Abstract
Purpose
The purpose of this study was to evaluate the utility of intravoxel incoherent motion diffusion-weighted imaging (IVIM DWI) parameters in identifying early renal function changes in diabetics.
Methods
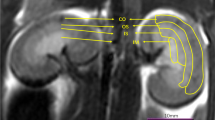
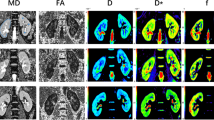
A total of 40 patients with type 2 diabetes mellitus and 20 healthy control subjects underwent multiple b value DWI. The diabetic patients were stratified into two groups based on albuminuria category: NAU (normal to mildly increased albuminuria; ACR < 30 mg/g) and MAU (moderately increased albuminuria; 30 ≤ ACR < 300 mg/g). The mean cortical and medullary IVIM parameters (D, D*, f, and ADC) were calculated and compared among the different groups, and the correlation of ACR and eGFR was also calculated.
Results
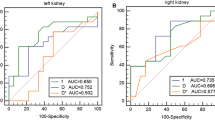
The present study revealed the limited water molecule diffusion and hyperperfusion of renal cortex and medulla in diabetic patients before proteinuria detection. Mean cortical and medullary D values negatively correlated with the ACR values in diabetics with 30 ≤ ACR < 300 mg/g, whereas no correlation was found between ACR values and other IVIM parameters.
Conclusion
IVIM DWI might be helpful in noninvasively identifying early-stage DN. The IVIM parametric values are more sensitive than the ACR in detecting early-stage kidney changes.




Similar content being viewed by others
References
Reutens AT, Atkins RC. (2011) Epidemiology of diabetic nephropathy. Contrib Nephrol 1–7.
Cakmak P, Yagci AB, Dursun B, Herek D, Fenkci SM (2014) Renal diffusion-weighted imaging in diabetic nephropathy: correlation with clinical stages of disease. Diagn Interv Radiol. 20(5):374–378
Caramori ML, Fioretto P, Mauer M (2003) Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes. 52(4):1036–1040
Fioretto P, Steffes MW, Mauer M (1994) Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes. 43(11):1358–1364
Caramori ML, Fioretto P, Mauer M (2000) The need for early predictors of diabetic nephropathy risk: is albumin excretion rate sufficient? Diabetes. 49(9):1399–1408
Parrish AE (1992) Complications of percutaneous renal biopsy: a review of 37 years’ experience. Clin Nephrol 38(3):135–141
Schrijvers BF, De Vriese AS, Flyvbjerg A (2004) From hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr Rev 25(6):971–1010
Dyvorne HA, Galea N, Nevers T, et al. (2013) Diffusion-weighted imaging of the liver with multiple b values: effect of diffusion gradient polarity and breathing acquisition on image quality and intravoxel incoherent motion parameters–a pilot study. Radiology. 266(3):920–929
Kim JW, Chang HL, Yoo KH, et al. (2016) Intravoxel incoherent motion magnetic resonance imaging to predict vesicoureteral reflux in children with urinary tract infection. Eur. Radiol 26(6):1–8
Rheinheimer S, Schneider F, Stieltjes B, et al. (2012) IVIM-DWI of transplanted kidneys: reduced diffusion and perfusion dependent on cold ischemia time. Eur J Radiol 81(9):951–956
Rheinheimer S, Stieltjes B, Schneider F, et al. (2012) Investigation of renal lesions by diffusion-weighted magnetic resonance imaging applying intravoxel incoherent motion-derived parameters–initial experience. Eur J Radiol 81(3):e310–316
Liang L, Chen WB, Chan KW, et al. (2016) Using intravoxel incoherent motion MR imaging to study the renal pathophysiological process of contrast-induced acute kidney injury in rats: Comparison with conventional DWI and arterial spin labelling. Eur Radiol 26(6):1597–1605
Cai XR, Yu J, Zhou QC, et al. (2016) Use of intravoxel incoherent motion MRI to assess renal fibrosis in a rat model of unilateral ureteral obstruction. J Magn Reson Imaging 44(3):698–706
Executive summary: standards of medical care in diabetes–2014. Diabetes Care 2014;37 Suppl 1:S5–13.
Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15(7):539–553
Kong X, Ma Y, Chen J, et al. (2013) Evaluation of the chronic kidney disease epidemiology collaboration equation for estimating glomerular filtration rate in the Chinese population. Nephrol Dial Transplant 28(3):641–651
Stevens PE, Levin A (2013) Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Internal Med 158(11):825–830
Welch WJ (2006) Intrarenal oxygen and hypertension. Clin Exp Pharmacol Physiol 33(10):1002–1005
Chen X, Xiao W, Li X, et al. (2014) In vivo evaluation of renal function using diffusion weighted imaging and diffusion tensor imaging in type 2 diabetics with normoalbuminuria versus microalbuminuria. Front Med 8(4):471–476
Yin WJ, Liu F, Li XM, et al. (2012) Noninvasive evaluation of renal oxygenation in diabetic nephropathy by BOLD-MRI. Eur J Radiol 81(7):1426–1431
Luo B, Wen S, Chen YC, et al. (2015) LOX-1-Targeted iron oxide nanoparticles detect early diabetic nephropathy in db/db mice. Mol Imaging Biol 17(5):652–660
Bakris GL (1996) Microalbuminuria: prognostic implications. Curr Opin Nephrol Hypertens 5(3):219–223
Peng XG, Bai YY, Fang F, et al. (2013) Renal lipids and oxygenation in diabetic mice: noninvasive quantification with MR imaging. Radiology. 269(3):748–757
Soininen P, Kangas AJ, Wurtz P, Suna T, Ala-Korpela M (2015) Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ Cardiovasc Genet 8(1):192–206
Xiao L, Zhu X, Yang S, et al. (2014) Rap1 ameliorates renal tubular injury in diabetic nephropathy. Diabetes. 63(4):1366–1380
Li Q, Li J, Zhang L, et al. (2014) Diffusion-weighted imaging in assessing renal pathology of chronic kidney disease: a preliminary clinical study. Eur J Radiol 83(5):756–762
Tervaert TW, Mooyaart AL, Amann K, et al. (2010) Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 21(4):556–563
Mise K, Hoshino J, Ueno T, et al. (2016) Prognostic value of tubulointerstitial lesions, urinary N-acetyl-beta-d-glucosaminidase, and urinary beta2-microglobulin in patients with type 2 diabetes and biopsy-proven diabetic nephropathy. Clin J Am Soc Nephrol 11(4):593–601
Lu L, Sedor JR, Gulani V, et al. (2011) Use of diffusion tensor MRI to identify early changes in diabetic nephropathy. Am J Nephrol 34(5):476–482
Su J, Li SJ, Chen ZH, et al. (2010) Evaluation of podocyte lesion in patients with diabetic nephropathy: Wilms’ tumor-1 protein used as a podocyte marker. Diabet Res Clin Pract 87(2):167–175
Luik PT, Hoogenberg K, Van Der Kleij FG, et al. (2002) Short-term moderate sodium restriction induces relative hyperfiltration in normotensive normoalbuminuric Type I diabetes mellitus. Diabetologia. 45(4):535–541
Le Bihan D (2008) Intravoxel incoherent motion perfusion MR imaging: a wake-up call. Radiology. 249(3):748–752
Cai XR, Zhou QC, Yu J, et al. (2015) Assessment of renal function in patients with unilateral ureteral obstruction using whole-organ perfusion imaging with 320-detector row computed tomography. PLoS ONE 10(4):e0122454
Notohamiprodjo M, Chandarana H, Mikheev A, et al. (2015) Combined intravoxel incoherent motion and diffusion tensor imaging of renal diffusion and flow anisotropy. Magn Reson Med 73(4):1526–1532
Nauta FL, Boertien WE, Bakker SJL, et al. (2011) Glomerular and tubular damage markers are elevated in patients with diabetes. Diabet Care 34(4):975–981
Xu X, Fang W, Ling H, Chai W, Chen K (2010) Diffusion-weighted MR imaging of kidneys in patients with chronic kidney disease: initial study. Eur Radiol 20(4):978–983
Acknowledgments
The authors wish to thank Zhong-Ping Zhang, advanced application specialist at GE Healthcare MR research China, Guangzhou, for his technical consultation for practicing the IVIM protocol.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by the Guangdong Science and Technology project in China (Grant No. 2014A020212442).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Feng, YZ., Chen, XQ., Yu, J. et al. Intravoxel incoherent motion (IVIM) at 3.0 T: evaluation of early renal function changes in type 2 diabetic patients. Abdom Radiol 43, 2764–2773 (2018). https://doi.org/10.1007/s00261-018-1555-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-018-1555-7