The Acoustic World of Harbor Porpoises
By Magnus Wahlberg, Meike Linnenschmidt, Peter Madsen, Danuta Wisniewska, Lee Miller
New research gives a clearer picture of how this specialized mammal perceives its underwater environment.
New research gives a clearer picture of how this specialized mammal perceives its underwater environment.

DOI: 10.1511/2015.112.46
The world as we experience it is a heavily filtered and modified version of the "real" world. For example, our hearing abilities are limited to a frequency range from about 20 hertz to 20 kilohertz, making infrasonic signals from elephants and ultrasonic echolocation signals from bats inaudible to us.

Photograph by Peter Verhoog, Fjord&Bælt.
Jakob von Uexküll, the 20th-century Estonian-German biologist and philosopher, introduced the concept of an animal's Umwelt: the world around the animal as experienced through its sense organs. One of the classic examples is von Uexküll's description of a tick's Umwelt, described in his book A Foray Into the Worlds of Animals and Humans. Ticks use chemical and temperature cues to 'decide' whether they should grab onto a possible host. They have neither eyes nor pressure- sensitive ears. Even though they inhabit the same fields that we may enjoy walking in, their Umwelt is completely different from ours. Similarly, the Umwelt of ants, birds, frogs, and snakes living in the same field will all be very different from each other, as their sensory organs will pick up and process subsamples of environmental information in very different ways.
Take the Umwelt of whales in aquatic environments. The ancestors of whales were terrestrial until some 40 million years ago. From this time whales have secondarily adapted to the aquatic environment in many physical and physiological ways. Among other traits, they have eyes and ears tuned to be functional under water, allowing for orientation and pursuit of prey such as krill, squid, fish, and in some cases, other marine mammal species. The Umwelt of humans in water is very different. Beyond the fact that we need special equipment to breathe, our own terrestrial adaptations reveal obvious disadvantages when we dive under water.
To understand another animal’s Umwelt, we first must understand our own—in this case, understanding what we do not perceive. Underwater, without a dive mask, a beautiful coral reef becomes blurred, and we lose our ability to tell the direction of sound sources such as the eerie calls of whales and fish. Weightlessness and difficulties in recognizing visual and acoustic landmarks lead to disorientation. These disadvantages for humans and other terrestrial mammals have been greatly reduced for whales through evolutionary time.
There are many reasons our senses do not work properly in water. Let us take hearing as an example. Briefly, airborne sound is collected by the outer ear, called the pinna, and led into the ear canal. At the end of the canal, the tympanic membrane is set into vibration from the pressure fluctuations created by the impinging sound wave. The three middle ear bones transfer the vibrations from the tympanic membrane to the oval window of the cochlea, putting the fluid inside it, called endolymph, into motion. A complex process of mechanical movements in the cochlea stimulates the auditory hair cells to evoke nerve impulses in the eighth cranial nerve, which leads to the brain stem. From there, neural information is transmitted to the auditory cortex after passing through synaptic connections in several ganglia. Eventually, more than 50 milliseconds after the sound arrived at the outer ear, we consciously experience having heard something.
Our ears are adapted for listening to airborne sound. The path from the outer to the inner ear only works properly in air, where the eardrum and middle ear bones efficiently transfer sound energy. Our directional hearing is achieved by the shadowing effect of the head, by the reflective properties of the pinna, and by the differences in time of arrival between the two ears. In water, sound can penetrate the body at many locations and be led directly to the inner ear through so-called bone conduction. Our directional hearing is seriously limited under water mainly because the mechanical and acoustical properties of water resemble those of the tissues of our bodies more so than air. In water the speed of sound and the wavelength for any given frequency are about 4.4 times greater than in air. So for humans in water the directional cues for finding a sound source are greatly diminished.
