Abstract
Background
Hospitalized patients with SARS-CoV2 develop acute kidney injury (AKI) frequently, yet gaps remain in understanding why adults seem to have higher rates compared to children. Our objectives were to evaluate the epidemiology of SARS-CoV2-related AKI across the age spectrum and determine if known risk factors such as illness severity contribute to its pattern.
Methods
Secondary analysis of ongoing prospective international cohort registry. AKI was defined by KDIGO-creatinine only criteria. Log-linear, logistic and generalized estimating equations assessed odds ratios (OR), risk differences (RD), and 95% confidence intervals (CIs) for AKI and mortality adjusting for sex, pre-existing comorbidities, race/ethnicity, illness severity, and clustering within centers. Sensitivity analyses assessed different baseline creatinine estimators.
Results
Overall, among 6874 hospitalized patients, 39.6% (n = 2719) developed AKI. There was a bimodal distribution of AKI by age with peaks in older age (≥60 years) and middle childhood (5–15 years), which persisted despite controlling for illness severity, pre-existing comorbidities, or different baseline creatinine estimators. For example, the adjusted OR of developing AKI among hospitalized patients with SARS-CoV2 was 2.74 (95% CI 1.66–4.56) for 10–15-year-olds compared to 30–35-year-olds and similarly was 2.31 (95% CI 1.71–3.12) for 70–75-year-olds, while adjusted OR dropped to 1.39 (95% CI 0.97–2.00) for 40–45-year-olds compared to 30–35-year-olds.
Conclusions
SARS-CoV2-related AKI is common with a bimodal age distribution that is not fully explained by known risk factors or confounders. As the pandemic turns to disproportionately impacting younger individuals, this deserves further investigation as the presence of AKI and SARS-CoV2 infection increases hospital mortality risk.
Similar content being viewed by others
Background
The SARS-CoV2 pandemic has killed more than 2.7 million people as of March 2021 [1]. Infection leads to a wide clinical spectrum from asymptomatic to severe multi-organ failure and death. Kidney involvement is increasingly recognized as an important complication of SARS-CoV2 infection, resulting in proteinuria, hematuria, and acute kidney injury (AKI) [2,3,4,5]. Kidney involvement is theorized to parallel severity of disease and associated common risk factors of hypoperfusion, ischemia and nephrotoxins. However, another hypothesis for kidney sequelae is related to the virus’ affinity for the ACE2 receptor with high density in the kidney [2, 6].
SARS-CoV2-related AKI has been reported in 25–60% of those critically ill, including up to 37% of critically ill children [7,8,9,10]. AKI has been associated with worse outcomes in those with Coronavirus Disease 2019 (COVID-19), the disease caused by SARS-CoV2. Initially, adult hospitals saw a rapid rise in the need for acute dialysis during COVID-19 waves [11, 12], yet this was not seen in pediatric hospitals. Overall, children seem less susceptible to infection and severe disease, so one hypothesis proposes lower rates of AKI/dialysis needs in children is a function of disease severity. Assessment of SARS-CoV2-related AKI across the age spectrum has not previously been reported.
The purpose of this study was to evaluate the incidence and epidemiology of SARS-CoV2-related AKI across the age spectrum and determine if age is an independent risk factor for AKI development in patients hospitalized with SARS-CoV2.
Methods
Study Design & Setting
This is a secondary analysis of the observational, international, prospective Viral Infection and Respiratory Illness Universal Study (VIRUS), initiated by Society of Critical Care Medicine (SCCM) in January 2020. VIRUS seeks to ascertain a wide range of clinical and outcome characteristics of patients hospitalized with SARS-CoV2 infection. The unique aspect of this registry is it captures both critically and non-critically ill hospitalized children and adults in the same cohort facilitating comparative evaluations.
Patients included in this analysis were admitted between January 2020 and March 2021; exact admission dates are confidential and not provided to investigators. Detailed methodologies have previously been described [13]. As this was deployed as a rapid registry early in the pandemic, detailed hospital-level characteristics are not available to investigators. Briefly, 298 centers from 26 countries contribute comprehensive pediatric and adult data from hospitalized patients encompassing intensive care units (ICUs) and non-ICUs. Ethical oversight was obtained at each local center and de-identified data stored in REDCap [14].
Patient population
We evaluated all participants in the registry if they had PCR- or antibody-confirmed presence of SARS-CoV2 infection, complete age and 28-day hospital outcome data, and at least one serum creatinine value. We excluded patients with clinical suspicion but no laboratory confirmation of SARS-CoV2, current pregnancy, chronic dialysis, or chronic kidney disease (CKD) stage 5.
Potential Bias
As this is an ongoing cohort registry, rapidly deployed during an evolving global pandemic, analyses were conducted by complete case analysis methods which could introduce some biases towards the more severe cases or because of imminent deaths. Nevertheless, the major exclusions were those without creatinine values or missing 28-day hospital outcomes as we assumed these patients to have the least complete data entry and highest risk for potential data entry errors.
Outcomes
The primary outcome of interest was AKI development as defined by Kidney Disease Improving Global Outcomes (KDIGO) serum creatinine-only criteria within the first 7 days of hospitalization [15]. AKI is defined as a rise in serum creatinine ≥0.3 mg/dL or > 50% from baseline. Urine output is considered part of the KDIGO AKI definition, but the registry data was determined to be insufficient as > 60% of our cohort was missing urine output values. We also further stratified AKI into stages and receipt of dialysis. Additional outcomes of interest included hospital mortality, hospital and ICU length of stay (LOS), and hospital-related complications.
The registry did not capture baseline creatinine (Crb) values (prior to hospitalization). It is therefore standard practice to estimate Crb [15,16,17]. However, the estimation of Crb is not standardized across the age spectrum. Using KDIGO guidelines for adults (≥18 years), we estimated a Crb by assuming an eGFR of 75 ml/min/1.73m2 and back calculating a creatinine with the modification of diet in renal disease (MDRD) equation [15]. No standard international guideline for estimating a Crb in children exists. We used the validated method of assuming eGFR of 120 ml/min/1.73m2 for children 2–17 years and median normative-based eGFR-for-age in children < 2 years and back calculating creatinine with the height-independent equation [18,19,20]. For patients with CKD, we used the minimum serum creatinine within the first 7 days of hospitalization as Crb estimation.
Though these are standard assumptions in AKI research in their respective fields of adult and pediatric nephrology [15,16,17], there is no standard acceptance of estimating Crb in the transition period from adolescents to adulthood. Therefore, given the lack of standardization for estimating Crb across the age spectrum, we conducted two sensitivity analyses: [1] using the full age spectrum (FAS) equation for both adults and children that does not assume a fixed eGFR by age but instead changes across the age spectrum to overcome this limitation [21] and [2] using the minimum serum creatinine as an assumed Crb for all patients. The FAS equation is limited as it has only been validated in Caucasian populations. The assumption of minimum creatinine as a baseline is limited as it assumes all patients return to their baseline within 7 days of hospitalization. In addition, we conducted a sensitivity analysis where race was removed from the MDRD calculation for adults [22].
Exposure
Primary exposure of interest was age; it was entered as years and months (children< 5 years), years (participants 5–90 years), and limited to ‘> 90’ for those > 90 years of age for privacy. For analysis, those > 90 were classified as 95 years. Age was evaluated as a continuous variable by years and categorical variable by 5-year and 20-year age increments to explore potential non-linear associations.
Additional variables
As this was an exploratory analysis, we included a variety of additional demographic, pre-hospital, and hospital-related variables from the registry. Sex and race/ethnicity were categorical. The registry de-identified center location except whether the center was in the United States or elsewhere. CDC classifications were used for weight categorization (underweight, normal weight, overweight, obese, severely obese) using BMI data for adults ≥18 years, BMI percentiles for children 2–17 years, and weight-for-height percentiles for children < 2 years [23]. CDC does not provide pediatric classification for severely obese, so those are grouped with obese for those < 18 years. SARS-CoV2 testing was determined by local centers. Other clinical data captured included comorbidities and recent pre-hospital medications as well as inpatient medications within the first 7 days. Comorbidities, including CKD, were determined by medical chart review by local investigators.
