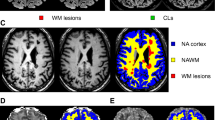
Abstract
Objectives
To evaluate the diffusion kurtosis and susceptibility change in the U-fiber region of patients with relapsing–remitting multiple sclerosis (pwRRMS) and their correlations with cognitive status and degeneration.
Materials and methods
Mean kurtosis (MK), axial kurtosis (AK), radial kurtosis (RK), kurtosis fractional anisotropy (KFA), and the mean relative quantitative susceptibility mapping (mrQSM) values in the U-fiber region were compared between 49 pwRRMS and 48 healthy controls (HCs). The U-fiber were divided into upper and deeper groups based on the location. The whole brain volume, gray and white matter volume, and cortical thickness were obtained. The correlations between the mrQSM values, DKI-derived metrics in the U-fiber region and clinical scale scores, brain morphologic parameters were further investigated.
Results
The decreased MK, AK, RK, KFA, and increased mrQSM values in U-fiber lesions (p < 0.001, FDR corrected), decreased RK, KFA, and increased mrQSM values in U-fiber non-lesions (p = 0.034, p < 0.001, p < 0.001, FDR corrected) were found in pwRRMS. There were differences in DKI-derived metrics and susceptibility values between the upper U-fiber region and the deeper one for U-fiber non-lesion areas of pwRRMS and HCs (p < 0.05), but not for U-fiber lesions in DKI-derived metrics. The DKI-derived metrics and susceptibility values were widely related with cognitive tests and brain atrophy.
Conclusion
RRMS patients show abnormal diffusion kurtosis and susceptibility characteristics in the U-fiber region, and these underlying tissue abnormalities are correlated with cognitive deficits and degeneration.
Clinical relevance statement
The macroscopic and microscopic tissue damages of U-fiber help to identify cognitive impairment and brain atrophy in multiple sclerosis and provide underlying pathophysiological mechanism.
Key Points
• Diffusion kurtosis and susceptibility changes are present in the U-fiber region of multiple sclerosis.
• There are gradients in diffusion kurtosis and susceptibility characteristics in the U-fiber region.
• Tissue damages in the U-fiber region are correlated with cognitive impairment and brain atrophy.




Similar content being viewed by others
Abbreviations
- AK:
-
Axial kurtosis
- DKI:
-
Diffusion kurtosis imaging
- DST:
-
Digit Span Test
- DTI:
-
Diffusion tensor imaging
- DWM:
-
Deep white matter
- EDSS:
-
Expanded Disability Status Scale
- FLAIR:
-
Fluid-attenuated inversion recovery
- FSS:
-
Fatigue Severity Scale
- HAMD:
-
Hamilton Depression
- HCs:
-
Healthy controls
- KFA:
-
Kurtosis fractional anisotropy
- MK:
-
Mean kurtosis
- MoCA:
-
Montreal Cognitive Assessment
- MPRAGE:
-
Magnetization prepared rapid gradient echo
- MRI:
-
Magnetic resonance imaging
- mrQSM:
-
Mean relative quantitative susceptibility mapping
- MS:
-
Multiple sclerosis
- NAWM:
-
Normal-appearing white matter
- Non-UFLs:
-
U-fiber non-lesions
- pwRRMS:
-
Patients with relapsing–remitting multiple sclerosis
- QSM:
-
Quantitative susceptibility mapping
- RK:
-
Radial kurtosis
- RRMS:
-
Relapsing-remitting MS
- SDMT:
-
Symbol Digit Modalities Test
- SWM:
-
Superficial white matter
- UFLs:
-
U-fiber lesions
References
Alotaibi A, Podlasek A, AlTokhis A, Aldhebaib A, Dineen RA, Constantinescu CS (2021) Investigating microstructural changes in white matter in multiple sclerosis: a systematic review and meta-analysis of neurite orientation dispersion and density imaging. Brain Sci 11:1151
Phillips OR, Nuechterlein KH, Asarnow RF et al (2011) Mapping corticocortical structural integrity in schizophrenia and effects of genetic liability. Biol Psychiatry 70:680–689
Nazeri A, Chakravarty MM, Felsky D et al (2013) Alterations of superficial white matter in schizophrenia and relationship to cognitive performance. Neuropsychopharmacology 38:1954–1962
Guevara M, Guevara P, Román C, Mangin JF (2020) Superficial white matter: a review on the dMRI analysis methods and applications. Neuroimage 212:116673
Movahedian Attar F, Kirilina E, Haenelt D et al (2020) Mapping Short Association Fibers in the Early Cortical Visual Processing Stream Using In Vivo Diffusion Tractography. Cereb Cortex 30:4496–4514
