Abstract
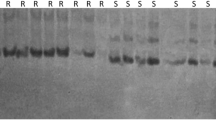
Little seed canary grass (Phalaris minor Retz.) populations resistant to herbicides that inhibit acetyl-CoA carboxylase (ACCase) represent an increasingly important weed control problem in northern India. The objective of this study was to develop DNA-based markers to differentiate herbicide-resistant and herbicide-susceptible population of P. minor. Primers were designed to amplify the conserved region carrying two reported mutations Trp2027 to Cys and Ile2041 to Asn conferring ACCase inhibitor resistance in several grass weeds and subjected to single-strand conformational polymorphism (SSCP) to detect the mutations. Five distinctive electrophoretic patterns on non-denaturing PAGE were observed, and four patterns were found to be associated with ACCase herbicide resistance in P. minor. The PCR-SSCP test developed in this study confirmed 17 resistant populations to contain mutations in CT domain of ACCase gene. This is the first report of rapid and easy molecular diagnosis of ACCase herbicide-resistant and herbicide-sensitive population of P. minor through PCR-SSCP analysis.


Similar content being viewed by others
References
Singh, S., Kirkwood, R. C., & Marshall, G. (1999) Biology and control of Phalaris minor Ritz. (littleseed canarygrass) in wheat. Crop Protection, 18, 1–16.
Chhokar, R. S., & Sharma, R. K. (2008) Multiple herbicide resistance in littleseed canarygrass (Phalaris minor): a threat to wheat production in India. Weed Biology and Management, 8, 112–123.
Rendina, A. R., Craig-Kennard, A. C., Beaudoin, J. D., & Breen, M. K. (1990) Inhibition of acetyl-coenzyme A carboxylase by two classes of grass-selective herbicides. Journal of Agricultural and Food Chemistry, 38, 1282–1287.
Burgos, N. R., Tranel, P. J., Streibig, J. C., Davis, V. M., Shaner, D., Norsworthy, J. K., & Ritz, C. (2013) Review: confirmation of resistance to herbicides and evaluation of resistance levels. Weed Science, 61, 4–20.
Orita, M., Iwahana, H., Kanazawa, H., Hayashi, K., & Sekiya, T. (1989) Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Proceedings of the National Academy of Sciences of the United States of America, 86, 2766–2770.
Untergasser, A., Nijveen, H., Rao, X., Bisseling, T., Geurts, R., & Leunissen, J. A. M. (2007) Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Research, 35, W71–W74.
Doyle, J. J., & Doyle, J. L. (1990) Isolation of plant DNA from fresh tissue. Focus, 12, 13–15.
Bassam, B. J., Caetano-Anollés, G., & Gresshoff, P. M. (1991) Fast and sensitive silver staining of DNA in polyacrylamide gels. Analytical Biochemistry, 196, 80–83.
Powels, S. B., & Yu, Q. (2010) Evolution in action: plants resistant to herbicides. Annual Review of Plant Biology, 61, 317–347.
Beckie, H. J., & Tardif, F. J. (2012) Herbicide cross resistance in weeds. Crop Protection, 35, 15–28.
Konstantinos, K. V., Panagiotis, P., Antonios, V. T., Agelos, P., & Argiris, N. V. (2008) PCR-SSCP: a method for the molecular analysis of genetic diseases. Molecular Biotechnology, 33, 155–163.
Kim, H. J., Hawthrone, D. J., Peters, T., Dively, G. P., & Clark, L. M. (2005) Application of DNA-based genotyping techniques for the detection of kdr-like pyrethroid resistance in field populations of Colorado beetle. Pesticide Biochemistry and Physiology, 81, 85–96.
Malekmohammadi, M., Hejazi, M. J., Mossadegh, M. S., Galehdari, H., Khanjani, M., & Goodarzi, M. T. (2012) Molecular diagnostic for detecting the acetylcholinesterase mutations in insecticide-resistant populations of Colorado potato beetle, Leptinotarsa decemlineata (Say). Pesticide Biochemistry and Physiology, 104, 150–156.
Kolkman, J. M., Slabaugh, M. B., Bruniard, J. M., Berry, S., Bushman, B. S., Olungu, C., Maes, N., Abratti, G., Zambelli, A., Miller, J. F., Leon, A., & Knapp, S. J. (2004) Acetohydroxyacid synthase mutations conferring resistance to imidazolinone or sulfonylurea herbicides in sunflower. Theoretical and Applied Genetics, 109, 1147–1159.
Hayashi, K. (1991) PCR-SSCP: a simple and sensitive method for detection of mutations in the genomic DNA. Genome Research, 1, 34–38.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, R., Sharma, D., Raghav, N. et al. Molecular Genotyping of Herbicide Resistance in P. minor: ACCase Resistance. Appl Biochem Biotechnol 175, 1617–1621 (2015). https://doi.org/10.1007/s12010-014-1363-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1363-7