Illness severity was categorized by variables that span the age spectrum. Severe illness was defined as a composite of received invasive mechanical ventilation, vasopressor(s) and/or inotrope(s), and/or extracorporeal membrane oxygenation (ECMO). Moderate illness was defined by ICU admission without any organ support therapies listed above. Mild illness was defined as hospitalization but without an ICU admission nor organ support therapies as listed above. As some of these therapies may be clustered within centers, we accounted for this potential in our analyses described below. More traditional markers of illness severity were captured but do not translate across pediatric and adult patients so are not the primary marker assessed in this analysis (e.g., sequential organ failure assessment (SOFA) scores for adults and pediatric risk of mortality (PRISM) scores for children).
Statistical analyses
Descriptive statistics compared demographic, pre-hospital and inpatient clinical characteristics within the first 7 days of hospitalization among those with and without AKI. Wilcoxon rank sum tests and chi-square tests were used for continuous and categorical variables, respectively. Given the large sample size which leads to highly significant p-values, Cohen’s effect size estimates were calculated for continuous variables to better express the magnitude of differences (small effect 0.1–0.3, medium effect 0.3–0.6, large effect > 0.6). Univariate risk differences (RD), odds ratios (OR), and 95% confidence intervals (CIs) were calculated for hospital mortality by AKI stage. To account for common clinical practices, clustering within centers was used via generalized estimating equations (GEE) with logistic regression models to determine if age is an independent risk factor for the development of AKI in SARS-CoV2-related hospitalizations, with adjustments for the potential confounding of sex, hypertension, diabetes mellitus, cancer, CKD, race/ethnicity, and severity of illness as defined above. Determination for potential confounders to include in models were determined by a priori clinical knowledge and directed acyclic graphs. Significance was set at an alpha-level of 0.05. Sensitivity analyses were conducted using different equations for estimating a Crb and stratifications by comorbidities and whether center was U.S.-based. All analyses were conducted in SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina).
Results
Demographics
6874 patients from 142 centers met inclusion criteria (Fig. 1). 28% of participants were from non-U.S. centers (Table 1). A total of 39.6% (n = 2719) developed AKI within the first 7 days of hospitalization; this was significantly higher among patients in ICUs (1926/4075, 47.3%) compared to non-ICUs (793/2799, 28.3%), p-value< 0.0001. Almost 60% of the cohort were admitted to the ICU (n = 4075). The median age was 60 years (range 0–95 years) and 9.0% (n = 621) were < 20 years (Table 1). Those with AKI were more likely to be older (median age 65 years) than those without AKI (median age 55 years), p-value< 0.0001 and effect size 0.45, and more likely to have comorbidities (median 3 versus 1 in those without AKI), p-value< 0.0001 and effect size 0.37. Among those < 20 years, 28% (171/621) developed AKI. Differences in AKI risk based on race/ethnicity (p-value< 0.0001) were noted. Supplementary Table 1 includes hospital-related associations with AKI.
Comparing patients excluded to those included revealed no significant difference by age, sex, or location of center (i.e., U.S.-based). However, those excluded were more likely to have no comorbidities (26%) compared to those included (20%), and only 31% of the excluded group were admitted to the ICU (compared to 59% in this analysis). As expected, those missing creatinine values were often missing other key variables; BMI data missing for 50% of excluded patients compared to 24% of patients in this analysis.
Hospital complications
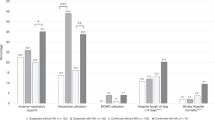
Among participants with AKI (n = 2719), 64% had Stage 1, 14% Stage 2, 19% Stage 3 without dialysis, and 4% Stage 3 with dialysis (Table 2). Of the patients requiring dialysis, the median duration was 5 days (IQR 2.4–12.4) ranging from 0.2–31.8 days (duration missing for 25/104 patients). Only 7% (n = 7) of those who received dialysis in the first week were from non-U.S. centers. Across AKI stages, there was a significant increase in hospital and ICU LOS (effect sizes 0.48 and 0.38, respectively), with the greatest increase being among those receiving dialysis; hospital LOS median 31 days (IQR 22–48) for those on dialysis compared to median 6 days (IQR 4–11) for those with no AKI (p-values all < 0.0001). Significant differences across AKI stages were also seen for intubation, new home oxygen requirement on discharge, vasopressor(s)/inotrope(s) use, development of thromboses, and inpatient mortality. The absolute risk of hospital mortality increased significantly (p-values< 0.0001) for each AKI stage compared to no AKI. Overall, the OR of hospital mortality in those with AKI compared to those without AKI was 4.0 (95% CI 3.5–4.5). These associations did not change significantly when alternative Crb estimators were used.
Association of age with AKI risk
Figure 2 depicts a bimodal distribution of AKI risk by age with those of young adolescence (10–15 years) having a higher risk than both very young children (< 5 years) and older adolescents/young adults (15–35 years), while those over age 65 years also have a high risk of AKI. Even after adjusting for potential confounders (sex, pre-existing hypertension, diabetes mellitus, cancer, CKD, race/ethnicity, and severity of illness) there remains increased risk of AKI in a bimodal distribution (odds ratio inset in Fig. 2). This pattern of AKI distribution did not change when using alternative Crb estimators (Supplementary Figs. 1, 2, 3 and 4), including the full-age spectrum equation. The pattern of AKI distribution held when evaluating those with no comorbidities versus those with comorbidities (Fig. 3) and again when we evaluated only those in the United States (Supplementary Fig. 5). Table 3 depicts a snapshot of representative age ranges and their adjusted OR of developing AKI in these different scenarios, i.e., by different Crb estimators and in a population with no pre-existing comorbidities. The data consistently shows an almost 2.5-fold increased odds of developing SARS-CoV2-related AKI for 10–15-year-olds and for 70–75-year-olds when compared to young adults (30–35 years old).
Age Distribution of Hospitalized Patients with SARS-CoV2 who Experienced AKI within First 7 days of Hospitalization. Main figure presents percentage per age bracket who developed acute kidney injury (AKI) among all hospitalized patients and further stratified by severity of illness status. Severe illness is defined as a composite indicator of invasive ventilation, use of vasopressor(s)/inotrope(s), and/or use of extracorporeal membrane oxygenation. Moderate illness is defined as admitted to an intensive care unit but without use of above organ support measures. Mild illness is defined as patient required hospitalization but not in an intensive care unit and without use of above organ support measures. Insert presents the adjusted odds ratio (OR) with 95% confidence intervals (CI) of developing AKI within the first week of hospitalization by age bracket compared to young adults (30–35-year-olds) as the referent category. Adjusted for sex, pre-existing hypertension, diabetes mellitus, cancer, chronic kidney disease, race/ethnicity, and severity of illness. AKI defined per KDIGO guidelines
Age Distribution of Hospitalized Patients with SARS-CoV2 who Experienced AKI within First 7 days of Hospitalization Stratified by Presence or Absence of Comorbidities. Presents percentage of hospitalized patients who developed acute kidney injury (AKI) among all hospitalized patients and further stratified by presence of any comorbidity versus no pre-existing comorbidities. AKI defined per KDIGO guidelines
Discussion
In a large and diverse cohort evaluating AKI in COVID-19, we found a high incidence of AKI (39.6%) and that it varies across the age spectrum with a bimodal distribution. Given our cohort’s wide age span, we demonstrate a more nuanced view of SARS-CoV2-related AKI than previous evaluations. In every context of our evaluations, there was consistently a bimodal age distribution of AKI risk with the older population and early adolescent (10–15 years) population at higher risk compared to the young adult populations. This is an interesting phenomenon as to date there are only descriptions of a linear relationship between age and COVID-19 severity and its complications [24, 25]. Other known risk factors for AKI were seen in this cohort, such as sex, pre-existing comorbidities (i.e., hypertension, diabetes mellitus, cancer), and race/ethnicity. However, even after controlling for these potential confounders, there remained an association producing a bimodal age distribution in AKI risk; a 10–15-year-old had a similar odds of AKI as a 70–75-year-old (compared to 30–35-year-olds). The bimodal distribution also persisted after controlling for severity of illness and within-center correlations, which suggests something additional is contributing to the AKI risk. This contradicts an early study on SARS-CoV2-related AKI that found illness severity to be the key risk factor for SARS-CoV2-related AKI, but it was a small study (n = 223) with results from the early waves (March–June 2020), and excluded children [26]. Interestingly this bimodal distribution differs from previous non-SARS-CoV2 AKI literature which suggests a U-shaped distribution (peaks in infancy and older adults) [27,28,29].