Hearing is the most important sensory modality for a whale because sound travels farther than light in water and can be used at night, or in murky waters. But how have adaptations in whales overcome the difficulties of hearing in water? Obviously, whales do not have an outer ear, even though the remnants of the opening of the ear canal can be seen as a small dot on the skin in some species. Instead, in toothed whales, sound is conducted to the middle and inner ears through specialized fat channels in the lower jaw. The structure (and probably the function) of the inner ear in toothed whales is similar to that of other mammals. For example, toothed whales and humans can pinpoint the direction of a sound source with similar precision, to a few degrees.
Just how this directionality is achieved in toothed whales is not well understood, but their inner ears are, unlike in humans, located in bony capsules below and separate from the skull. This positioning limits bone conduction and thus makes it possible to keep the two ears acoustically isolated from each other, which can help better determine the direction to a sound source or echo-reflecting prey. In general the underwater hearing range of toothed whales is vastly expanded compared to a human’s in air. For example, the harbor porpoise (Phocoena phocoena) can hear frequencies from about 100 hertz to 150 kilohertz. Higher frequencies mean shorter wavelengths, making it possible for them to echolocate smaller targets.
Although humans have limited senses in the underwater environment, we have the intriguing prospect of learning what that missing perception might be like by studying the creatures that have it. We have had the opportunity to study the acoustic Umwelt of the harbor porpoise at facilities in Kerteminde, Denmark: the Fjord&Bælt, an educational outreach facility with an outdoor enclosed water area, and the Marine Research Laboratory of the University of Southern Denmark. These facilities opened in 1997 when two 1- to 3-year-old harbor porpoises that had been by-caught in fishing nets were introduced to the outdoor pool of the Fjord&Bælt. At the ends of the pool are nets separating it from the narrow Kerteminde harbor, which is connected to the Great Belt at one end and to a shallow fjord at the other end. Tides, wind, and current cause large water movements in and out of the harbor, keeping the water in the facility fresh.
In 2004 a third harbor porpoise was added to the Fjord&Bælt. A female calf was born in 2007; this event marked the first successful birth and maturation of a harbor porpoise in captivity, offering a unique opportunity to study its development. Head trainer Jakob Kristensen and his team at Fjord&Bælt train the porpoises there using operant conditioning with positive reinforcement. In colloquial terms, this technique is called "clicker training" by, for example, dog and horse trainers. The trainer makes a movement signifying the porpoise should perform a certain behavior. After the animal accomplishes this task, the trainer gives what’s called a bridge signal (usually a whistle) indicating that the response was correct, and the porpoise returns to receive its reward (a fish).
The harbor porpoise is one of the smallest whales. It has been described as a whale “in the fast lane” by marine biologists Andrew Read (of Duke University) and Aleta Hohn (of the National Oceanic and Atmospheric Administration) because it breeds at yearly intervals, and it needs to eat nearly constantly because of its small size and its habitat in the temperate to cold waters of the Northern Hemisphere. Adult harbor porpoise females weigh about 60 kilograms and are about 170 centimeters in length whereas adult males are about 45 kilograms and 150 centimeters long. The details of their social system are not fully known. There seems to be no stable family structure, such as is found in many dolphin species. Instead, animals loosely aggregate when foraging, but otherwise seem to live solitary lives, except for the strong mother–calf bond that lasts for at least eight months. Observations of animals in human care indicate that females also can have strong bonds to other females.
Porpoises feed on small fish and squid and perform up to 10-minute-long dives down to a maximum of a few hundred meters, but most dives are short and shallow.
Many people are more familiar with dolphins than porpoises, but there are some significant differences between these two closely related families of animals. There are about 40 species of dolphins, but only six of porpoises. The most consistent physiological difference, across all these species, is the teeth: Porpoises have rounded ones; dolphins have pointed ones. Among other traits, commonly porpoises have less diverse vocalizations, and they tend to have rounded noses, unlike the beaked faces of dolphins. And dolphins tend to have about twice the life span of porpoises (about 40 versus 20 years, respectively).
