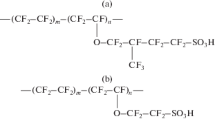Abstract
Data on the water uptake, proton conductivity, diffusion permeability, cation transport selectivity, and mechanical properties of short side chain Aquivion sulfonated perfluoropolymer membranes with an equivalent weight of 870 and 965 have been described. Properties of the membranes have been compared with those of a long side chain Nafion 212 membrane (equivalent weight of 1100). An increase in the equivalent weight leads to an increase in the sorption exchange capacity and water uptake of the membranes and a decrease in their proton conductivity. The conductivity of the Aquivion membrane with an equivalent weight of 870 is 1.4–1.5 times higher than that of the Nafion 212 membrane; it reaches 13.6 mS/cm in contact with water and 1.0 mS/cm at a relative humidity of 32% at 25°C. Diffusion permeability and cation transport selectivity exhibit a nonmonotonic dependence on the equivalent weight of the material. The lowest diffusion permeability, the highest Na+ cation transport selectivity (99.5%), and the best mechanical properties have been found for the Aquivion membrane with an equivalent weight of 965, which is characterized by the highest degree of crystallinity.
Similar content being viewed by others
References
S. J. Peighambardoust, S. Rowshanzamir, and M. Amjadi, Int. J. Hydrogen Energy 35, 9349 (2010).
S. Bose, T. Kuila, T. X. Hien Nguyen, et al., Prog. Polym. Sci. 36, 813 (2011).
E. Yu. Safronova and A. B. Yaroslavtsev, Pet. Chem. 56, 281 (2016).
K. A. Mauritz and R. B. Moore, Chem. Rev. 104, 4535 (2004).
Deuk Ju Kim, Min Jae Jo, and Sang Yong Nam, J. Ind. Eng. Chem. 21, 36 (2015).
C. M. Branco, A. El-kharouf, and S. Du, in Reference Module in Materials Science and Materials Engineering., Ed. by S. Hashmi (Elsevier, 2017). doi 10.1016/b978-0-12-803581-8.09261-4
A. B. Yaroslavtsev, Russ. Chem. Rev. 78 (11), 1013 (2009).
G. Pourcelly, Pet. Chem. 51, 480 (2011).
G. Alberti, R. Narducci, and M. Sganappa, J. Power Sources 178, 575 (2008).
E. Safronova, D. Safronov, A. Lysova, et al., Sens. Actuators B 240, 1016 (2017).
B. P. Tripathi and V. K. Shahi, Prog. Polym. Sci. 36, 945 (2011).
E. Yu. Safronova and A. B. Yaroslavtsev, Solid State Ionics 221, 6 (2012).
V. D. Noto, N. Boaretto, E. Negro, et al., Int. J. Hydrogen Energy 37, 6215 (2012).
H. Strathmann, A. Grabowski, and G. Eigenberger, J. Ind. Eng. Chem. Res. 52, 10364 (2013).
B. R. Matos, R. A. Isidoro, E. I. Santiago, et al., Int. J. Hydrogen Energy 40, 1859 (2015).
E. Gerasimova, E. Safronova, A. Ukshe, et al., Chem. Eng. J. 305, 121 (2016).
I. A. Prikhno, E. Yu. Safronova, and A. B. Yaroslavtsev, Int. J. Hydrogen Energy 41, 15585 (2016).
E. Yu. Safronova, I. A. Stenina, and A. B. Yaroslavtsev, Pet. Chem. 57, 299 (2017).
C. C. Ke, X. J. Li, S. G. Qu, et al., Polym. Adv. Technol. 23, 92 (2012).
H. Tang, Z. Wan, M. Pan, and S. P. Jiang, Electrochem. Commun. 9, 2003 (2007).
A. D’Epifanio, B. Mecher, E. Fabbri, et al., J. Electrochem. Soc. 154, B1148 (2007).
K. D. Kreuer, M. Schuster, B. Obliers, et al., J. Power Sources 178, 499 (2008).
P. Xiao, J. Li, H. Tang, et al., J. Membr. Sci. 442, 65 (2013).
J. Li, M. Pan, and H. Tang, RSC Adv. 4, 3944 (2014).
N. Berezina, S. Timofeev, and N. Kononenko, J. Membr. Sci. 209, 509 (2002).
G. Alberti, R. Narducci, and M. Sganappa, J. Power Sources 178, 575 (2008).
R. Kuwertz, C. Kirstein, T. Turek, and U. Kunz, J. Membr. Sci. 500, 225 (2016).
A. Skulimowska, M. Dupont, M. Zaton, et al., Int. J. Hydrogen Energy 39, 6307 (2014).
M. Amirinejad, S. S. Madaeni, M. A. Navarra, et al., J. Power Sources 196, 988 (2011).
M. Geise, H. J. Cassady, D. R. Paul, et al., Phys. Chem. Chem. Phys. 16, 21673 (2014).
V. I. Volkov, E. V. Volkov, S. V. Timofeev, et al., Russ. J. Inorg. Chem. 55, 315 (2010).
V. I. Roldugin and L. V. Karpenko-Jereb, Colloid J. 78, 795 (2016).
A. B. Yaroslavtsev and V. V. Nikonenko, Nanotechnol. Russ. 4, 137 (2009).
G. Gebel and R. B. Moore, Macromolecules 33, 4850 (2000).
K. D. Kreuer, M. Schuster, B. Obliers, et al., J. Power Sources 178, 499 (2008).
M. K. Mistry, N. R. Choudhury, N. K. Dutta, and R. Knott, Langmuir 26, 19073 (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.Yu. Safronova, A.K. Osipov, A.B. Yaroslavtsev, 2018, published in Membrany i Membrannye Tekhnologii, 2018, Vol. 8, No. 1, pp. 34–41.
Rights and permissions
About this article
Cite this article
Safronova, E.Y., Osipov, A.K. & Yaroslavtsev, A.B. Short Side Chain Aquivion Perfluorinated Sulfonated Proton-Conductive Membranes: Transport and Mechanical Properties. Pet. Chem. 58, 130–136 (2018). https://doi.org/10.1134/S0965544118020044
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544118020044