The differences in AKI risks across the age spectrum found here were not explained by different Crb estimators. KDIGO is a standard guideline for defining AKI, yet it lacks a standard method for estimating a Crb in children when one is not known. We therefore evaluated variety of Crb estimators in pediatric and adult populations. Yet, a bimodal distribution of AKI risk by age remained even with several sensitivity analyses, including a Crb estimator (FAS) validated across the age spectrum of 2–90 years. The FAS equation assumes a slow transitional change in eGFR from childhood into adulthood [18,19,20]. In addition, the bimodal age distribution of SARS-CoV2-related AKI development persisted when evaluating only hospitalized patients with no pre-existing comorbidities or evaluating only U.S.-based centers, suggesting that comorbidity differences nor center or country specifics do not explain the bimodal pattern.
The persistence of the bimodal pattern by age, despite multiple iterative analyses, suggests there may be something unique about SARS-CoV2 and its relationship with AKI. One could hypothesize that the propensity of the SARS-CoV2 virus to attack the endothelium could also contribute to the differences seen in the older population and their risk with AKI beyond illness severity [30], though it does not explain the higher risk in early adolescence. There may be a hormonal influence in early adolescence that makes the endothelium more prone to injury compared to younger adults, but this would not fully explain the higher AKI rates in the elderly. We postulate that the bimodal AKI distribution could perhaps be a combination of SARS-CoV2-related vasculopathy and hormonal influences. There may also be yet unknown biological mechanisms that are contributing to this bimodal pattern. For example, we could not account for the different strains or clinical spectrum of SARS-CoV2 presentations which may be an important driver of the bimodal age pattern. A recent report of 2600 hospitalized adults with SARS-CoV2 infection found similarly that high AKI rates are not fully explained by known risk factors and need further exploration [31]. Fully understanding the bimodal age distribution of SARS-CoV2-related AKI risk is even more important now as countries are seeing a shift in age distribution of SARS-CoV2 infections as children are not yet eligible worldwide for vaccinations and new variants may disproportionately affect younger populations. Further in-depth epidemiological studies and animal models may be needed to understand the biological mechanisms underpinning the age distribution in SARS-CoV2-related AKI.
Similar to other studies [7,8,9, 32], this cohort demonstrates a high rate of AKI in COVID-19 patients; among ICU patients the AKI rate was 47.3% and in non-critically ill patients was 28.3%. Only a few studies report SARS-CoV2-related AKI rates outside of ICUs [33], and our results suggest a high-percentage of non-critically ill patients are at risk.
Other literature has found that SARS-CoV2-related AKI has an increased risk of mortality [4, 8, 9, 34]. In addition to this, we report a strong relationship with mortality and other hospital complications that is proportional to AKI’s severity and seen even in non-critically ill patients and those with mild increases in serum creatinine (≥0.3 mg/dL). Very few reports thus far have explored the complications associated with the varying degrees of AKI severity [2, 10]. This is important as even the slightest degree of AKI may be associated with long-term morbidity and mortality among those hospitalized with SARS-CoV2. Interestingly, though young adolescents had higher risks of AKI compared to middle adulthood, the rates of dialysis were higher in middle adulthood (20–40 years) compared to children (< 20 years). These may be related to center practice differences or the overall small sample of dialysis needs in both of these groups in this cohort (n = 7 for 20–40 year-olds and n = 1 for < 20 year-olds).
Limitations
The VIRUS registry has been a real-time assessment of the COVID-19 pandemic, so we may have introduced bias by excluding participants missing data. However, the large sample size provides real-time insight to ongoing trends and allows comparisons across the ages. Comparing the cohort of those with and without creatinine values, we found that we likely had some selection bias toward sicker patients; however, 40% of our participants were never in the ICU. A limitation of evaluating AKI across the age spectrum is the lack of standard Crb estimators, but our results were similar when using multiple estimators, suggesting there is a true phenomenon of bimodal age distribution in SARS-CoV2-related AKI that deserves further exploration. The registry includes multiple centers and as such risks introducing bias through practice pattern differences between pediatric versus adult centers and regional variations, but we controlled for this in our analyses by accounting for clustering within centers. However, evaluating data from across multiple regions and centers allows a broader view of the epidemiology of SARS-CoV2-related AKI, which is needed to plan for more in-depth case-control or randomized clinical trials evaluating different management and treatment strategies for improved outcomes in SARS-CoV2-related AKI.
Conclusions
Patients hospitalized with SARS-CoV2 have a high risk of AKI, irrespective of illness severity. We demonstrate an interesting phenomenon of a bimodal age distribution of SARS-CoV2-related AKI risk – high in the elderly and early adolescence – that deserves more in-depth exploration as it was not explained by pre-existing comorbidities, illness severity, eGFR equations, or clustering within centers. Our study reiterates other findings that SARS-CoV2-related AKI at any stage increases patients’ morbidity and mortality. However, as the pandemic lingers, outbreaks will continue, and while younger children remain unvaccinated, it is even more important to understand if there are biological reasons or other unexplored risk factors behind this bimodal age distribution of AKI risk that may guide clinical care improvements in the management of SARS-CoV2 infections and/or provide insights into the pathophysiology of this unique virus.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author/society of critical care medicine on reasonable request.
References
World Health Organization. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/. Accessed 21 March 2021.
Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46(7). https://doi.org/10.1007/s00134-020-06153-9.
Zamoner W, Santos CA, Magalhães LE, Oliveira PG, Balbi AL, Ponce D. Acute kidney injury in COVID-19: 90 days of the pandemic in a Brazilian public hospital. Front Med. 2021;8. https://doi.org/10.3389/fmed.2021.622577.
Pei G, Zhang Z, Peng J, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31(6). https://doi.org/10.1681/ASN.2020030276.
Palevsky PM. COVID-19 and AKI: where do we stand? JASN. 2021.
Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2). https://doi.org/10.1016/j.cell.2020.02.052.
Neugarten J, Bellin E, Yunes M, et al. AKI in Hospitalized Patients with and without COVID-19: A Comparison Study. J Am Soc Nephrol. 2020;31(9). https://doi.org/10.1681/ASN.2020040509.
Kolhe NV, Fluck RJ, Selby NM, Taal MW. Acute kidney injury associated with COVID-19: A retrospective cohort study. PLoS Med. 2020;17(10). https://doi.org/10.1371/journal.pmed.1003406.
Zahid U, Ramachandran P, Spitalewitz S, et al. Acute kidney injury in COVID-19 patients: an inner City Hospital experience and policy implications. Am J Nephrol. 2020;51(10). https://doi.org/10.1159/000511160.
Bjornstad EC, Krallman KA, Askenazi D, Zappitelli M, Goldstein SL, Basu RK. Preliminary assessment of acute kidney injury in critically Ill children associated with SARS-CoV-2 infection. Clin J Am Soc Nephrol. 2020;16:CJN.11470720. https://doi.org/10.2215/cjn.11470720.
Goldfarb DS, Benstein JA, Zhdanova O, et al. Impending shortages of kidney replacement therapy for covid-19 patients. Clin J Am Soc Nephrol. 2020;15(6). https://doi.org/10.2215/CJN.05180420.
Sourial MY, Sourial MH, Dalsan R, et al. Urgent peritoneal dialysis in patients with COVID-19 and acute kidney injury: a single-center experience in a time of Crisis in the United States. Am J Kidney Dis. 2020;76(3). https://doi.org/10.1053/j.ajkd.2020.06.001.
