Abstract
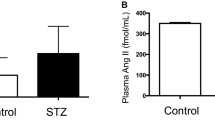
Intracellular accumulation of sorbitol, generated fromd-glucose via the aldose reductase pathway, is thought to play an important role in diabetic complications such as lens cataracts and neuropathy. In order to elucidate the effect of diabetes on the renal inner medulla, another sorbitol-rich tissue, male Wistar rats were treated with a single dose of streptozotocin (60 mg/kg body weight, i.p.). Six wecks later total inner medullary tissue (IM) or isolated inner medullary collecting duct (IMCD) cells were prepared. In diabetic IM tissue, sorbitol content was 1.8-fold higher than in control IM tissue (134±17 vs. 74±22 μmol/g tissue protein). Sorbitol production in both normal and diabetic IMCD cells was strongly dependent on extracellulard-glucose concentration. In normal cells, for example, sorbitol production was 90±9 μmol sorbitol/g protein x h at 45 mMd-glucose compared to 13±1 μmol/g protein x h at 5 mM. At identicald-glucose concentrations sorbitol synthesis in diabetic IMCD cells was, however, always significantly higher than in control cells (122% of control at 15 mM and 126% of control at 45 mM). In addition, aldose reductase activity in diabetic IM was found to be augmented. The maximal velocity was 4.2 times higher (97±22 U/g protein vs. 23±7 U/g protein) while theK m of the enzyme remained unchanged. Membrane permeability for sorbitol or the response to changes in extracellular osmolarity was not significantly different in diabetic IMCD cells and normal cells with correspondingly high intracellular sorbitol concentrations. Similarly the kinetic parameters ofd-glucose uptake were not altered by streptozotocin treatment. These results suggest that increased medullary sorbitol content in diabetic rats is a result of increased sorbitol synthesis due to a higher extracellulard-glucose concentration and augmented aldose reductase activity in face of an unaltered sorbitol permeability of the plasma membrane.
Similar content being viewed by others
References
Bagnasco SM, Balaban HM, Fales MH, Yang YM, Burg MB (1986) Predominant osmotically active organic solutes in rat and rabbit renal medullas. J Biol Chem 261:5872–5877
Bagnasco SM, Uchida S, Balaban RS, Kador PF, Burg MB (1987) Induction of aldose reductase and sorbitol in renal inner medullary cells by elevated extracellular NaCl. Proc Natl Acad Sci USA 84:1718–1720
Bagnasco SM, Murphy HR, Bedford JJ, Burg MB (1988) Osmoregulation by slow changes in aldose reductase and rapid changes in sorbitol flux. Am J Physiol 254:C788-C792
Beutler HO (1984) D-Sorbitol. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol III. Verlag Chemie, Weinheim Deerfield Beach Basel, pp 356–362
Beutler HO, Becker J (1977) Enzymatische Bestimmung von D-Sorbit und Xylit in Lebensmitteln. Deutsche Lebensmittel-Rundschau 6:182–187
Butlen D, Vadrot S, Roseau S, Morel F (1988) Insulin receptors along the rat nephron:125I Insulin-binding in microdissected glomeruli and tubules. Pflügers Arch 412:604–612
Chauncey B, Leite MV, Goldstein L (1988) Renal sorbitol accumulation and associated enzyme activities in diabetes. Enzyme 39:231–234
Das B, Srivastava SK (1985) Purification and properties of aldose reductase and aldehyde reductase II from human erythrocyte. Arch Biochem Biophys 238:670–679
Gabbay KH (1973) The sorbitol pathway and the complications of diabetes. New Engl J Med 288:831–836
Gabbay KH, Cathcart ES (1974) Purification and immunologic identification of aldose reductases. Diabetes 23:460–468
Goldstein DA, Massry SG (1978) Diabetic nephropathy — clinical course and effect of hemodialysis. Nephron 20:286–296
González RG, Miglior S, von Saltza I, Buckley L, Neuringer LJ, Cheng H-M (1988)31P NMR studies of the diabetic lens. Magn Reson Imaging 6:435–444
Grunewald RW, Kinne RKH (1988) Sorbitol metabolism in papillary collecting duct cells (PCD) of diabetic rats. Pflügrs Arch 412:R46
Grunewald RW, Kinne RKH (1988) Sugar transport in isolated rat kidney papillary collecting duct cells. Pflügers Arch 413:32–37
Grunewald RW, Kinne RKH (1989) Intracellular sorbitol content in isolated rat inner medullary collecting duct cells: Regulation by extracellular osmolarity. Pflügers Arch 414:178–184
Grupp C, Pavenstädt I, Grunewald RW, Stokes III JB, Kinne RKH (1989) A Na-K-Cl cotransporter in isolated rat papillary collecting duct cells. Kidney Int (in press)
Guder WG, Schmolke M, Wirthensohn G (1988) Zur physiologischen Funktion des renalen Sorbitstoffwechsels und seiner Veränderung beim experimentellen Diabetes mellitus. Akt Endokrinol Stoffw 9:88
Hermann RK, Kador PF, Kinoshita JH (1983) Rat lens aldose reductase: rapid purification and comparison with human placental aldose reductase. Exp Eye Res 37:467–474
Kunst A, Graeger B, Ziegenhorm J (1984) Colorimetric methods with glucose oxidase and peroxidase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol VI. Verlag Chemie, Weinheim Deerfield Beach Basel, pp 178–185
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurements with the Folin phenol reagent. J Biol Chem 193:265–275
Nakanishi T, Balaban RS, Burg MB (1988) Survey of osmolytes in renal cell lines. Am J Physiol 255:C181-C191
Needleman P, Passonneau JV, Lowry OH (1968) Distribution of glucose and related metabolites in rat kidney. Am J Physiol 215:655–659
Nguyen TH, Williams EH (1985) Effects of hyperglycemia on the expression of aldose reductase in lens epithelial cells. J Cell Biol 101:373
Noll F (1984)l-(+)-lactate. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol III. Verlag Chemie, Weinheim Deerfield Beach Basel, pp 582–592
Stokes III JB, Grupp C, Kinne RKH (1987) Purification of rat papillary collecting duct cells: Functional and metabolic assessment. Am J Physiol 253:F251-F262
Vassault A (1984) Lactate dehydrogenase. UV-method with pyruvate and NADH. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol III. Verlag Chemie, Weinheim Deerfield Beach Basel, pp 118–126
Wirthensohn G, Lefrank S, Schmolcke M, Guder WG (1989) Regulation of organic osmolyte concentration in tubules from rat renal inner medulla. Am J Physiol 256:F128-F135
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Willi, R., Kinne, R.K.H. Sorbitol metabolism in inner medullary collecting duct cells of diabetic rats. Pflugers Arch. 414, 346–350 (1989). https://doi.org/10.1007/BF00584637
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00584637