Abstract
Introduction
The presence of microembolic signals (MES) during the acute phase of stroke is poorly understood, and its role and clinical application in relation to risk stratification and prognosis in patients remain uncertain. We assessed the prevalence of spontaneous MES in acute stroke and their relationship with risk stratification, stroke recurrence, morbidity, and mortality.
Patients and methods
This was a prospective cohort study conducted in the Stroke Unit. The MES presence was evaluated by transcranial Doppler (TCD) in patients with ischemic stroke within 48 h. The outcomes (risk stratification, morbidity, mortality, and recurrence of a stroke) were followed up for 6 months. The relationship between risk stratification and MES was obtained by odds ratios and that between MES and stroke recurrence, morbidity, and mortality using multiple logistic regression; considering statistical significance at P < 0.05.
Results
Of the 111 patients studied, 70 were men (63.1%) and 90 were white (81.1%), with a median age of 68 years. The MES frequency was 7%. There was a significant relationship between MES and symptomatic carotid disease (OR = 22.7; 95% CI 4.1–125.7; P < 0.001), a shorter time to monitoring (OR = 12.4; 95% CI 1.4–105.4; P = 0.02), and stroke recurrence (OR = 16.83; 95% CI 2.01–141; P = .009).
Discussion
It was observed that the stroke recurrence adjusted for prior stroke was higher and earlier among patients with MES detection. In conclusion, MES demonstrated a significant correlation with symptomatic carotid disease and a shorter DELAY until monitoring, and could be a predictor for the early recurrence of stroke in the long term.
Similar content being viewed by others
Introduction
Transcranial Doppler (TCD) is an important tool used in health care to assess blood flow velocity in brain arteries, with extensive application in neurovascular clinical practice [1, 2]. TCD has potential diagnostic applications in locating embolic sources and long-term risk prediction of ischemic stroke [3]. The detection of embolic signals has been observed in a variety of potential embolic sources during patient assessment with TCD. Experimental studies have shown that this technique has a high sensitivity and specificity for the detection of various types of embolic elements [4, 5].
Data from many studies have demonstrated that the detection of spontaneous microembolic signals (MES) can predict the risk of stroke in patients with cardiac emboli or large artery disease, especially in cases of symptomatic internal carotid stenosis [6,7,8,9,10]. TCD is the only method that permits direct identification of embolic phenomena through the detection of an MES [11, 12].
The presence of MES on TCD during the acute phase of stroke is poorly understood, and its role and clinical application in relation to risk stratification and prognosis in patients remain uncertain, with no study having been conducted in the Latin American population. We assessed the hypothesis that investigating MES prevalence in the acute phase of stroke is related to risk stratification, stroke recurrence, disability, and death.
Materials and methods
Ethics approval and consent to participate
This trial was approved by a Committee for Ethics in Research involving human subjects from Ribeirão Preto School of Medicine. Upon inclusion, all patients or legal surrogates provided written informed consent in accordance with the Declaration of Helsinki II.
Study design, setting, and participants
This was a prospective cohort study of consecutive adult patients of both sexes diagnosed with ischemic stroke of the anterior circulation during the first 48 h of ictus. This study was conducted in the Stroke Unit at Botucatu Medical School (UNESP) from January 2015 to March 2016. The inclusion criteria for monitoring included a diagnosis of ischemic stroke of the anterior circulation territory confirmed by neuroimaging examinations (CT or MRI) and by OSCP (The Oxford Community Stroke Project classification) within the first 48 h of ictus, age above 18 years, monitoring with TCD for a minimum duration of 30 min, and performance of phenotypic classification and stroke risk stratification by Trial of Org 10172 in Acute Stroke Treatment (TOAST), preferably in the first 3 months after inclusion in the study [13].