Walkey AJ, Christopher Sheldrick R, Kashyap R, et al. Guiding principles for the conduct of observational critical care research for coronavirus disease 2019 pandemics and beyond: the society of critical care medicine discovery viral infection and respiratory illness universal study registry. Crit Care Med. 2020. https://doi.org/10.1097/CCM.0000000000004572.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009. https://doi.org/10.1016/j.jbi.2008.08.010.
Kellum AJ. Official journal of the international society of nephrology kdigo clinical practice guideline for acute kidney injury. Kidney Int. 2012. https://doi.org/10.1159/000339789.
Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL. Epidemiology of acute kidney injury in critically ill children and Young adults. N Engl J Med. 2017. https://doi.org/10.1056/NEJMoa1611391.
Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008. https://doi.org/10.2215/CJN.05431207.
Hoste L, Dubourg L, Selistre L, et al. A new equation to estimate the glomerular filtration rate in children, adolescents and young adults. Nephrol Dial Transplant. 2014;29(5). https://doi.org/10.1093/ndt/gft277.
Hessey E, Ali R, Dorais M, et al. Evaluation of height-dependent and height-independent methods of estimating baseline serum creatinine in critically ill children. Pediatr Nephrol. 2017. https://doi.org/10.1007/s00467-017-3670-z.
Pottel H, Delanaye P, Weekers L, et al. Age-dependent reference intervals for estimated and measured glomerular filtration rate. Clin Kidney J. 2017;10(4). https://doi.org/10.1093/ckj/sfx026.
Pottel H, Hoste L, Dubourg L, et al. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant. 2016. https://doi.org/10.1093/ndt/gfv454.
Zelnick LR, Leca N, Young B, Bansal N. Association of the estimated glomerular filtration rate with vs without a coefficient for race with time to eligibility for kidney transplant. JAMA Netw Open. 2021;4(1). https://doi.org/10.1001/jamanetworkopen.2020.34004.
Centers for Disease Control and Prevention. A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years). https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. Accessed 9 March 2021.
Gao YD, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy Eur J Allergy Clin Immunol. 2020. https://doi.org/10.1111/all.14657.
Ebinge JE, Achamallah N, Ji H, et al. Pre-existing traits associated with Covid-19 illness severity. PLoS One. 2020;15(7 July). https://doi.org/10.1371/journal.pone.0236240.
Hardenberg JHB, Stockmann H, Aigner A, et al. Critical illness and systemic inflammation are key risk factors of severe acute kidney injury in patients with COVID-19. Kidney Int Rep. 2021. https://doi.org/10.1016/j.ekir.2021.01.011.
Joyce EL, Kane-Gill SL, Fuhrman DY, Kellum JA. Drug-associated acute kidney injury: who’s at risk? Pediatr Nephrol. 2017;32(1). https://doi.org/10.1007/s00467-016-3446-x.
Fuhrman DY, Kane-Gill S, Goldstein SL, Priyanka P, Kellum JA. Acute kidney injury epidemiology, risk factors, and outcomes in critically ill patients 16–25 years of age treated in an adult intensive care unit. Ann Intensive Care. 2018;8(1). https://doi.org/10.1186/s13613-018-0373-y.
Chao CT, Wang J, Wu HY, Huang JW, Chien KL. Age modifies the risk factor profiles for acute kidney injury among recently diagnosed type 2 diabetic patients: a population-based study. GeroScience. 2018;40(2). https://doi.org/10.1007/s11357-018-0013-3.
Batlle D, Soler MJ, Sparks MA, et al. Acute kidney injury in COVID-19: Emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31(7). https://doi.org/10.1681/ASN.2020040419.
Moledina DG, Simonov M, Yamamoto Y, et al. The association of COVID-19 with acute kidney injury independent of severity of illness: a multicenter cohort study. Am J Kidney Dis. 2021. https://doi.org/10.1053/j.ajkd.2020.12.007.
Charytan DM, Parnia S, Khatri M, et al. Decreasing incidence of acute kidney injury in patients with COVID-19 critical illness in new York City. Kidney Int Rep. 2021. https://doi.org/10.1016/j.ekir.2021.01.036.
Guan W, Ni Z, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18). https://doi.org/10.1056/nejmoa2002032.
Ng JH, Hirsch JS, Hazzan A, et al. Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis. 2021;77(2). https://doi.org/10.1053/j.ajkd.2020.09.002.
Acknowledgements
The SCCM Discovery VIRUS registry is funded in part by the Gordon and Betty Moore Foundation, and Janssen Research & Development, LLC. They had no influence on analysis, interpretation and reporting of pooled data.
SCCM Discovery VIRUS Investigators Group.
Jean-Baptiste Mesland15, Pierre Henin15, Hélène Petre15, Isabelle Buelens15, Anne-Catherine Gerard15, Philippe Clevenbergh16, Rolando Claure-Del Granado17, Jose A. Mercado17, Esdenka Vega-Terrazas17, Maria F. Iturricha-Caceres17, Dragana Markotić18, Ivana Bošnjak18, Oscar Y Gavidia19, Felipe Pachon19, Yeimy A Sanchez19, Danijel knežević20, Tanja Kovacevic21, Josko Markic21, Tatjana Catipovic Ardalic21, Branka Polic21, Ivo Ivić21, Dominko Carev21, Robert Glavinic21, Mohamed El Kassas22, Mohamed Badr22, Ahmed Tawheed22, Ahmed Tawheed22, Hend Yahia22, Dimitrios Kantas23, Vasileios Koulouras23, Sierra-Hoffman24, Fernando Valerio24, Oscar Diaz24, Jose Luis Ramos Coello25, Guillermo Perez25, Ana Karen Vallecillo Lizardo25, Gabina María Reyes Guillen25, Helin Archaga Soto25, Csaba Kopitkó26, Ágnes Bencze26, István Méhész26, Zsófia Gerendai26, Phaneendra Doddaga27, Neethi Chandra27, Girish Vadgaonkar10, Rekha Ediga10, Shilpa Basety10, Shwetha Dammareddy10, Phani Sreeharsha Kasumalla10, Umamaheswara Raju28, Janaki Manduva28, Naresh Kolakani28, Shreeja Sripathi28, Sheetal Chaitanya28, Anusha Cherian29, Sreejith Parameswaran29, Magesh Parthiban29, Menu Priya A.29, Chetak Basavaraja30, Madhav Prabhu31, Vishal Jakati31, Puneet Rijhwani32, Ashish Jain32, Aviral Gupta32, Ram Mohan Jaiswal32, Ambika Tyagi32, Nimish Mathur32, Mradul Kumar Daga33, Munisha Agarwal33, Ishan Rohtagi33, Sridhar Papani34, Mahesh Kamuram34, Kamlesh Kumar Agrawal35, Vijendra Baghel35, Kirti Kumar Patel35, Surapaneni Krishna Mohan8, Ekambaram Jyothisree8, Mukur Petrolwala36, Bharat Ladva36, Yuki Itagaki37, Akira