Abstract
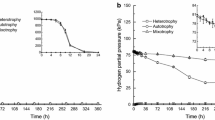
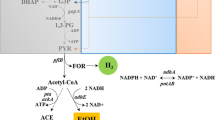
The hyperthermophilic anaerobic eubacterium Thermotoga maritima was grown on glucose as carbon and energy source. During growth 1 mol glucose was fermented to 2 mol acetate, 2 mol CO2 and 4 mol H2. The molar growth yicld on glucose (Yglucose) was about 45 g cell dry mass/mol glucose. In the presence of elemental sulfur growing cultures of T. maritima converted 1 mol glucose to 2 mol acetate, 2 mol CO2 about 0.5 mol H2 and about 3.5 mol H2S. Yglucose was about 45 g/mol. Cell extracts contained all enzymes of the Embden-Meyerhof pathway: hexokinase (0.29 U/mg, 50°C), glucose-6-phosphate isomerase (0.56 U/mg, 50°C), phosphofructokinase (0.19 U/mg, 50° C), fructose-1,6-bisphosphate aldolase (0.033 U/mg, 50°C), triosephosphate isomerase (6.3 U/mg, 50°C), glyceraldehyde-3-phosphate dehydrogenase (NAD+ reducing: 0.63 U/mg, 50°C), phosphoglycerate kinase (3.7 U/mg, 50°C), phosphoglycerate mutase (0.4 U/mg, 50°C); enolase (4 U/mg, 80°C), pyruvate kinase (0.05 U/mg, 50°C). Furthermore, cell extracts contained pyruvate: ferredoxin oxidoreductasee (0.43 U/mg, 60°C); NADH: ferredoxin oxidoreductase (benzylviologen reduction: 0.46 U/mg, 80°C); hydrogenase (benzylviologen reduction: 15 U/mg, 80°C), phosphate acetyltransferase (0.13 U/mg, 80°C), acetate kinase (1.2 U/mg, 55°C), lactate dehydrogenase (0.16 U/mg, 80°C) and pyruvate carboxylase (0.02 U/mg, 50°C). The findings indicate that the hyperthermophilic eubacterium T. maritima ferments sugars (glucose) to acetate, CO2 and H2 involving the Embden-Meyerhof pathway, phosphate acetyltransferase and acetate kinase. Thus, the organism differs from the hyperthermophilic archaeon Pyrococcus furiosus which ferments sugars to acetate, CO2 and H2 involving a modified non-phosphorylated Entner-Doudoroff pathway and acetyl-CoA synthetase (ADP forming).
Similar content being viewed by others
References
Altekar W, Rangaswamy V (1991) Ketohexokinase (ATP: D-fructose 1-phosphotransferase) intiates fructose breakdown via the modified EMP pathway in halophilic archaebacteria. FEMS Microbiol Lett 83: 241–246
Balch WF, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: reevaluation of a unique biological group. Microbiol Rev 43:260–296
Bode CH, Goebel H, Stähler H (1968) Zur Eliminierung von Trübungsfehlern bei der Eiweißbestimmung mit der Biuretmethode. Z Klin Chem Biochem 5:419–422
Cline JD (1969) Spectrometric determination of hydrogen sulfide in natural waters. Limnol Oceonogr 14:454–458
Danson MJ, Hough DW (1992) The enzymology of archaebacterial pathways of central metabolism. Biochem Soc Symp 58: 7–21
Decker K, Jungermann K, Thauer RK (1970) Energy production in anaerobic organisms. Angew Chem (Int Edn) 9:138–158
Dietrich G, Weiss N, Winter J (1988) Acetothermus paucivorans, gen. nov., sp. nov, a stricly anaerobic, thermophilic bacterium from scwage sludge, fermenting hexoses to acetate, CO2 and H2. Syst Appl Microbiol 10:174–179
Dorn M, Andreesen JR, Gottschalk G (1978) Fermentation of fumarte and l-malate by Clostridium formicoaceticum. J Bacteriol 133:26–32
Fuchs G, Stupperich E (1986) Carbon assimilation pathways in archaebacteria. Syst Appl Microbiol 7:364–369