The study excluded patients with a diagnosis of hemorrhagic stroke or with the presence of other types of brain lesions of non-vascular etiology, those who did not have a temporal acoustic window (TAW) to undergo TCD, those who underwent any surgical procedure that impeded the realization of monitoring, or those lost during the 6-month follow-up. Also excluded were examinations of poor technical quality during TCD due to various artifacts during recording.
Variables
Definition of risk factors
The risk factors evaluated were as follows: hypertension, smoking, obesity, diabetes mellitus (DM), at-risk drinkers, dyslipidemia, Chagas’ disease, prior stroke, congestive heart failure (CHF), prior acute myocardial infarction (AMI), coronary artery disease (CAD), depression, atrial fibrillation, atrial flutter, patent foramen ovale (PFO, confirmed with transthoracic and/or transesophageal echocardiogram), and extracardiac shunts. These data were collected from the clinical history of patients, such as previous use of antihypertensive medications, oral hypoglycemic agents or parenteral insulin, oral lipid-lowering drugs, or were confirmed clinically and by laboratory tests during hospitalization. Levels considered for confirmation were hypertension with systolic blood pressure ≥ 140/90 mmHg, dyslipidemia with cholesterol levels ≥ 240 mg/dL, diabetes mellitus with glycated hemoglobin level > 7%, obese patients with a body mass index ≥ 30 kg/m2, and a score > 8 in the Hospital Anxiety and Depression Scale (HADS) [14,15,16,17].
TCD examination
A portable TCD device (DWL, Doppler Box model, Compumedics, Singen, Baden-Württemberg, Germany) was placed between the lateral margin of the orbit and the ear, above the zygomatic arch. Low-power pulsed-wave 2-MHz transducers, 1.7 cm in diameter, were used with TCD-8 software (Version 8.00 K) at a pulse repetition frequency of 6500 Hz and a programmable high-pass filter of 50–600 Hz. The transducers were fixed bilaterally to a helmet to monitor both middle cerebral arteries at 45–60 mm.
All patients were initially evaluated by two independent neurologists for the presence of a temporal acoustic window (TAW) by TCD. Patients with bilateral TAWs underwent examination with TCD for at least 30 min. The researchers were single blinded during monitoring to the risk stratification of the patients. By the time of TCD, intravenous solution dripping was interrupted.
Microembolic signals detection
To evaluate MES, the following characteristics were considered according to the microembolus detection consensus [12]: randomly occurring during the cardiac cycle, short (< 0.1 s), high intensity (3–9 dB) above the wave spectrum, and characteristic audible sound. We used the Power Doppler M in all tests to identify the arteries and aid in the identification of microembolic signals (Fig. 1). In general, monitoring took 45 min. Automatic detection of microembolic signals was used in association with visual and sound discretion of microemboli. All tests were recorded for at least 30 min, filed, and independently reviewed by two blinded investigators to identify MES [12]. The presence or absence of MES was reported, as well the number of MES for each patient.
Clinical variables and risk stratification
The patients were classified according to TOAST, examination of brain imaging by computed tomography (CT) or magnetic resonance imaging (MRI), extensive laboratory examinations, electrocardiogram (ECG), transthoracic or transesophageal echocardiography, and duplex or CT angiography of the carotid and vertebrobasilar system. All patients underwent hospitalization in the Stroke Unit and received standard care according to the international protocols [18,19,20]. The degree of carotid stenosis was classified by the NASCET criteria, defined as < 50%, 50–69%, ≥ 70%, or occlusion [21]. TCD examination was performed in all patients to evaluate possible intracranial stenosis. MR angiography, CT angiography, and digital arteriography were performed on the suspicion of stenosis of intracranial or extracranial vessels. The examination of digital 24-h Holter monitoring was conducted in subjects over 55 years and suspected of paroxysmal atrial fibrillation.
Follow-up
Outpatient follow-up time was 6 months after the onset of stroke and was performed by a blinded investigator. Disability was monitored using the modified Rankin Scale (mRS), with mRS 0–2 being classified as favorable and mRS 6 as death, and the recurrence of stroke was monitored throughout the study. Recurrence of stroke was documented by means of clinical and radiological evaluation.
