Abstract
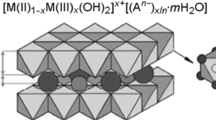
New experimental results and published data on sorption of strontium and yttrium radionuclides from aqueous solutions onto layered double hydroxides (LDHs) of various compositions are summarized. The effect of double-charged (Mg2+, Ba2+, Sr2+, Cu2+, Zn2+, Ni2+) and triple-charged (Al3+, Fe3+, Nd3+) cations incorporated in the LDH matrix and of anions incorporated in LDH (CO 2−3 , SO 2−4 , NO −3 , OH−, Cl−, C2O 2−4 , H2EDTA2−) on the physicochemical properties of this class of compounds, including the ability to sorb strontium and yttrium radionuclides, is examined.
Similar content being viewed by others
References
Goba, V.E., Stavitskaya, S.S., Petrenko, T.P., and Stavitskii, V.V., Khim. Tekhnol. Vody, 2003, vol. 25, no. 6, pp. 574–584.
Tarkovskaya, I.A., Antonova, L.S., Goba, V.E., et al., Zh. Prikl. Khim., 1996, vol. 68, no. 4, pp. 624–629.
Basharin, A.V., Vishnevskaya, A.A., Drugachenok, M.A., et al., Radiochemistry, 2003, vol. 45, no. 3, pp. 286–289.
Voroshilov, Yu.A., Logunov, M.V., Prokof’ev, N.N., and Zemlina, N.P., Radiochemistry, 2003, vol. 45, no. 1, pp. 64–67.
Myasoedova, G.V. and Nikashina, V.A., Ross. Khim. Zh., 2006, vol. 50, no. 5, pp. 55–63.
Panasyugin, A.S., Golikova, N.V., and Strukova, O.V., Radiochemistry, 2003, vol. 45, no. 3, pp. 290–292.
Sharygin, L.M. and Muromskii, A.Yu., Radiochemistry, 2004, vol. 46, no. 2, pp. 185–189.
Kameda, T. and Yoshioka, T., Metal, Ceramic and Polymer Composites for Various Uses, Cuppoletti, J., Ed., Rijeka (Croatia): INTECH, 2011, ch. 6, pp. 123–148.
Yan, H., Wei, M., Ma, J., et al., J. Phys. Chem. A, 2009, vol. 113, no. 21, pp. 6133–6141.
Kulyukhin, S.A., Krasavina, E.P., Rumer, I.A., and Gredina, I.V., Radiochemistry, 2012, vol. 54, no. 3, pp. 253–257.
Kulyukhin, S.A., Krasavina, E.P., Gredina, I.V., and Rumer, I.A., Radiochemistry, 2009, vol. 51, no. 6, pp. 616–621.
Kulyukhin, S.A., Krasavina, E.P., Gredina, I.V., et al., Radiochemistry, 2008, vol. 50, no. 5, pp. 493–501.
Ma, S., Fan, C., Du, L., et al., Chem. Mater., 2009, vol. 21, no. 15, pp. 3602–3610.
JCPDS—Int. Centre for Diffraction Data, PDF 70-2151, Mg2Al(OH)6[(CO3)1/2·1.5H2O.
Kameda, T., Fubasami, Y., and Yoshioka, T., J. Colloid Interface Sci., 2011, vol. 362, pp. 497–502.
Wang, Y. and Gao, H., J. Colloid Interface Sci., 2006, vol. 301, no. 1, pp. 19–26.
Abellán, G., Busolo, F., Coronado, E., et al., J. Phys. Chem. C, 2012, vol. 116, pp. 15 756–15 764.
Radha, A.V., Thomas, G.S., Kamath, P.V., and Shivakumara, C., J. Phys. Chem. B, 2007, vol. 111, no. 13, pp. 3384–3390.
Thomas, N. and Rajamathi, M.I., Langmuir, 2009, vol. 25, no. 4, pp. 2212–2216.
Radha, A.V., Kamath, P.V., and Shivakumara, C., J. Phys. Chem. B, 2007, vol. 111, no. 13, pp. 3411–3418.
Majoni, S. and Hossenlopp, J.M., J. Phys. Chem. A, 2010, vol. 114, no. 49, pp. 12858–12 869.
Kandare, E. and Hossenlopp, J.M., J. Phys. Chem. B, 2005, vol. 109, no. 17, pp. 8469–8475.
Frolov, Yu.G., Kurs kolloidnoi khimii. Poverkhnostnye yavleniya i dispersnye sistemy (Course of Colloid Chemistry. Surface Phenomena and Disperse Systems), Moscow: Khimiya, 1989.
Lidin, R.A., Andreeva, L.L., and Molochko, V.A., Konstanty neorganicheskikh veshchestv: Spravochnik (Constants of Inorganic Substances: Handbook), Moscow: Drofa, 2006, pp. 336–342.
