Abstract
The antiproliferative activity of [Mn(CO)3(N^N)Br] (N^N = phendione 1, bipy 3) and of the two newly synthesized Mn complexes [Mn(CO)3(acridine)(phendione)]OTf (2) and [Mn(CO)3(di-triazole)Br] (4) has been evaluated by MTS against three tumor cell lines A2780 (ovarian carcinoma), HCT116 (colorectal carcinoma), HCT116doxR (colorectal carcinoma resistant to doxorubicin), and in human dermal fibroblasts. The antiproliferative assay showed a dose-dependent effect higher in complex 1 and 2 with a selectivity toward ovarian carcinoma cell line 21 times higher than in human fibroblasts. Exposure of A2780 cells to IC50 concentrations of complex 1 and 2 led to an increase of reactive oxygen species that led to the activation of cell death mechanisms, namely via intrinsic apoptosis for 2 and autophagy and extrinsic apoptosis for 1. Both complexes do not target DNA or interfere with cell cycle progression but are able to potentiate cell migration and neovascularization (for 2) an indicative that their application might be directed for initial tumor stages to avoid tumor invasion and metastization and opening a new avenue for complex 2 application in regenerative medicine. Interestingly, both complexes do not show toxicity in both in vivo models (CAM and zebrafish).
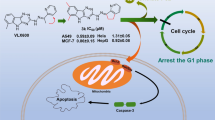
Graphical abstract













Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Abbas Z, Rehman S (2018) An overview of cancer treatment modalities. In: Neoplasm. INTECH. https://doi.org/10.5772/intechopen.76558
Makovec T (2019) Cisplatin and beyond: molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol Oncol 53:148–158. https://doi.org/10.2478/raon-2019-0018
Dasari S, Tchounwou PB (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740:364–378. https://doi.org/10.1016/j.ejphar.2014.07.025
Kostova I (2006) Ruthenium complexes as anticancer agents. Curr Med Chem 13:1085–1107. https://doi.org/10.2174/092986706776360941
Mjos KD, Orvig C (2014) Metallodrugs in medicinal inorganic chemistry. Chem Rev 114:4540–4563. https://doi.org/10.1021/cr400460s
Ndagi U, Mhlongo N, Soliman M (2017) Metal complexes in cancer therapy – an update from drug design perspective. Drug Des Devel Ther 11:599–616. https://doi.org/10.2147/dddt.s119488
Tarushi A, Geromichalos GD, Lafazanis K et al (2018) A step-ladder manganese(iii) metallacrown hosting mefenamic acid and a manganese(ii)–mefanamato complex: synthesis, characterization and cytotoxic activity. New J Chem 42:6955–6967. https://doi.org/10.1039/c8nj01182f
Vidhyapriya P, Divya D, Bala M, N S, (2018) Photoactivated [Mn(CO)3Br(μ-bpcpd)]2 induces apoptosis in cancer cells via intrinsic pathway. J Photochem Photobiol B 188:28–41. https://doi.org/10.1016/j.jphotobiol.2018.08.021
Ashok Kumar C, Divya D, Nagarajaprakash R et al (2017) Self-assembly of manganese(I) and rhenium(I) based semi-rigid ester functionalized M 2 L 2 -type metallacyclophanes: Synthesis, characterization and cytotoxicity evaluation. J Organomet Chem 846:152–160. https://doi.org/10.1016/j.jorganchem.2017.06.003
Chakraborty I, Carrington SJ, Roseman G, Mascharak PK (2017) Synthesis, structures, and CO release capacity of a family of water-soluble PhotoCORMs: assessment of the biocompatibility and their phototoxicity toward human breast cancer cells. Inorg Chem 56:1534–1545. https://doi.org/10.1021/acs.inorgchem.6b02623
Hu M, Yan Y, Zhu B et al (2019) A series of Mn(i) photo-activated carbon monoxide-releasing molecules with benzimidazole coligands: synthesis, structural characterization, CO releasing properties and biological activity evaluation. RSC Adv 9:20505–20512. https://doi.org/10.1039/c9ra01370a