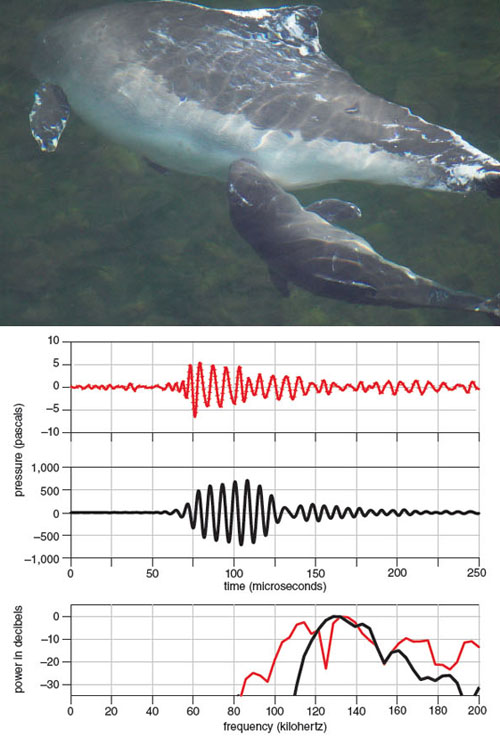
Two decades of research on porpoises in our facility have given us many insights into their acoustic Umwelt. To a large degree, they rely on acoustics to find their prey and navigate underwater. Almost constantly they emit short and powerful clicks of extremely high frequency, which were first described in 1971 by Nikolai Dubrovskiy and his colleagues, now of the Russian Academy of Sciences, and in 1973 by Søren Andersen and Bertel Møhl, both at the University of Copenhagen at the time. The clicks are only 50 to 100 microseconds long and have a frequency centered around 130 kilohertz, making them some of the most high-pitched signals produced by any animal. Naturally the clicks are inaudible to humans, but they are still of extremely high intensity: If we could hear these frequencies well under water, their most powerful clicks repeated at a high rate could actually cause hearing damage in humans, even at several meters’ distance.
When the clicks bounce off a fish or another item in the water, a faint echo returns. If the echo is audible to the porpoise, the delay time from the emitted click to the returning echo tells the porpoise the distance to the fish and, with its sensitive hearing, the porpoise can also determine the direction to the prey. Thus, the porpoise has a built-in echo sounder it can use for echolocating prey and for orientation. We call this biological sonar or biosonar for short, and toothed whales share this trait with bats, the only other animals that use biosonar while capturing prey. When swimming and searching for prey, harbor porpoises emit clicks about 20 times a second. When homing in on prey, the click rate increases and ends at several hundred clicks per second in what’s called a terminal buzz when the prey is captured. The pattern of emitted signals during prey capture is very similar among harbor porpoises and other toothed whales, as well as in insectivorous bats.
Photograph courtesy of Fjord&Bælt. Illustration by Tom Dunne.
Besides echolocation, porpoises also use their high-pitched clicks for communication. Actually these are the only signals heard from harbor porpoises. Most dolphins, unlike porpoises, use a wide range of whistles and clicks for communication. By varying the repetition rate of clicks, porpoises can express various types of signals, but the meaning of these click patterns is still largely unknown. However, work by one of our former Master’s students, Karin Clausen, suggests that a signal with a very high repetition rate indicates aggression, whereas an upsweep in repetition rate seems to be used as a contact call.
We were fortunate to follow the development of biosonar in a newborn calf at the Fjord&Bælt. Just after birth, the calf started to emit relatively low-pitched signals, audible to humans. Within an hour, it started to produce clicks with high frequencies centered around the main frequency of the adult clicks (see figure above). After a few days, the young porpoise’s biosonar seemed fully functional, but it did not catch and eat fish until it was weaned (after more than eight months).
Harbor porpoises make their click sounds with a pair of special organs called the phonic lips, located in the nasal air passage just below the blowhole. The blowhole is really the fused nostrils of the whale, having migrated upwards during evolutionary time to facilitate breathing. The porpoise forces air through the phonic lips, which causes these structures to vibrate and produce clicks. We have shown that they use primarily only the right pair of phonic lips for click generation, as do other toothed whales, for reasons that remain unknown. The sound leaves the head through the fatty roundish tissue between the rostrum and the blowhole, called the melon.