Primary outcome measures
Disability and death in 6 months.
Secondary outcome measures
Recurrence of stroke in 6 months.
Study size and sampling
We needed a minimum of 100 subjects to obtain a maximum sampling error of 7.5% and a confidence level of 95%. Type I and II error probabilities equaled 0.05 and 0.20, respectively, and it was estimated that the power to test the association between the presence of MES with stroke < 48 h post-ictus, atheromatous carotid, and symptomatic carotid artery disease was above 80%. For clinical follow-up data, it was estimated that the power to test the association between the presence of MES and recurrence, disability, and death was below 65%.
Statistical methods
The interobserver agreement of the presence of MES was classified by Cohen’s κ coefficient. The risk stratification of patients with acute ischemic stroke and the relationship with MES had point and interval estimations of the odds ratios, depending on the statistically related independent variables calculated by Fisher’s exact test, taking into account confounding factors (thrombolysis, level of anticoagulation, and antiplatelet therapies) at monitoring. The selected variables with P < 0.05 in the univariate analysis were included in the multiple logistic regression to determine the independent variables associated with the presence of MES.
The relationship between morbidity and mortality in patients and MES (presence or absence and number for patient) during follow-up was evaluated using univariate analysis for potential confounders, such as age, sex, race, hypertension, NIHSS at admission, previous mRS, hemorrhagic transformation, smoking, obesity, diabetes, dyslipidemia, Chagas’ disease, prior stroke, prior AMI, CAD, depression, wake-up stroke, carotid dissection, carotid atheroma, symptomatic carotid disease, intracranial stenosis, atrial flutter, atrial fibrillation, cardiac heart failure (CHF), patent foramen ovale (PFO), extracardiac shunt, and thrombolysis. A subsequent multiple logistic regression analysis was performed with backward selection correcting for the effect of potential confounders.
The variables more strongly associated (P < 0.20) with the incidence rate of stroke recurrence were included in a Cox proportional regression model. Statistical analysis was carried out with SPSS software (Version 21.0, SPSS, Chicago, IL, USA).
Results
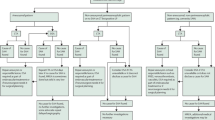
A total of 254 patients were screened in this study, with 105 excluded by the exclusion criteria. Only 149 patients were considered for monitoring, and 38 patients (26%) showed no TAW during TCD evaluation (Fig. 2).
A high level of concordance was demonstrated between the two experts who rated the presence of MES (κ = 0.95; P < 0.01, % agreement = 98%). The total number of patients monitored with TCD was 111; 70 were men (63.1%) and 90 were white (81.1%), with a median age of 68 years. Hypertension was found to be the main risk factor (83.8%). MES were found in eight patients (7%). Participant characteristics are displayed in Table 1.
In relation to MES detection, thrombolytic therapy and the level of use of therapeutic oral anticoagulation (INR > 2) and antiplatelet therapies at the time of monitoring were analyzed as potential confounders. There was no statistically significant relationship to these variables. Demographic variables, such as sex and race, were also not statistically significant for MES detection.
MES detection was higher in patients with atherosclerotic carotid (OR = 15.5, 95% CI 3.2 to 74.7; P = 0.001) and symptomatic carotid artery disease (OR = 22.7; 95% CI 4.1 to 125.7; P = 0.001). MES were not detected in patients with small vessel disease or atrial fibrillation. MES detection was higher among patients who were monitored earlier in relation to the onset of symptoms of stroke (OR = 2.4, 95% CI 1.4 to 105.4; P = 0.02) (Table 2).