Kodate37, Reina Suzuki37, Akira Kodate37,Yuki Takahashi37, Koyo Moriki37, Michihito Kyo38, Hidenobu Shigemitsu39, Yuka Mishima39, Nobuyuki Nosaka39, Michio Nagashima39, Abdulrahman Al-Fares40, Rene Rodriguez-Gutierrez41, Jose Gerardo Gonzalez-Gonzalez41, Alejandro Salcido-Montenegro41, Adrian Camacho-Ortiz41, Fatimah Hassan-Hanga42, Hadiza Galadanci42, Abubakar Shehu Gezawa42, Halima M. S. Kabara42, Taiwo Gboluwaga Amole42, Halima Kabir42, Dalha Gwarzo Haliru42, Abdullahi S Ibrahim42, Muhammad Sohaib Asghar43, Mashaal Syed43, Syed Anosh Ali Naqvi43, Sidra Ishaque44, Ali Faisal Saleem44, Naveed Ur Rehman Siddiqui44, Salima Sherali44, Yasmin Hashwani44, Shafia Ishaque44, Igor Borisovich Zabolotskikh45, Konstantin Dmitrievich Zybin45, Sergey Vasilevich Sinkov45, Tatiana Sergeevna Musaeva45, Marwa Ridha Amer46, Mohammed Abdullah Bawazeer46, Talal I. Dahhan46, Eiad Kseibi46, Abid Shahzad Butt46, Syed Moazzum Khurshid46, Muath Rabee46, Mohammed Abujazar46, Razan Alghunaim46, Maal Abualkhair46, Abeer Turki AlFirm46, Eiad Kseibi46, Syed Moazzum Khurshid46, Muath Rabee46, Mohammed Abujazar46, Razan Alghunaim46, Razan K Alamoudi47, Hassan M. AlSharif47, Sarah A. Almazwaghi47, Mohammed S Elsakran47, Mohamed A Aid47, Mouaz A Darwich47, Omnia M Hagag47, Salah A Ali47, Alona Rocacorba47, Kathrine Supeña47, Efren Ray Juane47, Jenalyn Medina47, Jowany Baduria47, Mohammed A Almazyad48, Mohammed I Alarifi48, Jara M Macarambon48, Ahmad Abdullah Bukhari48, Hussain A. Albahrani48, Kazi N Asfina48, Kaltham M Aldossary48, Ana Andrijevic49, Srdjan Gavrilovic49, Vladimir Carapic49, Pedja Kovacevic50, Predrag D Stevanovic51, Dejan S Stojakov51, Duska K Ignjatovic51, Suzana C Bojic51, Marina M Bobos51, Irina B Nenadic51, Milica S Zaric51, Marko D Djuric51, Vladimir R Djukic51, Santiago Y. Teruel52, Belen C. Martin52, Santiago Y. Teruel52, Varsha P Gharpure53, Usman Raheemi53, Kenneth W. Dodd54, Nicholas Goodmanson54, Kathleen Hesse54, Paige Bird54, Chauncey Weinert54, Nathan Schoenrade54, Abdulrahman Altaher54, Esmael Mayar54, Matthew Aronson54, Tyler Cooper54, Monica Logan54, Brianna Miner54, Gisele Papo54, Suzanne Barry55, Christopher Woll55, Gregory Wu55, Erin Carrole55, Kathryn Burke55, Mustafa Mohammed55, Catherine A. St. Hill4, Roman R. Melamed4, David M. Tierney4, Love A. Patel4, Vino S. Raj4, Barite U. Dawud4, Narayana Mazumder4, Abbey Sidebottom4, Alena M. Guenther4, Benjamin D. Krehbiel4, Nova J. Schmitz4, Stacy L. Jepsen4, Lynn Sipsey4, Anna Schulte4, Whitney Wunderlich4, Cecely Hoyt4, Abhijit A Raval56, Andrea Franks56, Katherine Irby57, Ronald C. Sanders Jr.57, Glenda Hefley57, Jennifer M. Jarvis58, Anmol Kharbanda59, Sunil Jhajhria59, Zachary Fyffe59, Stephen Capizzi60, Bethany Alicie60, Martha Green60, Lori Crockarell60, Amelia Drennan60, Kathleen Dubuque60, Tonya Fambrough60, Nikole Gasaway60, Briana Krantz60, Peiman Nebi60, Jan Orga60, Margaret Serfass60, Alina Simion60, Kimberly Warren60, Cassie Wheeler60, CJ Woolman60, Amy B. Christie7, Dennis W. Ashley7, Rajani Adiga7, Andrew S. Moyer61, George M. Verghese61, Andrea Sikora Newsome62, Christy C. Forehand62, Rebecca Bruning62, Timothy W. Jones62, Moldovan Sabov63, Fatema Zaidi63, Fiona Tissavirasingham63, Dhatri Malipeddi63, Jarrod M Mosier9, Karen Lutrick9, Beth Salvagio Campbell9, Cathleen Wilson9, Patrick Rivers9, Jonathan Brinks9, Mokenge Ndiva Mongoh9, Boris Gilson9, Donna Lee Armaignac64, Don Parris64, Maria Pilar Zuniga64, Ilea Vargas64, Viviana Boronat64, Anneka Hutton64, Navneet Kaur64, Prashank Neupane64, Nohemi Sadule-Rios64, Lourdes M. Rojas64, Aashish Neupane64, Priscilla Rivera64, Carlos Valle Carlos64, Gregory Vincent64, Christopher M Howard65, Cameron McBride65, Jocelyn Abraham65, Orlando Garner65, Katherine Richards65, Keegan Collins65, Preethi Antony65, Sindhu Mathew65, Valerie C. Danesh66, Gueorgui Dubrocq66, Amber L. Davis66, Marissa J Hammers66, ill M. McGahey66, Amanda C. Farris66, Elisa Priest66, Robyn Korsmo66, Lorie Fares66, Kathy Skiles66, Susan M. Shor66, Kenya Burns66, Corrie A Dowell66, Gabriela “Hope” Gonzales66, Melody Flores66, Lindsay Newman66, Debora A Wilk66, Jason Ettlinger66, Jaccallene Bomar66, Himani Darji66, Alejandro Arroliga66, Alejandro C Arroliga66, Corrie A. Dowell66, Gabriela Hope Conzales66, Melody Flores66, Lindsay Newman66, Debora A. Wilk66, Jason Ettlinger66, Himani Darji66, Jaccallene Bomar66, Paras B. Khandhar67, Elizabeth Kring67, Valerie M. Banner-Goodspeed6, Somnath Bose6, Lauren E. Kelly6, Melisa Joseph6, Marie McGourty6, Krystal Capers6, Benjamin Hoenig6, Maria C. Karamourtopoulos6, Anica C. Law6, Elias N. Baedorf Kassis6, Allan J. Walkey11, Sushrut S. Waikar11, Michael A. Garcia11, Mia Colona11, Zoe Kibbelaar11, Michael Leong11, Daniel Wallman11, Kanupriya Soni11, Jennifer Maccarone11, Joshua Gilman11, Ycar Devis11, Joseph Chung11, Munizay Paracha11, David N. Lumelsky11, Madeline DiLorenzo11, Najla Abdurrahman11, Shelsey Johnson11, Maj Andrew M. Hersh68, CPT Stephanie L Wachs68, Brittany S. Swigger68, CPT Stephanie L Wachs68, Capt Lauren A. Sattler68, Capt Michael N. Moulton68, Aaron S. Miller69, Edwin L. Anderson69, Rosemary Nagy69, Ravali R. Inja69, Pooja A. Nawathe70, Isabel Pedraza70, Jennifer Tsing70, Karen Carr70, Anila Chaudhary70, Kathleen Guglielmino70, Raghavendra Tirupathi71, Alymer Tang71, Arshad Safi71, Cindy Green71, Jackie Newell71, Katja M. Gist14, Imran A Sayed14, John Brinton14, Larisa Strom14, Kathleen Chiotos72, Allison M. Blatz72, Giyoung Lee72, Ryan H. Burnett72, Guy I. Sydney72, Danielle M. Traynor72, Karissa Nauert73, Annika Gonzalez73, Mariel Bagley73, Anita Santpurkar73, Sreekanth Cheruku74, Farzin Ahmed74, Christopher Deonarine74, Ashley Jones74, Mohammad-Ali Shaikh74, David Preston74, Jeanette Chin74, Vidula Vachharajani75, Abhijit Duggal75, Prabalini Rajendram75, Omar Mehkri75, Siddharth Dugar75, Michelle Biehl75, Gretchen Sacha75, Stuart Houltham75, Alexander King75, Kiran Ashok75, Bryan Poynter75, Mary Beukemann75, Richard Rice75, Susan Gole75, Valerie Shaner75, Adarsh Conjeevaram75, Michelle Ferrari75, Narendrakumar Alappan75, Steven Minear75, Jaime Hernandez-Montfort75, Syed Sohaib Nasim75, Ravi