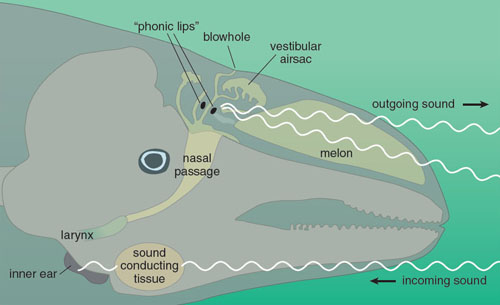
Illustration by Tom Dunne.
Properties of the melon and structures around it cause the sound to be emitted in a narrow beam, about 12 degrees wide. This beam, together with the high frequencies, enables the porpoise to focus sound on the target while reducing echoes from nearby objects. Also, high frequencies will in general improve the resolution of the biosonar system, making it possible for the porpoise to obtain information about small objects and prey. Finally, and perhaps most important, using very high frequencies makes it difficult for a predator such as killer whales to hear the porpoises’ signals.
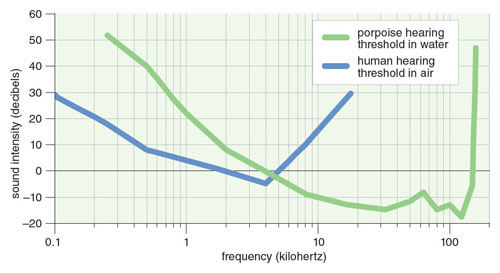
Another important aspect of an animal’s Umwelt is sound reception. There would be little function to biosonar without pairing the outgoing signals with the harbor porpoise’s excellent hearing. Ronald A. Kastelein and his colleagues of the Sea Mammal Research Company in the Netherlands found the best sensitivity at about 125 kilohertz with an extremely low auditory threshold (see figure below). The auditory sensitivity of the harbor porpoise is about the same as the most sensitive bat, Megaderma lyra.
Data adapted by Tom Dunne from Kastelein et al. 2010 and the American National Standards Institute Audiometer Standard 3.6 (1969).
Hearing can be studied using psychophysics, where the animal is trained to reply whether or not it can hear a sound presented to it. This process is very similar to how hearing is normally measured in humans. Such experiments, however, take intensive training of the animal and consequently a very long time. Therefore scientists increasingly rely on direct electrophysiological measurements of the neural response to sound. The deflection of the hair cells in the cochlea causes neurons in the eighth cranial nerve to generate action potentials.
Watch a video that shows an example of one such experiment where two different spheres–one aluminum and the other plastic–are introduced to a blindfolded porpoise that must locate the aluminum sphere:
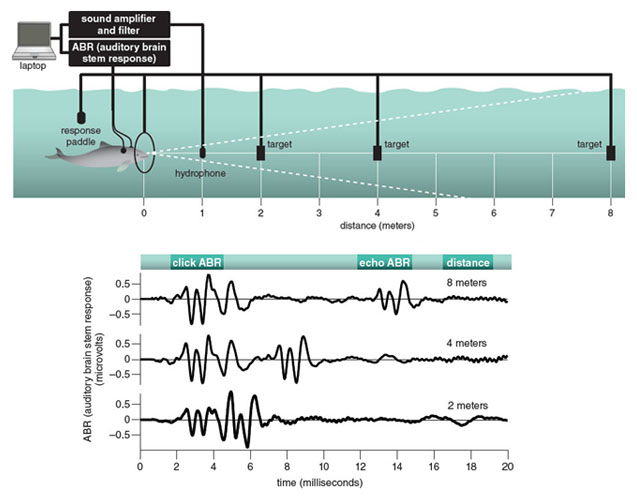
The auditory ganglia in the brain stem contain numerous large neurons that produce big electrical responses and rapidly conduct neural signals to the auditory cortex. This neural activity can readily be recorded via electrodes attached to the skin near the source. This is the auditory brain stem response (ABR) to sound stimuli. The same technique is used to measure the hearing abilities of newborn babies. When working with harbor porpoises, we attach suction cup electrodes to the skin of the head and back regions and, with the appropriate recording equipment, we can measure ABR to their echolocation clicks as well as to the echoes from objects they ensonify (see figure below).