Medrano Martorell S, Cuadrado Blázquez M, García Figueredo D, González Ortiz S, Capellades Font J (2012) Imágenes puntiformes hiperintensas en la sustancia blanca: una aproximación diagnóstica [Hyperintense punctiform images in the white matter: a diagnostic approach]. Radiologia 54:321–335
Shukla DK, Keehn B, Smylie DM, Müller RA (2011) Microstructural abnormalities of short-distance white matter tracts in autism spectrum disorder. Neuropsychologia 49:1378–1382
Miki Y, Grossman RI, Jk U et al (1998) Isolated U-fiber involvement in MS: preliminary observations. Neurology 50:1301–1306
Mesulam MM (1990) Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol 28:597–613
Wu M, Kumar A, Yang S (2016) Development and aging of superficial white matter myelin from young adulthood to old age: mapping by vertex-based surface statistics (VBSS). Hum Brain Mapp 37:1759–1769
Liewald D, Miller R, Logothetis N, Wagner HJ, Schüz A (2014) Distribution of axon diameters in cortical white matter: an electron-microscopic study on three human brains and a macaque. Biol Cybern 108:541–557
Phillips OR, Clark KA, Luders E et al (2013) Superficial white matter: effects of age, sex, and hemisphere. Brain Connect 3:146–159
Butt AM, Berry M (2000) Oligodendrocytes and the control of myelination in vivo: new insights from the rat anterior medullary velum. J Neurosci Res 59:477–488
Haroutunian V, Katsel P, Roussos P, Davis KL, Altshuler LL, Bartzokis G (2014) Myelination, oligodendrocytes, and serious mental illness. Glia 62:1856–1877
Buyukturkoglu K, Vergara C, Fuentealba V et al (2022) Machine learning to investigate superficial white matter integrity in early multiple sclerosis. J Neuroimaging 32:36–47
Oishi K, Zilles K, Amunts K et al (2008) Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage 43:447–457
Smith SM, Jenkinson M, Johansen-Berg H et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505
Kirilina E, Helbling S, Morawski M et al (2020) Superficial white matter imaging: Contrast mechanisms and whole-brain in vivo mapping. Sci Adv 6:eaaz9281
Stojanovski S, Nazeri A, Lepage C, Ameis S, Voineskos AN, Wheeler AL (2019) Microstructural abnormalities in deep and superficial white matter in youths with mild traumatic brain injury. Neuroimage Clin 24:102102
Bigham B, Zamanpour SA, Zemorshidi F, Boroumand F, Zare H (2020) Identification of superficial white matter abnormalities in Alzheimer’s disease and mild cognitive impairment using diffusion tensor imaging. J Alzheimers Dis Rep 4:49–59
Rocca MA, Amato MP, De Stefano N et al (2015) Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol 14:302–317
Benedict RHB, Amato MP, DeLuca J, Geurts JJG (2020) Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol 19:860–871
Jandric D, Lipp I, Paling D et al (2021) Mechanisms of network changes in cognitive impairment in multiple sclerosis. Neurology 97:e1886–e1897
Li HQ, Yin B, Quan C et al (2018) Evaluation of patients with relapsing-remitting multiple sclerosis using tract-based spatial statistics analysis: diffusion kurtosis imaging. BMC Neurol 18:108
van der Weijden CW, van der Hoorn A, Potze JH et al (2022) Diffusion-derived parameters in lesions, peri-lesion, and normal-appearing white matter in multiple sclerosis using tensor, kurtosis, and fixel-based analysis. J Cereb Blood Flow Metab 42:2095–2106
Rahmanzadeh R, Galbusera R, Lu PJ et al (2022) A new advanced mri biomarker for remyelinated lesions in multiple sclerosis. Ann Neurol 92:486–502
Schweser F, Hagemeier J, Dwyer MG et al (2021) Decreasing brain iron in multiple sclerosis: the difference between concentration and content in iron MRI. Hum Brain Mapp 42:1463–1474
Thompson AJ, Banwell BL, Barkhof F et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17:162–173
Chan KS, Marques JP (2021) SEPIA-Susceptibility mapping pipeline tool for phase images. Neuroimage 227:117611
Haacke EM, Ayaz M, Khan A et al (2007) Establishing a baseline phase behavior in magnetic resonance imaging to determine normal vs. abnormal iron content in the brain. J Magn Reson Imaging 26:256–264
Chen W, Zhang Y, Mu K et al (2017) Quantifying the susceptibility variation of normal-appearing white matter in multiple sclerosis by quantitative susceptibility mapping. AJR Am J Roentgenol 209:889–894
Lu P, Yuan T, Liu X, Tian G, Zhang J, Sha Y (2020) Role of diffusional kurtosis imaging in differentiating neuromyelitis optica-related and multiple sclerosis-related acute optic neuritis: comparison with diffusion-weighted imaging. J Comput Assist Tomogr 44:47–52