Sunderkrishnan75, Debasis Sahoo75, Steven K. Daugherty76, Sam Atkinson76, Kelly Shrimpton76, Sidney Ontai77, Brian Contreras77, Uzoma Obinwanko77, Nneka Amamasi77, Amir Sharafi77, Sarah Lee78, Zahia Esber78, Chetna Jinjvadia78, Christine Waller79, Kara Kallies79, Jonean Thorsen79, Alec Fitzsimmons79, Haley Olsen79, Heda R. Dapul80, Sourabh Verma80, Alan Salas80, Ariel Daube80, Michelle Korn80, Michelle Ramirez80, Logi Rajagopalan80, Laura Santos80, Héctor Collazo Santiago81, Ricardo Alan Hernandez81, Orma Smalls82, Atul Malhotra83, Abdurrahman Husain83, Qais Zawaydeh83, J.H. Steuernagle84, Steven Q. Davis85, Valentina Jovic85, Valentina Jovic85, Max Masuda85, Amanda Hayes85, Kristen Lee Gossett86, Jennifer Nason86, Sarah Morris86, Sarah Deans86, Stephanie Houston86, Michael Smith87, William Snow87, Riley Liptak87, Hannah Durant87, Valerie Pendleton87, Alay Nanavati87, Risa Mrozowsk87, Namrata Nag88, Jeff Brauer88, Ashwin Dharmadhikari88, Sahib Singh88, Franco Laghi88, Ghania Naeem88, Andrew Wang88, Kevin Bliden88, Amit Rout88, Jaime Barnes88, Martin Gesheff88, Asha Thomas88, Melbin Thomas88, Alicia R. Liendo88, Jovan Milosavljevic88, Kenan Abbasi88, Nicholas B. Burley88, Nicole Rapista88, Samuel Amankwah88, Sanjay K Poudel88, Saroj Timilsina88, Sauradeep Sarkar88, Oluwasayo Akinyosoye88, Shashi K. Yalamanchili88, Sheena Moorthy88, Sonia Sugumar88, Jonathan Ford88, Martin C. Taylor88, Charlotte Dunderdale88, Alyssa Henshaw88, Mary K. Brunk88, Jessica Hagy88, Shehryar Masood88, Sushrutha Sridhar88, Manoj K Gupta89, Franscene E. Oulds89, Akshay Nandavar89, Yuk Ming Liu90, Sarah Zavala90, Sarah Zavala90, Esther Shim90, Andy Y. Wen91, Allie DaCar91, Ronald A. Reilkoff92, Julia A. Heneghan92, Sarah Eichen92, Lexie Goertzen92, Scott Rajala92, Ghislaine Feussom92, Ben Tang92, Christine C. Junia93, Robert Lichtenberg93, Hasrat Sidhu93, Diana Espinoza93, Shelden Rodrigues93, Maria Jose Zabala93, Daniela Goyes93, Ammu Susheela93, Buddhi Hatharaliyadda93, Naveen Rameshkumar93, Amulya Kasireddy93, Genessis Maldonado93, Lisseth Beltran93, Akshata Chaugule93, Hassan Khan93, Namrata Patil94, Ruhi Patil94, Rodrigo Cartin-Ceba95, Ayan Sen95, Amanda Palacios95, Giyth M. Mahdi95, Rahul Kashyap12, Ognjen Gajic12, Vikas Bansal12, Aysun Tekin12, Amos Lal12, John C. O’Horo12, Neha N. Deo12, Mayank Sharma12, Shahraz Qamar12, Juan Pablo Domecq12, Romil Singh12, Alex Niven12, Marija Bogojevic12, Abigail La Nou96, Barbara Mullen96, Devang Sanghavi2, Pablo Moreno Franco2, Pramod Guru2, Karthik Gnanapandithan2, Hollie Saunders2, Zachary Fleissner2, Juan Garcia2, Alejandra Yu Lee Mateus2, Siva Naga Yarrarapu2, Nirmaljot Kaur2, Abhisekh Giri2, Syed Anjum Khan97, Juan Pablo Domecq97, Nitesh Kumar Jain97, Thoyaja Koritala97, Alexander Bastidas98, Gabriela Orellana98, Adriana Briceno Bierwirth98, Eliana Milazzo98, Juan Guillermo Sierra98, Thao Dang98, Rahul S Nanchal99, Paul A Bergl99, Jennifer L Peterson99, Travis Yamanaka100, Nicholas A. Barreras100, Michael Markos100, Anita Fareeduddin100, Rohan Mehta100, Chakradhar Venkata101, Miriam Engemann101, Annamarie Mantese101, Yasir Tarabichi5, Adam Perzynski5, Christine Wang5, Dhatri Kotekal5, Adriana C Briceno Bierwirth102, Gabriela M Orellana102, Gerardo Catalasan102, Shohana Ahmed102, Carlos F Matute102, Ahmad Hamdan102, Ivania Salinas102, Genesis Del Nogal102, Angel Tejada102, Anna Eschler103, Mary Hejna103, Emily Lewandowski103, Kristen Kusmierski103, Clare Martin103, Nasar A Siddiqi104, Lesly Jurado104, Lindsey Tincher104, Carolyn Brown104, Prithvi Sendi105, Meghana Nadiger105, Balagangadhar Totapally105, Bhagat S. Aulakh106, Sandeep Tripathi106, Jennifer A. Bandy106, Lisa M. Kreps106, Dawn R. Bollinger106, Jennifer A. Bandy106, Roger Scott Stienecker107, Andre G. Melendez107, Tressa A. Brunner107, Sue M Budzon107, Jessica L. Heffernan107, Janelle M. Souder107, Tracy L. Miller107, Andrea G. Maisonneuve107, Roberta E. Redfern108, Jessica Shoemaker108, Jennifer Micham108, Lynn Kenney108, Gabriel Naimy108, Holly Balcer109, Sara Utley109, Dawn Bouknight109, Radha Patel109, Lama Alfehaid109, Majdi Hamarshi110, Jeannette Ploetz110, Nick Bennett110, Kyle Klindworth110, Moustafa Younis110, Adham Mohamed110, Antonia L. Vilella111, Sara B. Kutner111, Kacie Clark111, Danielle Moore111, Shina Menon3, John K McGuire3, Deana Rich3, Howard A. Zaren112, Stephanie J. Smith112, Grant C. Lewis112, Lauren Seames112, Cheryl Farlow112, Judy Miller112, Gloria Broadstreet112, Anthony Martinez113, Micheal Allison113, Aniket Mittal113, Rafael Ruiz113, Aleta Skaanland113, Robert Ross113, Umang Patel114, Jordesha Hodge114, Krunal Kumar Patel114, Shivani Dalal114, Himanshu Kavani114, Sam Joseph114, Paul K Mohabir115, Connor G O′Brien115, Komal Dasani115, William Marx116, Ioana Amzuta116, Asad J. Choudhry116, Mohammad T. Azam116, Neha Gupta117, Tracy L Jones117, Shonda C Ayers117, Amy B Harrell117, Brent R Brown117, Utpal S. Bhalala118, Joshua Kuehne118, Melinda Garcia118, Morgan Beebe118, Heather Herrera118, Chris Fiack119, Stephanie Guo119, May Vawer119, Beth Blackburn119, Katherine A. Belden120, Michael Baram120, Devin M. Weber120, Rosalie DePaola120, Yuwei Xia120, Hudson Carter120, Aaron Tolley120, Mary Barletta120, Mark Steele121, Laurie Kemble121, Joshua L. Denson122, A. Scott Gillet122, Margo Brown122, Rachael Stevens122, Andrew Wetherbie122, Kevin Tea122, Mathew Moore122, Benjamin J Sines123, Thomas J Bice123, Rajany V. Dy124, Alfredo Iardino124, Jill Sharma124, Julia Christopher124, Marwan Mashina124, Kushal Patel124, Erica C. Bjornstad1, Nancy M. Tofil1, Scott House1, Isabella Aldana1, Nikhil K. Meena125, Jose D. Caceres125, Nikhil K Meena125, Sarenthia M. Epps125, Harmeen Goraya125, Kelsey R. Besett125, Ryan James125, Lana Y. Abusalem125, Akash K. Patel125, Lana S Hasan125, Casey W Stulce126, Grace Chong126, Ahmeneh Ghavam126, Anoop Mayampurath126, Dina Gomaa127, Michael Goodman127, Devin Wakefield127, Anthony Spuzzillo127, John O. Shinn II127, Patrick W. McGonagill128, Colette Galet128, Janice Hubbard128, David Wang128, Lauren Allan128, Aditya Badheka128, Madhuradhar Chegondi128, Usman Nazir129, Garrett Rampon129, Jake Riggle129, Nathan