Table 3 shows the association between MES and outcomes. The chance for favorable outcomes were reduced with increasing age (OR = 0.96; 95% CI 0.91 to 1.00, P = 0.036), increasing NIHSS at admission (OR = 0.80; 95% CI 0.73 to 0.87, P < 0.001), and higher prior mRS (OR = 0.31; 95% CI 0.14 to 0.66, P = 0.002). The probability for favorable outcomes tended to be higher in patients who smoked (OR = 2.83; 95% CI 0.98 to 8.15, P = 0.054). There was no significant relationship between MES and favorable outcomes (mRS 0–2) (OR = 2.4, 95% CI 0.36 to 16.36, P = 0.366). The probability of death was higher with increasing age (OR = 1.07; 95% CI 1.01 to 1.12, P = 0.014) and increasing NIHSS (OR = 1.16; 95% CI 1.07 to 1.26, P < 0.001). There was no significant relationship between MES and the occurrence of death (mRS 6) (OR = 2.27, 95% CI 0.19 to 26.50, P = 0.513).
The absence of a statistically significant association was found between favorable outcomes (mRS 0–2) (P = 0.429), and death (P = 0.154) and positive association between the number of MES for patient and recurrence (P = 0.017). However, multivariate analysis was not performed due to the low number of patients with MES (great asymmetry of the sample). The OR for the outcomes as a function of the number of MES was analyzed for the total sample, and the analysis showed no statistical differences in terms of favorable outcomes for lower number of MES for patient (OR = 0.85 95% CI 0.56 to 1.30). The mortality (OR = 1.41 95% CI 0.92 to 2.16) and recurrence (OR = 1.55 95% CI 0.88 to 2.76) increased with the more number of MES for patient, but none of the three associations was statistically significant.
Four patients (3.6%) had a recurrence of stroke within 6 months of follow-up, and the TOAST classifications of these strokes were small vessels, cardioembolic, atherosclerosis of large vessels, and indeterminate nature, respectively. There were no confounding factors for stroke recurrence, and there was a significant relationship between the detection of MES and stroke recurrence during follow-up (OR = 16.83, 95% CI 2.01 to 141.08; P = 0.009).
Table 4 and Fig. 3 demonstrate the identified factors that were more strongly associated with the incidence rate of stroke recurrence.
It was observed that the stroke recurrence adjusted for prior stroke was higher and earlier among patients with MES detection (RRadjusted = 36 (4–323), P < 0.001).
During hospitalization and after discharge, 16 patients started using anticoagulant therapy, while all others were given antiplatelet therapies. The treatment of carotid artery disease was performed in 5 patients exclusively with endovascular therapy (stent). No patient was treated with endarterectomy due to an intrinsic characteristic of the hospital.
Discussion
Of the total number of patients involved in this study, 26% did not have a TAW on TCD examination. These results were demonstrated in a previous study with the same sample by our stroke team [22]. The other studies in the literature showed a range of 3–34% absence of acoustic window, including populations from healthy volunteers and patients with cerebrovascular disease [23].
The MES detected by TCD have been described in patients with carotid artery stenosis, dissection of large cervical vessels, atrial fibrillation, catheter-based procedures, and atheromatous plaques in the aortic arch, but their clinical application remains uncertain [24, 25]. The number of patients with MES in this study was 8 patients (7%). Other samples showed variations in the rate of MES detection in the acute phase of stroke ranging between 9.3 and 71%, with most studies demonstrating a relationship with large artery disease and less frequently with cardioembolic disease [26,27,28,29]. MES were not detected in cases with intracranial stenosis, in contrast to a prevalence of 22% reported in other studies [3]. The high percentage of patients who underwent thrombolytic treatment or who were using antiplatelet therapies during transcranial Doppler monitoring, despite not presenting a statistically significant correlation with the detection of MES, may have influenced the low detection rate of these MES. However, it must be emphasized that these monitoring conditions in the acute and subacute stages of a stroke correspond to the conditions that most resemble the real world.