Jimenez J, Chakraborty I, Dominguez A et al (2018) A luminescent manganese PhotoCORM for CO delivery to cellular targets under the control of visible light. Inorg Chem 57:1766–1773. https://doi.org/10.1021/acs.inorgchem.7b02480
Jimenez J, Pinto MN, Martinez-Gonzalez J, Mascharak PK (2019) Photo-induced eradication of human colorectal adenocarcinoma HT-29 cells by carbon monoxide (CO) delivery from a Mn-based green luminescent photoCORM. Inorganica Chim Acta 485:112–117. https://doi.org/10.1016/j.ica.2018.09.088
Kawahara B, Gao L, Cohn W et al (2020) Diminished viability of human ovarian cancer cells by antigen-specific delivery of carbon monoxide with a family of photoactivatable antibody-photoCORM conjugates. Chem Sci 11:467–473. https://doi.org/10.1039/c9sc03166a
Kumar CA, Nagarajaprakash R, Victoria W et al (2016) Synthesis, characterisation and cytotoxicity studies of Manganese(I) and Rhenium(I) based metallacrown ethers. Inorg Chem Commun 64:39–44. https://doi.org/10.1016/j.inoche.2015.12.011
Kumar U, Jose S, Divya D et al (2019) Self-assembly of manganese(i) based thiolato bridged dinuclear metallacycles: synthesis, characterization, cytotoxicity evaluation and CO-releasing studies. New J Chem 43:7520–7531. https://doi.org/10.1039/c8nj06271d
Kumar U, Roy S, Jha RK et al (2019) Selenolato-bridged manganese(I)-based dinuclear metallacycles as potential anticancer agents and photo-CORMs. ACS Omega 4:1923–1930. https://doi.org/10.1021/acsomega.8b03177
Musib D, Raza MK, Martina K, Roy M (2019) Mn(I)-based photoCORMs for trackable, visible light-induced CO release and photocytotoxicity to cancer cells. Polyhedron 172:125–131. https://doi.org/10.1016/j.poly.2019.04.008
Niesel J, Pinto A, Peindy N’Dongo HW et al (2008) Photoinduced CO release, cellular uptake and cytotoxicity of a tris(pyrazolyl)methane (tpm) manganese tricarbonyl complex. Chem Commun (Camb). https://doi.org/10.1039/b719075a
Oyarzo J, Bosque R, Toro P et al (2019) A novel type of organometallic 2-R-2,4-dihydro-1H-3,1-benzoxazine with R = [M(η5-C5H4)(CO)3] (M = Re or Mn) units. Experimental and computational studies of the effect of substituent R on ring-chain tautomerism. Dalton Trans 48:1023–1039. https://doi.org/10.1039/c8dt03265c
Pinto MN, Chakraborty I, Jimenez J et al (2019) Therapeutic potential of two visible light responsive luminescent photoCORMs: Enhanced cellular internalization driven by lipophilicity. Inorg Chem 58:14522–14531. https://doi.org/10.1021/acs.inorgchem.9b02121
Üstün E, Özgür A, Coşkun KA et al (2016) CO-releasing properties and anticancer activities of manganese complexes with imidazole/benzimidazole ligands. J Coord Chem 69:3384–3394. https://doi.org/10.1080/00958972.2016.1231921
Liu J, Guo W, Li J et al (2015) Tumor-targeting novel manganese complex induces ROS-mediated apoptotic and autophagic cancer cell death. Int J Mol Med 35:607–616. https://doi.org/10.3892/ijmm.2015.2073
Luís DV, Silva J, Tomaz AI et al (2014) Insights into the mechanisms underlying the antiproliferative potential of a Co(II) coordination compound bearing 1,10-phenanthroline-5,6-dione: DNA and protein interaction studies. J Biol Inorg Chem 19:787–803. https://doi.org/10.1007/s00775-014-1110-0
Lenis-Rojas OA, Fernandes AR, Roma-Rodrigues C et al (2016) Heteroleptic mononuclear compounds of ruthenium(ii): synthesis, structural analyses, in vitro antitumor activity and in vivo toxicity on zebrafish embryos. Dalton Trans 45:19127–19140. https://doi.org/10.1039/c6dt03591d