Coutu JP, Chen JJ, Rosas HD, Salat DH (2014) Non-Gaussian water diffusion in aging white matter. Neurobiol Aging 35:1412–1421
Bian W, Tranvinh E, Tourdias T et al (2016) In vivo 7T MR quantitative susceptibility mapping reveals opposite susceptibility contrast between cortical and white matter lesions in multiple sclerosis. AJNR Am J Neuroradiol 37:1808–1815
Wisnieff C, Ramanan S, Olesik J, Gauthier S, Wang Y, Pitt D (2015) Quantitative susceptibility mapping (QSM) of white matter multiple sclerosis lesions: Interpreting positive susceptibility and the presence of iron. Magn Reson Med 74:564–570
Sheng H, Zhao B, Ge Y (2019) Blood perfusion and cellular microstructural changes associated with iron deposition in multiple sclerosis lesions. Front Neurol 10:747
Zhang Y, Gauthier SA, Gupta A et al (2016) Quantitative susceptibility mapping and R2* measured changes during white matter lesion development in multiple sclerosis: myelin breakdown, myelin debris degradation and removal, and iron accumulation. AJNR Am J Neuroradiol 37:1629–1635
Aljishi RH, Almatrafi RJ, Alzayer ZA, Alkhamis BA, Yaseen EE, Alkhotani AM (2021) Prevalence of anxiety and depression in patients with multiple sclerosis in Saudi Arabia: a cross-sectional study. Cureus 13:e20792
Broch L, Flemmen HØ, Simonsen CS et al (2022) Fatigue in multiple sclerosis is associated with socioeconomic factors. Mult Scler Relat Disord 64:103955
Moore H, Nair KPS, Baster K, Middleton R, Paling D, Sharrack B (2022) Fatigue in multiple sclerosis: a UK MS-register based study. Mult Scler Relat Disord 64:103954
Phillips OR, Joshi SH, Narr KL et al (2018) Superficial white matter damage in anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry 89:518–525
Migliore S, Ghazaryan A, Simonelli I et al (2017) Cognitive impairment in relapsing-remitting multiple sclerosis patients with very mild clinical disability. Behav Neurol 2017:7404289
Haider L, Prados F, Chung K et al (2021) Cortical involvement determines impairment 30 years after a clinically isolated syndrome. Brain 144:1384–1395
Fujimori J, Fujihara K, Wattjes M, Nakashima I (2021) Patterns of cortical grey matter thickness reduction in multiple sclerosis. Brain Behav 11:e02050
Narayana PA, Govindarajan KA, Goel P et al (2012) Regional cortical thickness in relapsing remitting multiple sclerosis: a multi-center study. Neuroimage Clin 2:120–131
Tsagkas C, Chakravarty MM, Gaetano L et al (2020) Longitudinal patterns of cortical thinning in multiple sclerosis. Hum Brain Mapp 41:2198–2215
Wang C, Barnett MH, Yiannikas C et al (2019) Lesion activity and chronic demyelination are the major determinants of brain atrophy in MS. Neurol Neuroimmunol Neuroinflamm 6:e593
Hidalgo de la Cruz M, Valsasina P, Meani A et al (2021) Differential association of cortical, subcortical and spinal cord damage with multiple sclerosis disability milestones: A multiparametric MRI study. Mult Scler 28:406–417
Bergsland N, Horakova D, Dwyer MG et al (2018) Gray matter atrophy patterns in multiple sclerosis: A 10-year source-based morphometry study. Neuroimage Clin 17:444–451
Tarasiuk J, Kapica-Topczewska K, Czarnowska A, Chorąży M, Kochanowicz J, Kułakowska A (2021) Co-occurrence of fatigue and depression in people with multiple sclerosis: a mini-review. Front Neurol 12:817256
Funding
This study received grants from the Key Project of Technological Innovation and Application Development of Chongqing Science and Technology Bureau (CSTC2021 jscx-gksb-N0008).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yongmei Li.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all participants.
Ethical approval
This retrospective study was approved by the Institutional Review Board of the First Affiliated Hospital of Chongqing Medical University.
Study subjects or cohorts overlap
None.
Methodology
• Retrospective
• Cross-section study
• Performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, D., Peng, Y., Zhu, Q. et al. U-fiber diffusion kurtosis and susceptibility characteristics in relapsing–remitting multiple sclerosis may be related to cognitive deficits and neurodegeneration. Eur Radiol 34, 1422–1433 (2024). https://doi.org/10.1007/s00330-023-10114-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10114-3