Hecht K, Wrba A, Jaenicke R (1989) Catalytic properties of thermophilic lactate dehydrogenase and halophilic malate dehydrogenase at high temperature and low water activity. Eur J Biochem 183:69–74
Huber R, Stetter KO (1992) The order Thermotogales. In: Balows A, Trüger HG, Dworkin M, Harder W, Schleifer K-H (eds) The prokaryotes. Springer, New York, pp 3809–3815
Huber R, Langworthy TA, König H, Thomm M, Woese CR, Sleytr UB, Stetter KO (1986) Thermotoga maritima sp. nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90°C. Arch Microbiol 144:324–333
Huber R, Woese CR, Langworthy TA, Fricke H, Stetter KO (1989) Thermosipho africanus gen. nov., represents a new genus of thermophilic eubacteria within the “Thermotogales”, Syst Appl Microbiol 12:32–37
Huber R, Woese CR, Langworthy TA, Kristjansson JK, Stetter KO (1990) Fervidobacterium islandicum sp. nov., a new extremely thermophilic eubacterium belonging to the “Thermotogales”. Arch Microbiol 154:105–111
Jannasch HW, Huber R, Belkin S, Stetter KO (1988) Thermotoga neapolitana sp. nov of the extremely thermophilic, eubacterial genus thermotoga. Arch Microbiol 150:103–104
Janssen PH, Morgan HW (1992) Heterotrophic sulfur reduction by Thermotoga sp. strain FjSS3.B1. FEMS Microbiol Lett 96: 213–218
Juszczak A, Aono S, Adams MWW (1991) The extremely thermophilic eubacterium, Thermotoga maritima, contains a novel iron-hydrogenase whose cellular activity is dependent upon tungsten. J Biol Chem 13834–13841
Kunst A, Draeger B, Ziegenhorn J (1981) Colorimetric methods with glucose oxidase and peroxidase. In: Bergmeyer H-U (ed) Methods of enzymatic analysis, 3rd edn, vol 6. Verlag Chemie, Weinheim, pp 178–185
Ma K, Schicho RN, Kelly RM, Adams MWW (1993) Hydrogenase of the hyperthermophile Pyrococcus furiosus is an elemental sulfur reductase or sulfhydrogenase: evidence for a sulfur-reducing hydrogenase ancestor. Proc Natl Acad Sci USA 90:5341–5344
Reeves RE, Warren LG, Susskind B, Lo HS (1977) An energyconserving pyruvate-to-acetate pathway in Entamoeba histolytica: pyruvate synthase and a new acetate thiokinase. J Biol Chem 252:726–731
Schäfer T, Schönheit P (1991) Pyruvate metabolism of the hyperthermophilic archaebacterium Pyrococcus furiosus. Acetate formation from acetyl-CoA and ATP synthesis are catalyzed by an acetyl-CoA synthetase (ADP forming). Arch Microbiol 155:366–377
Schäfer T, Schönheit P (1992) Maltose fermentation to acetate, CO2 and H2 in the anaerobic hyperthermophilic archaeon Pyrocossus furiosus: evidence for the operation of a novel sugar fermentation pathway. Arch Microbiol 158:188–202
Schäfer T, Schönheit P (1993) Gluconeogenesis from pyruvate in the hyperthermophilic archaeon Pyrococcus furiosus: involvement of reactions of the Embden-Meyerhof pathway. Arch Microbiol 159:354–363
Schäfer T, Selig M, Schönheit P (1993) Acetyl-CoA synthetase (ADP forming) in archaea, a novel enzyme involved in acetate formation and ATP synthesis. Arch Microbiol 159:72–83
Schauder R, Kröger A (1993) Bacterial sulphur respiration. Arch Microbiol 159:491–497
Schönheit P, Wäscher C, Thauer RK (1978) A rapid procedure for the purification of ferredoxin from Clostridia using polycthylene imine. FEBS Lett 89:219–222