Lenis-Rojas OA, Robalo MP, Tomaz AI et al (2018) RuII(p-cymene) compounds as effective and selective anticancer candidates with no toxicity in vivo. Inorg Chem 57:13150–13166. https://doi.org/10.1021/acs.inorgchem.8b01270
Lenis-Rojas OA, Roma-Rodrigues C, Fernandes AR et al (2017) Dinuclear RuII(bipy)2Derivatives: structural, biological, and in vivo zebrafish toxicity evaluation. Inorg Chem 56:7127–7144. https://doi.org/10.1021/acs.inorgchem.7b00790
Lenis-Rojas OA, Cabral R, Carvalho B, Friães S, Roma-Rodrigues C, Fernández JAA et al (2021) Triazole-based half-sandwich ruthenium(II) compounds: from in vitro antiproliferative potential to in vivo toxicity evaluation. Inorg Chem. https://doi.org/10.1021/acs.inorgchem.1c00527 (Epub ahead of print. PMID: 33973771)
Staal LH, Oskam A, Vrieze K (1979) The syntheses and coordination properties of M(CO)3X(DAB) (M = Mn, Re; X = Cl, Br, I; DAB = 1,4-diazabutadiene). J Organomet Chem 170:235–245. https://doi.org/10.1016/s0022-328x(00)81088-8
Stanbury M, Compain J-D, Trejo M et al (2017) Mn-carbonyl molecular catalysts containing a redox-active phenanthroline-5,6-dione for selective electro- and photoreduction of CO 2 to CO or HCOOH. Electrochim Acta 240:288–299. https://doi.org/10.1016/j.electacta.2017.04.080
Hohloch S, Kaiser S, Duecker FL et al (2015) Catalytic oxygenation of sp3 “C-H” bonds with Ir(III) complexes of chelating triazoles and mesoionic carbenes. Dalton Trans 44:686–693. https://doi.org/10.1039/c4dt02879a
Krause L, Herbst-Irmer R, Sheldrick GM, Stalke D (2015) Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J Appl Crystallogr 48:3–10. https://doi.org/10.1107/S1600576714022985
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr C Struct Chem 71:3–8. https://doi.org/10.1107/S2053229614024218
Hübschle CB, Sheldrick GM, Dittrich B (2011) ShelXle: a Qt graphical user interface for SHELXL. J Appl Crystallogr 44:1281–1284. https://doi.org/10.1107/S0021889811043202
Farrugia LJ (2012) WinGXandORTEP for windows: an update. J Appl Crystallogr 45:849–854. https://doi.org/10.1107/s0021889812029111
Pedrosa P, Mendes R, Cabral R et al (2018) Combination of chemotherapy and Au-nanoparticle photothermy in the visible light to tackle doxorubicin resistance in cancer cells. Sci Rep 8:1. https://doi.org/10.1038/s41598-018-29870-0
Svahn N, Moro AJ, Rodrigues C et al (2018) The important role of the nuclearity, rigidity and solubility of phosphane ligands on the biological activity of gold(I) complexes. Chemistry. https://doi.org/10.1002/chem.201802547
Vinhas R, Fernandes AR, Baptista PV (2017) Gold nanoparticles for BCR-ABL1 gene silencing: Improving tyrosine kinase inhibitor efficacy in chronic myeloid leukemia. Mol Ther Nucleic Acids 7:408–416. https://doi.org/10.1016/j.omtn.2017.05.003
Morais TS, Jousseaume Y, Piedade MF et al (2018) Important cytotoxic and cytostatic effects of new copper(i)–phosphane compounds with N, N, N, O and N, S bidentate ligands. Dalton Trans 47:7819–7829. https://doi.org/10.1039/c8dt01653d
Roma-Rodrigues C, Heuer-Jungemann A, Fernandes AR et al (2016) Peptide-coated gold nanoparticles for modulation of angiogenesis in vivo. Int J Nanomedicine 11:2633–2639. https://doi.org/10.2147/IJN.S108661
Reigosa-Chamorro F, Raposo LR, Munín-Cruz P et al (2021) In vitro and in vivo effect of palladacycles: targeting A2780 ovarian carcinoma cells and modulation of angiogenesis. Inorg Chem 60:3939–3951. https://doi.org/10.1021/acs.inorgchem.0c03763
OECD (2013) Test no. 236: fish embryo acute toxicity (FET) test. OECD. https://doi.org/10.1787/9789264203709-en.
Directive 2010/63/EU of the european parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes.