A statistically significant relationship between the MES and patients with large vessel disease was found. There was also a significant association between detecting MES and symptomatic carotid artery disease. Of patients with symptomatic carotid disease, two had arterial dissection at the time of monitoring by TCD, and in one of these, MES were found. Some other studies have demonstrated the importance of microembolism in TCD monitoring as a risk factor for stroke in carotid dissection [30,31,32]. One of the patients examined in the present study was diagnosed with pulmonary thromboembolism and left-to-right shunts could be detected with a simple bubble test, without contrast. This patient had extensive ischemia in the territory of both the middle cerebral arteries with bilateral MES during TCD monitoring, a relatively rare finding in the literature [33]. TCD associated with the use of contrast is a useful tool in the investigation of right-left deviations, such as patent foramen ovale and other rare medical conditions, such as pulmonary fistula.
This study showed a higher chance of MES detection the sooner the monitoring time was in relation to the onset of symptoms of stroke. Studies conducted in the 1990s showed that early monitoring with TCD in acute stroke increases the likelihood of the detection of MES [34].
No relationship was found between the detection of MES and the degree of disability and death; correlations were found with aging, NIHSS, and prior mRS. These findings were similar to other studies in the literature [6, 34]. In this study, smoking seemed to be a protective factor. This paradoxical finding was shown in other studies and can be explained by the better vascular recanalization in patients who smoke [35].
The presence of MES showed a good correlation with early stroke recurrence. A study that analyzed 467 patients showed that the risk of ischemic stroke occurrence increased 5.57 times over the course of 2 years of follow-up when MES were identified and that when MES were detected, there was a greater chance of new cerebral ischemic events occurring [27, 28]. In a meta-analysis from 2009, the authors found a twofold increase in the chance of new cerebral ischemic events when they identified embolic signals in TCD monitoring [3].
In relation to the recurrence of cerebral ischemic events, the present study showed a relationship between coronary artery disease (CAD) and stroke recurrence, demonstrating the probable role of systemic atherosclerotic disease and the role and coexistence of atherosclerotic cardiac comorbidities in these patients, even though we cannot rule out the presence of hidden atrial fibrillation in patients with high burden of atherosclerotic diseases. The low rate of stroke recurrence at 6 months of follow-up may be justified by the clinical and endovascular therapies instituted.
Clinical implications
Early detection of MES in acute phase of stroke may be a clinical indicator to determine carotid disease and recurrences of stroke. This indicator should promptly request a confirmation of possible carotid disease with other methods.
Limitations and strengths
The main limitation of this study is the small number of patients included, as well as the non-inclusion of patients with strokes in the posterior circulation. Also, a limitation of TCD is that the TAW can be obtained from a variable percentage of patients, as well as the fact that this technique is operator dependent. Other limitations of this study are related to the characteristics of the recruited patients (the study was performed in a single center), in addition to technical difficulties with the settings of the monitoring helmet, which generated a greater number of artifacts. The fact that the patients were in the acute phase of stroke and the presence of neurological symptoms, such as unilateral spatial neglect, aphasia, psychomotor agitation, or delirium, made TCD monitoring difficult. In relation to the MES detection points, care must be taken during the examination and the interpretation of the findings due to the need to discriminate MES from possible artifacts.
Conclusion
In conclusion, it was found that MES detection showed a higher correlation with symptomatic carotid disease and a shorter time between ictus and TCD monitoring. In addition, the presence of MES and CAD could be predictors for stroke recurrence.
Availability of data and materials
Data are available with the main author, who can provide all information in case of the editor’s requirement.