Adapted by Tom Dunne from Linnenschmidt et al., 2012.
Recently we used combined methods to study echolocation and hearing in harbor porpoises at the same time. First, we designed a psychophysical experiment around echolocation. The porpoise was sent down to a hoop, where it remained stationed in front of an opaque screen and an acoustic screen. In some trials, a target (a hollow 18-centimeter-long cylinder) was lowered down one meter on the other side of the opaque and acoustic screens at different distances (two, four, and eight meters) from the porpoise. When the acoustic screen was removed, the animal could echolocate through the opaque screen, but could not see the target. A hydrophone on the acoustic axis recorded the level of the emitted clicks. For a target present, the animal should leave the hoop and indicate that it can detect the cylinder by touching the tip of its rostrum on a response paddle at the surface. If the target is not there, the animal should stay at the station for a certain time. If the animal makes the correct choice, the trainer reinforces the porpoise by blowing a whistle and giving it a fish reward.
Watch a video that visualizes this experiment:
While the animal is performing the behavioral experiment, ABR electrodes in suction cups are attached so we can also obtain information on its hearing abilities. With this method we can record the ABR of sounds made by the porpoise, the emitted click level, as well as that of echoes returning from the target. This experimental setup is similar to that used on dolphins at the Hawaii Institute of Marine Biology spearheaded by Paul Nachtigall, Alexander Supin, and their colleagues.
As the target range increases, the sound level of the echo decreases, meaning that the ABR elicited by the echo should also decrease. But the surprising result in our studies, and in those on dolphins too, was that the ABR generated by the echo remained nearly unchanged, independent of target distance. It seems that the animal can adjust the perceived sound to a convenient level and thus compensate for attenuation caused by changes in distance to the target. This ability may help the animal detect, localize, and classify targets.
Several mechanisms can explain these behaviors. First of all, the emitted sound level is reduced when the target range decreases. Second, the powerful outgoing signal affects the echo reception, a well-described phenomenon not only in whales but also in other mammals. Our harbor porpoise study suggests that besides these two mechanisms, there is a third one in play. This mechanism seems to be able to reduce the hearing sensitivity in a way not explained by the other two.
Combining these data about harbor porpoise’s sensory abilities with a view of its Umwelt can help with understanding, and hopefully solving, a threat to these animals. Danish porpoises live in shallow waters mainly less than 50 meters deep, and will go for minute-long dives to hunt for their favorite prey, a variety of small fish and squid. Fishermen accidentally by-catch harbor porpoises in gill nets, where the porpoises can drown. But the properties of porpoise biosonar indicate they should be able to detect gill nets at distances of 5 to 10 meters.
In fact it seems porpoises can detect nets at much greater distances. Porpoises are found very close to the coast at our Northern Funen field site. Here our former Master’s student, Torben Nielsen, tracked porpoises from land while at intervals he deployed a fishing net in the area. When comparing the distribution of porpoises, he could see that the porpoises reacted to the fishing net at very long distances, beyond 50 meters and perhaps close to 100 meters. These are much longer detection ranges than previously assumed. The discrepancy in detection distance might be explained by our discovery of the porpoise’s ability to regulate their auditory perception.
If the porpoises can detect fishing nets at such great distances, then why do they get by-caught? One obvious possibility is that they are directing their biosonar somewhere other than at the nets. We know porpoises like to feed on bottom-dwelling fish and in this case they would not detect a gill net because the animals would be directing their biosonar downward instead of forward.
One technique that has proven useful for reducing by-catch is placing acoustic alarms on fishing nets. Such alarms either scare porpoises away or draw their attention to the net, hopefully causing the porpoise to avoid it. Studies by Finn Larsen at the Technical University of Denmark and others show that acoustic alarms on nets indeed reduce porpoise by-catch.