Schönheit P, Moll J, Thauer RK (1980) Growth parameters (Ks, μmax, Ys of Methanobacterium thermoautotrophicum. Arch Microbiol 127:59–65
Schultes V, Deutzmann R, Jaenicke P (1990) Complete aminoacid sequence of glyceraldehyde-3-phosphate dehydrogenase from the hyperthermophilic eubacterium Thermotoga maritima. Eur J Biochem 192:25–31
Siebers B, Hensel R (1993) Glucose catabolism of the hyperthermophilic archaeum Thermoproteus tenax. FEMS Microbiol Lett 111:1–8
Soutschek E, Winter J, Schindler F, Kandler O (1984) Acetomicrobium flavidum, gen. nov., sp. nov., a thermophilic, anaerobic bacterium from sewage sludge, forming acetate, CO2 and H2 from glucose. Syst Appl Microbiol 5:377–390
Stetter KO (1993) Life at the upper temperature border. In: Tran Thanh Van J, Tran Thanh Van K, Mounolou JC, Schneider J, McKay C (eds) Frontiers of life. Editions Frontières, Gif-sur-Yvette, pp 195–219
Stouthammer AH (1979) The search for correlation between theoretical and experimental growth yields. In: Quale JR (ed) Microbial biochemistry, vol 21. University Park Press, Baltimore, pp 1–47
Tewes FJ, Thauer RK (1980) Regulation of ATP-synthesis in glucose fermenting bacteria involved in interspecies hydrogen transfer. In: Gottschalk G, Pfennig N, Werner H (eds), Anaerobes and anaerobic infections. Gustav Fischer, Stuttgart New York, pp 97–104
Thauer RK, Morris G (1984) Metabolism of chemotrophic anaerobes: old views and new aspects. In: Kelly DP, Carr NG (eds) The microbe. Part II. Prokaryotes and eukaryotes. Society for General Microbiology Symposium 36. The Society for General Microbiology Ltd University Press, Cambridge, pp 123–168
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Tomlinson GA, Koch TK, Hochstein LI (1974) The metabolism of carbohydrates by extremely halophilic bacteria: glucose metabolism via a modified Entner-Doudoroff pathway. Can J Microbiol 20:1085–1091
Tomschy A, Glockshuber R, Jaenicke R (1993) Functional expression of d-glyceraldehyde 3-phosphate dehydrogenase from the hyperthermophilic eubacterium Thermotoga maritima in Escherichia coli. Eur J Biochem 214:43–50
Windberger E, Huber R, Trincone A, Fricke H, Stetter KO (1989) Thermotoga thermarum sp. nov. and Thermotoga neapolitana occurring in African continental solfataric springs. Arch Microbiol 151:506–512
Winter J, Zellner G (1990) Thermophilic anaerobic degradation of carbohydrates-metabolic properties of microorganisms from the different phases. FEMS Microbiol Rev 75:139–154
Woese CR (1987) Bacterial evolution. Microbiol Rev 51:221–271
Woese CR, Kandler O, Wheelis ML (1990) Towards a natural system of organisms: proposal for the domains archaea, bacteria, and eucarya. Proc Natl Acad Sci USA 87:4576–4579
Wrba A, Schweiger A, Schultes V, Jaenicke R, Závodszky P (1990) Extremely thermostable d-glyderaldehyde-3-phosphate dehydrogenase from the eubacterium Thermotoga maritima. Biochem 29:7584–7592
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schröder, C., Selig, M. & Schönheit, P. Glucose fermentation to acetate, CO2 and H2 in the anaerobic hyperthermophilic eubacterium Thermotoga maritima: involvement of the Embden-Meyerhof pathway. Arch. Microbiol. 161, 460–470 (1994). https://doi.org/10.1007/BF00307766
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00307766