References
Garami Z, Alexandrov AV (2008) Neurosonology. Neurol Clin 27:89–108
Sloan MA, Alexandrov AV, Tegeler CH, Spencer MP, Caplan LR, Feldmann E et al (2004) Therapeutics and technology assessment subcommittee of the American Academy of Neurology. Assessment: transcranial Doppler ultrasonography. Neurology 62:1468–1481
King A, Markus HS (2009) Doppler embolic signals in cerebrovascular disease and Prediction of stroke risk: a systematic review and meta-analysis. Stroke 40:3711–3717
Markus HS, Brown MM (1993) Differentiation between different pathological cerebral embolic materials using transcranial Doppler in an in vitro model. Stroke 24:1–5
Markus HS, Droste DW, Brown MM (1994) Detection of asymptomatic cerebral embolic signals with Doppler ultrasound. Lancet 343:1011–1012
Markus HS, MacKinnon A (2005) Asymptomatic embolization detected by Doppler ultrasound predicts stroke risk in symptomatic carotid artery stenosis. Stroke 36:971–975
Batista P, Oliveira V, Ferro JM (1999) The detection of microembolic signals in patients at risk of recurrent cardioembolic stroke: possible therapeutic relevance. Cerebrovasc Dis 9:314–319
Spence JD, Tamayo A, Lownie SP, Ng WP, Ferguson GG (2005) Absence of microemboli on transcranial Doppler identifies low-risk patients with asymptomatic carotid stenosis. Stroke 36:2373–2378
Spence JD (2017) Transcranial Doppler monitoring for microemboli: a marker of a high-risk carotid plaque. Semin Vasc Surg 301:62–66
Spence JD, Coates V, Li H, Tamayo A, Hackam DG, DiCicco M et al (2010) Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 67:180–186
Jiang J, Jiang Y, Feng S, Feng S, Sun D, Zhuang A, Zeng Q et al (2013) Microembolic signal monitoring of TOAST-classified cerebral infarction patients. Mol Med Rep 8(4):1135–1142
Sliwka U, Job FP, Wissuwa D, Diehl RR, Flachskampf FA, Hanrath P et al (1995) Occurrence of transcranial Doppler high-intensity transient signals in patients with potential cardiac sources of embolism. Stroke 26:2067–2070
Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL et al (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Stroke 24:35–41
Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M et al (2013) The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 31:1281–1357
Jellinger PS, Smith DA, Mehta AE, Ganda O, Handelsman Y, Rodbard HW et al (2012) American Association of Clinical Endocrinologists’ Guidelines for management of dyslipidemia and prevention of atherosclerosis. Endocr Pract 18(Suppl 1):1–78
American Diabetes Association (2008) Standards of medical care in diabetes. Diabetes Care 31(Suppl 1):S12–S54
Wirth A, Wabitsch M, Hauner H (2014) The prevention and treatment of obesity. Dtsch Arztebl Int 111:705–713
Adams HP Jr, Brott TG, Furlan AJ, Gomez CR, Grotta J, Helgason CM et al (1996) Guidelines for thrombolytic therapy for acute stroke: a supplement to the guidelines for the management of patients with acute ischemic stroke. Circulation 94:1167–1174
Hacke W, Kaste M, Skyhoj Olsen T, Bogousslavsky J, Orgogozo JM (2000) Acute treatment of ischemic stroke. Cerebrovasc Dis 10(Suppl 3):S22–S33
European Stroke Initiative (EUSI): recommendations for stroke management. Cerebrovasc Dis 2000; 10(Suppl 3):S1–S34
Barnett HJ, Taylor W, Eliasziw M, Fox AJ, Fergunson GG, Haynes RB et al (1998) Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 339:1415–1425
Bazan R, Braga GP, Luvizutto GJ, Hueb JC, Hokama NK, Zanati Bazan SG et al (2015) Evaluation of the temporal acoustic window for transcranial Doppler in a multi-ethnic population in Brazil. Ultrasound Med Biol 41:2131–2134
Klotzsch C, Popescu O, Berli P (1998) A new 1 MHz probe for transcranial Doppler sonography in patients with inadequate temporal bone windows. Ultrasound Med Biol 24:101–103