Russell WMS, Burch LR (1959) The principles of humane experimental technique. Methuen
Groom CR, Bruno IJ, Lightfoot MP, Ward SC (2016) The Cambridge structural database. Acta Crystallogr B Struct Sci Cryst Eng Mater 72:171–179. https://doi.org/10.1107/S2052520616003954
Chen S, Cheng A-C, Wang M-S, Peng X (2008) Detection of apoptosis induced by new type gosling viral enteritis virus in vitro through fluorescein annexin V-FITC/PI double labeling. World J Gastroenterol 14:2174–2178. https://doi.org/10.3748/wjg.14.2174
Katiyar SK, Roy AM, Baliga MS (2005) Silymarin induces apoptosis primarily through a p53-dependent pathway involving Bcl-2/Bax, cytochrome c release, and caspase activation. Mol Cancer Ther 4:207–216. https://doi.org/10.4161/cbt.4.2.1442
Galluzzi L, Vitale I, Aaronson SA et al (2018) Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ 25:486–541. https://doi.org/10.1038/s41418-017-0012-4
Pierroz V, Joshi T, Leonidova A, Mari C, Schur J, Ott I, Spiccia L, Ferrari S, Gasser G (2012) Molecular and cellular characterization of the biological effects of Ruthenium(II) complexes incorporating 2-Pyridyl-2-pyrimidine-4-carboxylic acid. J Am Chem Soc 134:20376–20387. https://doi.org/10.1021/ja307288s
Mizushima N, Yoshimori T, Levine B (2010) Methods in mammalian autophagy research. Cell 140:313–326. https://doi.org/10.1016/j.cell.2010.01.028
Tan C, Lai S, Wu S, Hu S, Zhou L, Chen Y, Wang M, Zhu Y, Lian W, Peng W, Ji L, Xu A (2010) Nuclear permeable ruthenium(II) β-carboline complexes induce autophagy to antagonize mitochondrial-mediated apoptosis. J Med Chem 53:7613–7624. https://doi.org/10.1021/jm1009296
Riahi R, Yang Y, Zhang DD, Wong PK (2012) Advances in wound-healing assays for probing collective cell migration. J Lab Autom 17:59–65. https://doi.org/10.1177/2211068211426550
Gutiérrez-Lovera C, Martínez-Val J, Cabezas-Sainz P et al (2019) In vivo toxicity assays in zebrafish embryos: a pre-requisite for xenograft preclinical studies. Toxicol Mech Methods 29:478–487. https://doi.org/10.1080/15376516.2019.1611980
Howe K, Clark MD, Torroja CF et al (2013) The zebrafish reference genome sequence and its relationship to the human genome. Nature 496:498–503. https://doi.org/10.1038/nature12111
Acknowledgements
This work was funded by national funds through FCT–Fundação para a Ciência e a Tecnologia, I.P., Project MOSTMICRO-ITQB with refs UIDB/04612/2020 and UIDP/04612/2020. The NMR spectrometers at CERMAX are integrated in the National NMR, Network (PTNMR) and are partially supported by Infrastructure Project No. 022161 (co-financed by FEDER through COMPETE 2020, POCI and PORL and FCT through PIDDAC). This work is financed by national funds from FCT—Fundação para a Ciência e a Tecnologia, I.P., in the scope of the project UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences—UCIBIO and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy—i4HB. Oscar A. Lenis-Rojas acknowledges Project LISBOA-01-0145-FEDER-007660 (Microbiologia Molecular, Estrutural e Celular) funded by FEDER funds through COMPETE2020-Programa Operacional Competitividade e Internacionalização (POCI) and by national funds through FCT, POPH-Programa Operacional Potencial Humano, and FSE (European Social Fund) for the CEEC 2017 Initiative. Jhonathan A.A Fernández acknowledges Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) and the program CAPES/PRINT Proc. 88887.470075/2019-00 for the financial support. Sabela F Vila, Juan A. Rubiolo, and Laura Sanchez acknowledge ACUIGEN (GI-1251) from the Universidad de Santiago de Compostela and Xunta de Galicia proyect ED431C 2018/28 for the financial support. Clara S. B. Gomes acknowledges the Associate Laboratory for Green Chemistry–LAQV and the Applied Molecular Biosciences Unit–UCIBIO. LAQV is financed by national funds from Fundação para a Ciência e a Tecnologia (UIDB/50006/2020, UIDP/50006/2020). X-ray infrastructure financed by FCT-MCTES through project RECI/BBB-BEP/0124/2012. The authors acknowledge M. Baleia and I. Goncalves for pDNA, gDNA, and preliminary BAX/BCL-2 data, respectively.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lenis-Rojas, O.A., Carvalho, B., Cabral, R. et al. Manganese(I) tricarbonyl complexes as potential anticancer agents. J Biol Inorg Chem 27, 49–64 (2022). https://doi.org/10.1007/s00775-021-01910-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-021-01910-7