Cordier, G. and Popa, J.-M., FR Patent 96/01406, Sept, 12, 1996, Publ. March 20, 1997.
JCPDS—Int. Centre for Diffraction Data, PDF 70-2150, [Mg6Fe2(OH)16(CO3)·4.5H2O]0.375, Pyroaurite.
JCPDS—Int. Centre for Diffraction Data, PDF 86-0441, Mg(OH)2.
JCPDS—Int. Centre for Diffraction Data, PDF 85-1049, 83-2256, 77-0114, Al(OH)3.
JCPDS—Int. Centre for Diffraction Data, PDF 38-0032, Fe(OH)3.
JCPDS—Int. Centre for Diffraction Data, PDF 70-0714, FeOOH.
JCPDS—Int. Centre for Diffraction Data, PDF 86-0182, Mg6Fe2(OH)16(CO3)·4H2O, Sjoegrenite.
JCPDS—Int. Centre for Diffraction Data, PDF 86-0181, Mg6Fe2(OH)16(CO3)·4.5H2O, Pyroaurite.
JCPDS—Int. Centre for Diffraction Data, PDF 26-1217, Mg10Fe2(OH)24(CO3)·2H2O, Coalingite.
JCPDS-Int. Centre for Diffraction Data, PDF 14-0365, Mg6Fe(OH)13(CO3)·4H2O, Brugnatellite.
JCPDS—Int. Centre for Diffraction Data, PDF 78-1830, Sr(OH)2·2H2O.
JCPDS—Int. Centre for Diffraction Data, PDF 74-0407, Sr(OH)2.
JCPDS—Int. Centre for Diffraction Data, PDF 79-1898, Ba(OH)2·3H2O.
JCPDS—Int. Centre for Diffraction Data, PDF 77-2334, Ba(OH)2·H2O.
JCPDS—Int. Centre for Diffraction Data, PDF 44-0585, Ba(OH)2.
JCPDS—Int. Centre for Diffraction Data, PDF 84-0503, BaAl2(OH)4(CO3)2·3H2O, Hydrodresserite.
JCPDS—Int. Centre for Diffraction Data, PDF 31-0116, BaAl2(OH)4(CO3)2·2H2O, Hydrodresserite.
JCPDS—Int. Centre for Diffraction Data, PDF 20-0617, Ba2Al4(OH)8(CO3)4·3H2}O, Dresserite
Tretyakov, Yu.D., Lukashin, A.V., and Eliseev, A.A., Russ. Chem. Rev., 2004, vol. 73, no. 9, pp. 889–922.
JCPDS—Int. Centre for Diffraction Data, PDF 37-0630, Cu6Al2(OH)16(CO3)·4H2O.
JCPDS—Int. Centre for Diffraction Data, PDF 15-0087, Ni6Al2(OH)16(CO3,OH)·4H2O, Takovite.
JCPDS—Int. Centre for Diffraction Data, PDF 130420, Cu(OH)2.
JCPDS—Int. Centre for Diffraction Data, PDF 84-0599, Cu2(OH)3NO3.
JCPDS—Int. Centre for Diffraction Data, PDF 75-1485, Cu3(OH)5NO3.
JCPDS—Int. Centre for Diffraction Data, PDF 73-1520, Ni(OH)2.
JCPDS—Int. Centre for Diffraction Data, PDF 06-0075, NiOOH.
JCPDS—Int. Centre for Diffraction Data, PDF 22-0752, Ni(OH)4(NO3)2.
JCPDS—Int. Centre for Diffraction Data, PDF 74-0094, Zn(OH)2.
JCPDS—Int. Centre for Diffraction Data, PDF 27-0627, Zn5(OH)8(NO3)2·2H2O.
JCPDS—Int. Centre for Diffraction Data, PDF 48-0594, Ni2Al(OH)3(CO3)8.
JCPDS—Int. Centre for Diffraction Data, PDF 48-0593, NiAl(OH)3(CO3).
JCPDS—Int. Centre for Diffraction Data, PDF 49-0188, Ni2Fe2(OH)8(CO3)·2H2O.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.A. Kulyukhin, E.P. Krasavina, I.A. Rumer, I.V. Klimovich, 2014, published in Radiokhimiya, 2014, Vol. 56, No. 6, pp. 506–517.
Rights and permissions
About this article
Cite this article
Kulyukhin, S.A., Krasavina, E.P., Rumer, I.A. et al. Sorption of strontium and yttrium radionuclides from aqueous solutions onto layered double hydroxides of various compositions. Radiochemistry 56, 593–606 (2014). https://doi.org/10.1134/S1066362214060046
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1066362214060046