Dismang129, Ozan Akca130, Rainer Lenhardt130, Rodrigo S. Cavallazzi130, Ann Jerde130, Alexa Black130, Allison Polidori130, Haily Griffey130, Justin Winkler130, Thomas Brenzel130, Pauline Park131, Andrew Admon131, Sinan Hanna131, Rishi Chanderraj131, Maria Pliakas131, Ann Wolski131, Jennifer Cirino131, Dima Dandachi132, Hariharan Regunath132, Maraya N. Camazine132, Grant. E. Geiger132, Abdoulie O. Njai132, Baraa M. Saad132, Faraaz Ali Shah133, Byron Chuan133, Sagar L. Rawal133, Manal Piracha133, Joseph E. Tonna134, Nicholas M. Levin134, Kayte Suslavich134, Rachel Tsolinas134, Zachary T. Fica134, Chloe R. Skidmore134, Renee D. Stapleton135, Anne E. Dixon135, Olivia Johnson135, Sara S. Ardren135, Stephanie Burns135, Anna Raymond135, Erika Gonyaw135, Kevin Hodgdon135, Chloe Housenger135, Benjamin Lin135, Karen McQuesten135, Heidi Pecott-Grimm135, Julie Sweet135, Sebastian Ventrone135, Murtaza Akhter136, Rania Abdul Rahman136, Mary Mulrow136, Erin M. Wilfong137, Kelsi Vela137, Markos G. Kashiouris138, Tamas Gal138, Manasi Mahashabde138, Alexandra Vagonis138, Rebecca Uber138, Haseeb Mahmud138, Stefan Leightle138, Zoe Zhang138, Nicole Vissichelli138, Oliver Karam138, Alia O’Meara138, Heloisa De Carvalho138, Katie Rocawich138, Ashish K. Khanna139, Lynne Harris139, Bruce Cusson139, Jacob Fowler139, David Vaneenenaam139, Glen McKinney139, Imoh Udoh139, Kathleen Johnson139, Patrick G. Lyons140, Andrew P Michelson140, Sara S. Haluf140, Lauren M. Lynch140, Nguyet M. Nguyen140, Aaron Steinberg140, Vishwanath Pattan141, Jessica Papke141, Ismail Jimada141, Nida Mhid141, Samuel Chakola141.
15Centre Hospitalier Jolimont, Belgium.
16The Brugmann University Hospital, Belgium.
17Clinica Los Olivos, Bolivia.
18Univresity Clinical Hospital, Mostar, Bosnia and Herzegovina.
19Clinica Medical SAS, Columbia.
20Clinical Hospital Center Rijeka, Croatia.
21University Hospital of Split, Croatia.
22Helwan University, Egypt.
23ICU University Hospital of Ioannina, Greece.
24CEMESA Hospital, Honduras.
25Honduras Medical Center, Honduras.
26Uzsoki Teaching Hospital, Hungary.
27ACSR Government Medical College and Hospital, India.
28Gandhi Medical College and Hospital, Hyderabad, India.
29Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry, India.
30JSS Medical College, India.
31KLEs Dr. Prabhakar Kore Hospital, India.
32Mahatma Gandhi Hospital, Jaipur, India.
33Maulana Azad Medical College and Lok Nayak Hospital, India.
34Medicover Hospitals, India.
35Om Superspecialty Hospital, Raipur, India.
36Tristar Hospital, India.
37Sapporo City General Hospital, Japan.
38Hiroshima University, Japan.
39Tokyo Medical and Dental University, Japan.
40Al-Amiri and Jaber Al-Ahmed Hospitals, Kuwait Extracorporeal Life Support Program, Kuwait.
41Hospital Universitario, Universidad Autonoma de Nuevo León, Mexico.
42Aminu Kano Teaching Hospital/Bayero University, Kano, Nigeria.
43Dow University Hospital, Pakistan.
44The Aga Khan University Hospital, Pakistan.
45Kuban State Medical University with affiliation Territorial Hospital #2, Russia.
46King Faisal Specialist Hospital & Research Centre, Riyadh, Saudi Arabia.
47King Fahad Armed Forces Hospital, Saudi Arabia.
48King Saud University, Saudi Arabia.
49Institute for Pulmonary Diseases of Vojvodina, Sremska Kamenica, Serbia.
50University Clinical Centre of the Republic of Srpska, Banja Luke, Bosnia and Herzegovina, Serbia.
51University Hospital Center ‘Dr Dragisa Misovic-Dedinje’, Serbia.
52Hospital Universitario La Paz, Spain.
53Advocate Children’s Hospital, IL, USA.
54Advocate Christ Medical Center, USA.
55Albany Medical Center, USA.
56AnMed Health, USA.
57Arkansas Children’s Hospital, USA.
58Ascension St. Mary’s Hospital, USA.
59Ascension St. Vincent Hospital, USA.
60Ascension/St. Thomas Research Institute West Campus, USA.
61Augusta Health, USA.
62Augusta University Medical Center, USA.
63Aultman Hospital, USA.
64Baptist Health South Florida, USA.
65Baylor College of Medicine, Baylor St. Lukes Medical Center, USA.
66Baylor Scott & White Health, USA.
67Beaumont Children’s Hospital, USA.
68Brooke Army Medical Center, USA.
69Cardinal Glennon Children’s Hospital, USA.
70Cedars Sinai Medical Center, USA.
71Chambersburg Hospital, USA.
72Children’s Hospital of Philadelphia, USA.
73CHRISTUS Santa Rosa Health System, USA.
74Clements University Hospital at UT Southwestern Medical Center, USA.
75Cleveland Clinic (Main Campus, Fairview Hospital, Florida-Weston, Hillcrest Hospital, Marymount Hospital), USA.
76Cox Medical Center Springfield, USA.
77Detar Family Medicine Residency, USA.
78Detroit Medical Centre, USA.
79Gundersen Health System La Crosse Wisconsin, USA.
80Hassenfeld Children’s Hospital at NYU Langone, USA.
81Hospital Auxilio Mutuo, USA.
82Howard University Hospital, USA.
83Jacobs Medical Center UC San Diego Health – La Jolla, USA.
84Johns Hopkins School of Medicine, USA.
85JPS Health Network, USA.
86KCPCRU at Norton Children’s Hospital Louisville, USA.
87Lakes Region General Hospital, USA.
88LifeBridge Health/Sinai and Northwest Hospitals, USA.
89Lincoln Medical Center, USA.
90Loyola University Medical Center, USA.
91Lucile Packard Children’s Hospital Stanford, USA.
92M Health-Fairview, University of Minnesota, USA.
93MacNeal Hospital Loyola Medicine, USA.
94Mass General Brigham Hospital, USA.
95Mayo Clinic Arizona, USA.
96Mayo Clinic, Eau Claire, USA.
97Mayo Clinic, Mankato, USA.
98Medical Center Health System, Odessa, USA.
99Medical College of Wisconsin, USA.
100Mercy Hospital and Medical Center, Chicago, USA.
101Mercy Hospital, Saint Louis, USA.
102Midland Memorial Hospital, Texas Tech Uninversity Health Sciences Center, USA.
103Millard Fillmore Suburban Hospital, USA.
104New Hanover Regional Medical Center, USA.
105Nicklaus Children’s Hospital, USA.
106OSF Saint Francis Medical Center, USA.
107Parkview Health System, Fort Wayne, USA.
108ProMedica Toledo Hospital, USA.
109Roper St. Francis Healthcare, USA.
110Saint Luke’s Hospital, USA.
111Sarasota Memorial Hospital, USA.
112St. Joseph’s Candler Health System, USA.
113St. Agnes Hospital, USA.
114St. Mary Medical Center, Langhorne, USA.
115Stanford Hospital and Clinics, USA.