Rundek T, Di Tullio MR, Sciacca RR, Titova IV, Mohr JP, Homma S et al (1999) Association between large aortic arch atheromas and high intensity transient signals in elderly stroke patients. Stroke 30:2683–2686
Oliveira V, Batista P, Soares F, Ferro JM (2001) HITS in internal carotid dissections. Cerebrovasc Dis 11:330–334
Iguchi Y, Kimura K, Kobayashi K, Ueno Y, Shibazaki K, Inoue T (2008) Microembolic signals at 48 hours after stroke onset contribute to new ischaemia within a week. J Neurol Neurosurg Psychiatry 79:253–259
Poppert H, Sadikov S, Sander K, Wolf O, Sander D (2006) Embolic signals in unselected stroke patients: prevalence and diagnostic benefit. Stroke 37:2039–2043
Markus HS, King A, Shipley M, Topakian R, Cullinane M, Reihill S et al (2010) Asymptomatic embolisation for prediction of stroke in the asymptomatic carotid emboli study (ACES): a prospective observational study. Lancet Neurol 9:663–671
Wong KS, Chen C, Fu J, Chang HM, Suwanwela NC, Huang YN, CLAIR study investigators et al (2010) Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol 9(5):489–497
Markus HS, Droste DW, Kaps M, Larrue V, Lees KR, Siebler M et al (2005) Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: the clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation 111:2233–2240
Molina CA, Alvarez-Sabín J, Schonewille W, Montaner J, Rovira A, Abilleira S, Codina A (2000) Cerebral microembolism in acute spontaneous internal carotid artery dissection. Neurology. 55:1738–1740
Brunser AM, Lavados PM, Hoppe A, Muñoz-Venturelli P, Sujima E, López J et al (2017) Transcranial Doppler as a predictor of ischemic events in carotid artery dissection. J Neuroimaging 27(2):232–236
Zanati Bazan SG, Braga GP, Luvizutto GJ, Trindade AP, Pontes-Neto OM, Bazan R (2016) Bihemispheric paradoxical cerebral embolism in a patient with pulmonary thromboembolism and presumptive fistula right-to-left shunt. J Stroke Cerebrovasc Dis 25:e95–e97
Del Sette M, Angeli S, Stara I, Finocchi C, Gandolfo C (1997) Microembolic signals with serial Transcranial Doppler monitoring in acute focal ischemic deficit. Stroke 28:1311–1313
Barbash GI, White HD, Modan M, Diaz R, Hampton JR, Heikkila J et al (1993) Significance of smoking in patients receiving thrombolytic therapy for acute myocardial infarction. Experience gleaned from the international tissue plasminogen activator/streptokinase mortality Trial. Circulation 87:53–58
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualization: RB, JPL, OMPN. Data curation: RB, GJL, GPB, SGZB, JCH, APT, MLS, GPM. Formal analysis: RB, GJL, SGZB, JCH, PTHF, HRCN. Investigation: RB, GPB, JCH, CCMF, APT, MLS, GPM. Methodology: RB, SGZB, JCH, HRCN. Project administration: RB, PTHF, JPL. Writing-original-draft: RB, GJL. Writing-review and editing: RB, PTHF, JPL, OMPN. Validation: HRCN. Supervision: JPL, OMPN. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This trial was approved by a Committee for Ethics in Research involving human subjects from Ribeirão Preto School of Medicine. Upon inclusion, all patients or legal surrogates provided written informed consent in accordance with the Declaration of Helsinki II.
Consent for publication
All authors of the manuscript have read and agreed to its content and are accountable for all aspects of the accuracy and integrity of the manuscript in accordance with ICMJE criteria. The article is original, has not already been published in a journal, and is not currently under consideration by another journal. We agree to the terms of the SpringerOpen Copyright and License Agreement.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bazan, R., Luvizutto, G.J., Braga, G.P. et al. Relationship of spontaneous microembolic signals to risk stratification, recurrence, severity, and mortality of ischemic stroke: a prospective study. Ultrasound J 12, 6 (2020). https://doi.org/10.1186/s13089-020-0156-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13089-020-0156-1