The adult porpoises at Fjord&Bælt react promptly when we throw a dead fish into the enclosure. It does not take many seconds for a porpoise to localize, approach, and capture the fish. This behavior has been studied in great detail. The animals are trained to wear suction cups on their eyes, occluding visual cues, but they can still find and capture the fish by emitting clicks and listening for the returning echoes.
This experiment has taught us much about how porpoises use their biosonar. First, just like other echolocators, porpoises avoid "stepping on their own echoes." That is, after emitting a click they will wait for all relevant echoes to return before emitting the next click. While searching for prey, porpoises usually emit clicks of rather long inter-click intervals (about 50 milliseconds), allowing echoes to return from ranges of up to a few tens of meters before the next signal is emitted. The animal is constantly moving its head, and thus the sound beam, from side to side and up and down while searching for interesting echoes from different directions.
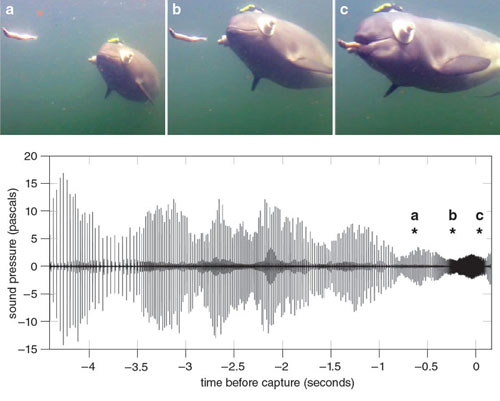
Adapted by Tom Dunne from Wisniewska et al., 2012.
Once the direction to the prey item has been determined, the animal keeps its sound beam on the prey while approaching. During this phase, the intervals between clicks as well as the intensity of the clicks are progressively decreasing. At very close range the porpoise switches to the so-called buzz phase where it can emit up to 500 clicks per second during prey capture (see figure above). With the progressive decrease in intensity of the emitted biosonar click while approaching the prey, and the auditory control adaptation mentioned above, the perceived levels of echoes from the prey remain relatively constant and independent of the distance to the prey.
Even though we have a good understanding of these various parts of the chase in terms of echolocation behavior, our understanding of how the porpoise perceives the situation is less clear. To picture the acoustic world of the porpoise during the search, we can imagine using a stroboscopic light when walking through a dark forest. We get a visual snapshot of our surroundings for each flash, and if the trees are far apart, we can navigate with a low flash rate to save batteries. If we want to pick an apple off a branch, we will have to increase the flash rate to precisely localize the apple. However, light is processed in parallel, whereas sound is processed sequentially; this means that the “picture” obtained by echolocation is probably not three-dimensional, as is the case with vision, but rather one- or two-dimensional. Light is processed without delay whereas echoes from biosonar signals are processed sequentially with delay. Thus the harbor porpoises’ acoustic Umwelt is updated in a discrete fashion for every new click, making it much more limited than our visual Umwelt.
Porpoises can adjust a perceived sound to a convenient level and thus compensate for attenuation caused by changes in distance to the target.
What is the acoustic Umwelt of the porpoise like during the chase? How does the animal make up its mind within a split second whether a detected echo is an edible item worth pursuit, an inedible item it should ignore, or perhaps a predator it should actively avoid? What does the auditory world look like to the porpoise when it finds a fish and goes into the final stage of capture, emitting clicks at a rate of up to 500 per second? Would it appear as a motion picture, as it would to us if we increased the flash rate to over our flicker-fusion-frequency of about 50 flashes per second?
Some of the above questions are very suitable to study with trained porpoises. By using external video recordings and an archival digital recording tag (which is fitted with two hydrophones and inertial sensors) built by Mark Johnson at St. Andrews University, we have studied the detailed searching behavior of porpoises when choosing between two spherical targets of various materials, each with a tiny hydrophone to measure the impinging porpoise signal.