116SUNY Upstate Medical University, USA.
117The Children’s Hospital at OU Medicine, USA.
118The Children’s Hospital of San Antonio, Baylor College of Medicine, USA.
119The Queen’s Medical Center, USA.
120Thomas Jefferson University Hospital, USA.
121Truman Medical Centers, USA.
122Tulane University Medical Center and University Medical Center New Orleans, USA.
123UNC Medical Center, USA.
124University Medical Center of Southern Nevada Las Vegas, University of Nevada Las Vegas, USA.
125University of Arkansas for Medical Sciences, USA.
126University of Chicago, USA.
127University of Cincinnati, USA.
128University of Iowa Carver College of Medicine, USA.
129University of Kansas Medical Center, USA.
130University of Louisville Hospital, USA.
131University of Michigan Health System.
132University of Missouri, Columbia, USA.
133University of Pittsburgh, USA.
134University of Utah Health, USA.
135University of Vermont Larner College of Medicine, USA.
136Valleywise Health (formerly Maricopa Medical Center), USA.
137Vanderbilt University Medical Center, USA.
138Virginia Commonwealth University Medical Center, USA.
139Wake Forest University School of Medicine, Wake Forest Baptist Health Network, USA.
140Washington University School of Medicine and Barnes-Jewish Hospital, USA.
141Wyoming Medical Center, USA.
Funding
The SCCM Discovery VIRUS registry is funded in part by the Gordon and Betty Moore Foundation, and Janssen Research & Development, LLC.
Author information
Authors and Affiliations
Consortia
Contributions
ECB, GC, PG, SM, KMG researched literature and conceived the study and analysis plan. ECB, GC, KMG, AJW, RK, VKK, VB, KB, MS, MB, ND, LR, OG were involved in protocol development. All authors were responsible for gaining local ethical approval and data collection. ECB and GC were responsible for data analysis. ECB and IA wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All research was conducted in accordance with the Declaration of Helsinki, and the University of Alabama at Birmingham declared the study exempt as there was no human-to-human interaction and consent was waived. The ethical approval boards at all individual VIRUS participating sites also declared exempt or approved the study. No participant interaction occurred and consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare no financial or competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Initial Hospital-related Associations with SARS-CoV2 -related AKI. These therapies or complications occur within the first 7 days of hospitalization when SARS-CoV2-related AKI is defined. Data presented as number (column percentiles), except where specified. ACE-I = angiotensin-converting enzyme-inhibitors; AKI = acute kidney injury; ARB = angiotensin receptor blockers; IVIG = intravenous immunoglobulin; NSAID = non-steroidal anti-inflammatory drugs; PRISM = Pediatric Risk of Mortality Score; SOFA = Sequential Organ Failure Assessment. aInitial PRISM score missing for 497 pediatric patients. Baseline SOFA score missing for 2741 adult patients; maximum SOFA score missing for 2016 adult patients.
Additional file 2: Supplementary Fig. 1.
Age Distribution of Hospitalized Patients with SARS-CoV2 who Experienced AKI within First 7 days of Hospitalization-different baseline creatinine estimators. Main figure presents percentage per age bracket who developed acute kidney injury (AKI) among all hospitalized patients. The original AKI definition (blue) assumes a baseline creatinine based on KDIGO guidelines for adults (eGFR 75 ml/min/1.73m2 and back calculates using MDRD equation) and common pediatric definitions assuming an eGFR of 120 ml/min/1.73m2 and back calculating using height-independent equation, except for patients with CKD when minimum serum creatinine during first 7 days of hospitalization is assumed to be their baseline creatinine value. Orange line assumes that the minimum creatinine during the first 7 days of hospitalization is the baseline creatinine for all participants. Gray line uses the KDIGO guidelines but back calculates the baseline creatinine for all participants using the FAS equation. Yellow line uses the original definition but uses the MDRD equation minus the race variable. Abbreviations: AKI = acute kidney injury, CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate, FAS = full age spectrum, KDIGO=Kidney Disease Improving Global Outcomes, MDRD = modification of diet in renal disease.
Additional file 3: Supplementary Fig. 2.
Age Distribution of Hospitalized Patients with SARS-CoV2 who Experienced AKI within First 7 days of Hospitalization-baseline creatinine estimator FAS equation. Main figure presents percentage per age bracket who developed acute kidney injury (AKI) among all hospitalized patients and further stratified by severity of illness status. Severe illness is defined as a composite indicator of invasive ventilation, use of vasopressor(s)/inotrope(s), and/or use of extracorporeal membrane oxygenation. Moderate illness is defined as admitted to an intensive care unit but without use of above organ support measures. Mild illness is defined as patient required hospitalization but not in an intensive care unit and without use of above organ support measures. Insert presents the adjusted odds ratio (OR) with 95% confidence intervals (CI) of developing AKI within the first week by age bracket compared to young adults (30–35-year-olds) as the referent category. Adjusted for sex, race/ethnicity, pre-existing hypertension, diabetes mellitus, cancer, chronic kidney disease, and severity of illness. AKI defined per KDIGO guidelines, but baseline creatinine estimator uses full-age spectrum (FAS) equation for all participants.
Additional file 4: Supplementary Fig. 3.
Age Distribution of Hospitalized Patients with SARS-CoV2 who Experienced AKI within First 7 days of Hospitalization-baseline creatinine estimator MDRD equation removing race. Main figure presents percentage per age bracket who developed acute kidney injury (AKI) among all hospitalized patients and further stratified by severity of illness status. Severe illness is defined as a composite indicator of invasive ventilation, use of vasopressor(s)/inotrope(s), and/or use of extracorporeal membrane oxygenation. Moderate illness is defined as admitted to an intensive care unit but without use of above organ support measures. Mild illness is defined as patient required hospitalization but not in an intensive care unit and without use of above organ support measures. Insert presents the adjusted odds ratio (OR) with 95% confidence intervals (CI) of developing AKI within the first week by age bracket compared to young adults (30–35-year-olds) as the referent category. Adjusted for sex, race/ethnicity, pre-existing hypertension, diabetes mellitus, cancer, chronic kidney disease, and severity of illness. AKI defined per KDIGO guidelines, but baseline creatinine estimator uses modified MDRD equation removing race component for adults (≥18 years) and height-independent equation for children (< 18 years).
Additional file 5: Supplementary Fig. 4.
Age Distribution of Hospitalized Patients with SARS-CoV2 who Experienced AKI within First 7 days of Hospitalization-baseline creatinine estimator as minimum serum creatinine. Main figure presents percentage per age bracket who developed acute kidney injury (AKI) among all hospitalized patients and further stratified by severity of illness status. Severe illness is defined as a composite indicator of invasive ventilation, use of vasopressor(s)/inotrope(s), and/or use of extracorporeal membrane oxygenation. Moderate illness is defined as admitted to an intensive care unit but without use of above organ support measures. Mild illness is defined as patient required hospitalization but not in an intensive care unit and without use of above organ support measures. Insert presents the adjusted odds ratio (OR) with 95% confidence intervals (CI) of developing AKI within the first week by age bracket compared to young adults (30–35-year-olds) as the referent category. Adjusted for sex, race/ethnicity, pre-existing hypertension, diabetes mellitus, cancer, chronic kidney disease, and severity of illness. AKI defined per KDIGO guidelines, but baseline creatinine estimator uses minimum serum creatinine value within first 7 days of hospitalization for all participants.
Additional file 6: Supplementary Fig. 5.
Age Distribution of Hospitalized Patients with SARS-CoV2 who Experienced AKI within First 7 days of Hospitalization Stratified by U.S. versus non-U.S. Hospital. Presents percentage of hospitalized patients who developed acute kidney injury (AKI) among all hospitalized patients and further stratified by hospital center based in the United States versus not in the United States. AKI defined per KDIGO guidelines.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bjornstad, E.C., Cutter, G., Guru, P. et al. SARS-CoV-2 infection increases risk of acute kidney injury in a bimodal age distribution. BMC Nephrol 23, 63 (2022). https://doi.org/10.1186/s12882-022-02681-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-022-02681-2