Exploration of the Sea, Copenhagen (Comm Meet Pap Rep, K:5)
Ihssen PE, Booke HE, Casselman JM, McGlade JM, Payne NR, Utter FM (1981) Stock identification, materials and methods. Can J Fish aquat Sciences 38:1838–1855
Jamieson A, Jones BW (1967) Two races of cod at Faroe. Heredity, Lond 22:610–612
Kimura M, Weiss GH (1964) The stepping stone model of population structure and the decrease of genetic correlation with distance. Genetics, Austin, Tex 49:561–576
Koehn RK, Hilbish TJ (1987) The adaptive importance of genetic variation. Am Scient 75:134–141
Lewontin RC (1974) Genetic basis of evolutionary change. Columbia University Press, New York
Lum-Kong A (1989) The reproductive biology of female Loligo forbesi Steenstrup (Cephalopoda: Myopsida). Unpublished thesis. University of Aberdeen
Mangold K (1987) Reproduction. In: Boyle PR (ed) Cephalopod life cycles. Vol. II. Comparative reviews. Academic Press, London, pp 387–400
Martins H (1982) Biological studies of the exploited stock of Loligo forbesi (Mollusca: Cephalopoda) in the Azores J mar biol Ass UK 62:799–808
Maynard Smith J (1989) Evolutionary genetics. Oxford University Press, Oxford
Mayr E (1970) Populations, species and evolution. Harvard University Press, Cambridge, Massachusetts
Morton JE, Yonge CM (1964) Classification and structure of the Mollusca. In: Wilbur KM, Yonge CM (eds) Physiology of the Mollusca. Vol. 1. Academic Press, New York, pp 1–58
Nei M (1972) Genetic distance between populations. Am Nat 106:283–292
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Nesis KN (1987) Cephalopods of the world. T.F.H. Publications Inc., Neptune City, New Jersey
O'Dor RK, Coelho ML (1993) Big squid, big currents and big fisheries. In: Okutani T, O'Dor RK, Kubodera T (eds) Recent advances in cephalopod fishery biology. Tokai University Press, Tokyo, Japan, pp 385–396
Pickard GL, Emery WJ (1982) Descriptive physical oceanography, an introduction. 4th enlarged edn. Pergamon Press, Oxford
Pierce GJ, Hastie LC, Guerra A, Thorpe RS, Howard FG, Boyle PR (1994a) Morphometric variation in Loligo forbesi and Loligo vulgaris: regional, seasonal, maturity and worker differences. Fish Res 21:127–148
Pierce GJ, Thorpe RS, Hastie LC, Brierley AS, Guerra A, Boyle PR, Jamieson R, Avila P (1994b) Geographic variation in Loligo forbesi in the Northeast Atlantic Ocean: analysis of morphometric data and tests of causal hypotheses. Mar Biol 119:541–547
Richardson BJ, Baverstock PR, Adams M (1986) Allozyme electrophoresis — a handbook for animal systematics and population studies. Academic Press, Sydney
Roper CFE, Sweeney MJ, Naun CE (1984) FAO species catalogue. Vol. 3. Cephalopods of the world. An annotated and illustrated guide to species of interest to fisheries. FAO Fish Synopsis 125(3):1–277
Ryman N, Utter F (eds) (1987) Population genetics and fishery management. Washington Sea Grant Program. University of Washington Press, Seattle, Washington
Slatkin M (1985) Gene flow in natural populations. Rev Ecol Syst 16:393–430
Smith PF, Mattlin RH, Roeleveld MA, Okutani T (1987) Arrow squids of the genus Nototodarus in New Zealand waters: systematics, biology and fisheries. NZ J mar Freshwat Res 21:315–326
Swofford DL, Selander RB (1981) BBIOSYS-1: a FORTRAN program for the comprehensive analysis of electrophoretic data in population genetics and systematics. J Hered 72:281–283
Thorpe JP (1979) Enzyme variation and taxonomy: the estimation of sampling errors in measurement of interspecific genetic similarity. Biol J Linn Soc 11:369–386
Thorpe JP (1982) The molecular clock hypothesis: biochemical evolution, genetic differentiation and systematics. A Rev Eol Syst 13:139–168
Thorpe JP (1983) Enzyme variation, genetic distance, and evolutionary divergence in relation to levels of taxanomic separation. In: Oxford GS, Rollinson D (eds) Protein polymorphism: adaptive and taxanomic significance. Academic Press, London, pp 131–152
Voss GL (1977) Present status and new trends in cephalopod systematics. Symp zool Soc Lond 38:49–60
Waples RS (1987) A multispecies approach to the analysis of gene flow in marine shore fishes. Evolution 41:385–400
Ward RD (1989) Molecular population genetics of marine organisms. In: Ryland JS, Tyler PA (eds) Reproduction genetics and distribution of marine organisms. Olsen & Olsen Fredensborg, Denmark, pp 235–249
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Wright S (1965) The interpretation of population structure by F-statistics with special regard to systems of mating. Evolution 19:395–420
Yeatman J, Benzie JAH (1993) Cryptic speciation in Loligo from Northern Australia. In: Okutani T, O'Dor RK, Kubodera T (eds) Recent advances in cephalopod fishery biology. Tokai University Press, Tokyo, Japan, pp 641–652
Author information
Authors and Affiliations
Additional information
Communicated by J. Mauchline, Oban
Rights and permissions
About this article
Cite this article
Brierley, A.S., Thorpe, J.P., Pierce, G.J. et al. Genetic variation in the neritic squid Loligo forbesi (Myopsida: Loliginidae) in the northeast Atlantic Ocean. Marine Biology 122, 79–86 (1995). https://doi.org/10.1007/BF00349280
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00349280