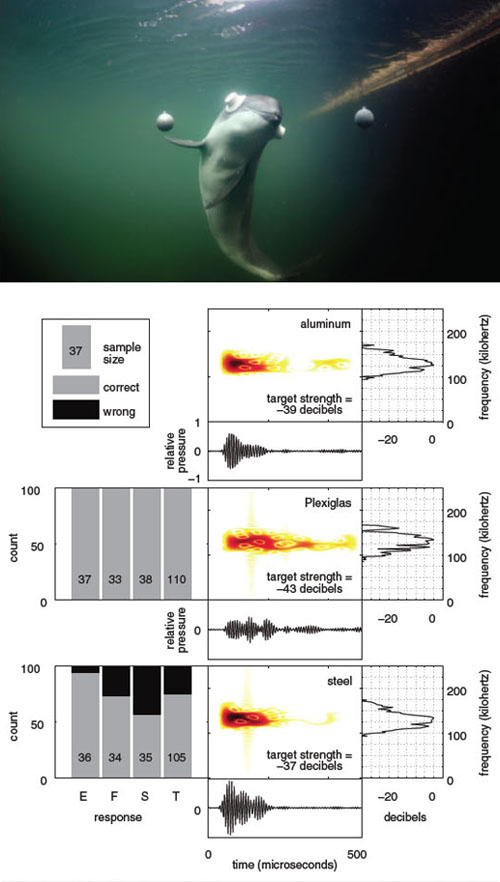
Top photograph by Solvin Zankl, Fjord&Bælt; data adapted by Tom Dunne from Wisniewska et al., 2012.
There are small, subtle differences in the echoes from the balls made of different materials. The porpoise can use these cues to discriminate between the objects. It first sends a biosonar click toward one of the balls, then directs its "acoustic gaze" toward the other one. By shifting its attention back and forth between the targets, the porpoise can build up a comparison between them and make the correct decision in most cases. From a set of two balls, the porpoises were supposed to always choose the aluminum ball (the standard) over the comparison ball of different material. The porpoises made most mistakes when trying to discriminate between the aluminum sphere and the steel sphere, likely because the echoes from these two spheres were quite similar. They had no problem discriminating between the aluminum and Plexiglas spheres (see figure above).
The example above illustrates an important difference between the porpoise’s acoustic Umwelt and our visual Umwelt in that, had the aluminum and Plexiglas spheres been painted black, the porpoise could still hear the difference between the materials, but we could not see the difference. Also, the porpoise can receive indirect echoes reflected from objects outside of its acoustic beam, such as echoes from the water surface (see figure below), whereas visual cues outside our field-of-view are not detectable to us.
These features make the porpoise’s acoustic Umwelt potentially more complex than otherwise expected. Naturally they have passive hearing like us. However, we know little about how sounds—such as pile driving during offshore wind farm construction, seismic explosions used for oil and gas exploration, and ship noise—influence their behavior. We are now using digital recording tags on wild harbor porpoises to provide some answers to these questions.
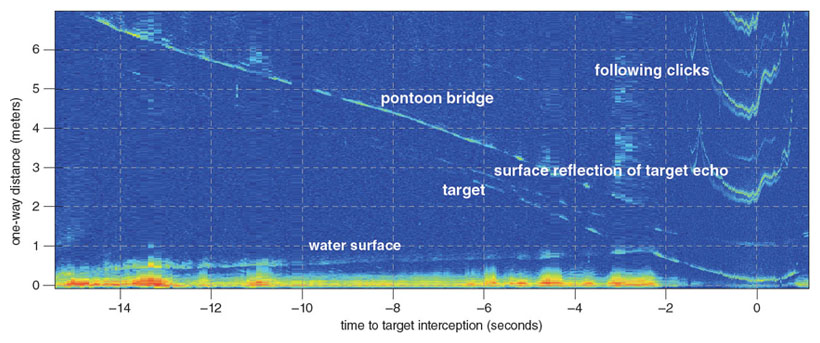
Adapted by Tom Dunne from Wisniewska et al., 2012.
Toothed whales and bats have a very special way of perceiving their surroundings through sound. By combining knowledge gained from tagged wild harbor porpoises with the results of experiments performed on trained animals, we are improving our knowledge of these elusive small whales, so that we can better understand their acoustic Umwelt, and in this way refine our ways of protecting them and the environment in which they live.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.