Abstract
Nanoparticles usage are now emerging as hazardous nanopollutants due to inappropriate usage and improper disposal. Superparamagnetic Iron Oxide Nanoparticles (SPIONs) is a widely used nanoparticle with various applications. In this study, SPIONs was evaluated for its impact against Vigna radiata and Eudrilus eugeniae. SPIONs were synthesized by chemical co-precipitation method in presence of cobalt chloride. The produced SPIONs was characterized using UV-Visible Spectroscopy, SEM (Scanning electron microscopy), EDX (Energy dispersive X-ray spectroscopy), XRD (X-ray diffraction), TEM (Transmission electron microscopy), AFM (Atomic force microscopy), XPS (X-ray photoelectron spectroscopy) and Zeta potential. The synthesized SPIONs were crystalline and monodispersed with size ranging between 15 nm and 20 nm. The seedlings of SPIONs treated Vigna radiata were found to have reduced root and shoot growth. The bioaccumulation of iron oxide in the treated plants was confirmed by ICP-OES (Inductively coupled plasma - optical emission spectrometry) analysis and Prussian blue staining. Cellular destruction and reduced reproduction rate were found in SPIONs exposed Eudrilus eugeniae and ICP-OES analysis of earthworm samples affirmed the bioaccumulation of SPIONs.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nanotechnology is a blooming field of science and has variety of applications in the field of science (Kreuter et al 2003). Applications of nanoparticles in various fields include agriculture, drug delivery (Samrot et al 2019a), imaging (Justin et al 2017, Justin et al 2018, Sruthi et al 2018, Samrot et al 2018a), mosquito larvicidal activity (Samrot et al 2019b), environmental remediation (Samrot et al 2019c), bioactivity including anticancer and antibacterial activity (Samrot et al 2016, Samrot et al 2018b, Samrot et al 2019a). The increased usage creates demand in the production alleviating the risks associated with improper disposal of metal nanoparticles. Several studies have assessed the negative impacts of inappropriate nanoparticle disposal in the environment (Taghavi et al 2013). Nanoparticles cause cell damage when they get accumulated inside cells by inducing oxidative stress, DNA damage and apoptosis (Ahamed et al 2010). The toxicity of the nanoparticles depends on the size, shape, surface chemistry, degree of internalization, dosage etc (Sharifi et al 2012). Nanoparticles when disposed in soil or water are unseeingly not readily metabolized by plants and so get accumulated in them. When these plants are consumed by animals/human, the particles enter the next tropic level leading to biomagnification. SPIONs in turn are biocompatibility and hence they can be used in targeted drug delivery as they can be directed to site such as an organ or tumor by applying external magnetic field (Yang et al 2008). They are also used in a wide range of biomedical applications like MRI scanning (Bulte and Kraitchman 2004), magnetic isolation of cells etc (Xu et al 2011). SPIONs can bind to drug, protein, antibody, enzymes however the uptake of SPIONs by the cells depends on the type of cells (Osaka et al 2009).
Since the SPIONs are versatile and explored in various fields, there exists a pressing need to meticulously observe the impact it creates in the living system to alleviate the potential risks connected with indecent disposal. Consequently we present out report on the SPIONs prepared by chemical co-precipitation method in the presence of cobalt, and its characteristics well documented by instrumental analysis. And look for their impact against a plant model, Vigna radiata and an animal model, Eudrilus eugeniae. E. eugeniae was taken as test model for its faster reproductivity, low cost and easy maintenance (Butt and Lowe 2010).
2. Materials and methods
2.1. Materials
Ammonia solution, cobalt chloride hexahydrate, ferric chloride hexahydrate, ferrous sulphate heptahydrate and sodium hydroxide were purchased with extrapure from SRL, India. Sodium hypochlorite was purchased from M/s Merck, India. Nitrogen purged Millipore water was used for the nanoparticle synthesis.
2.2. Synthesis of SPIONs
100 ml of 1 M ferric chloride (FeCl3.6H2O) and 1 M of ferrous sulphate (FeSo4.7H2O) were mixed together. A solution of CoCl2–NaOH was prepared with 50 ml 1 M cobalt chloride hexahydrate (CoCl2.6H2O) and 50 ml 5 M sodium hydroxide. To the precursor iron solution, 100 ml of CoCl2–NaOH mixture was added in drops with vigorous stirring maintained at 60 °C. To these drops of ammonia was added until the contents turns black. Precipitates collected in external magnetic field were washed several times with Nitrogen purged Millipore water and lyophilized.
2.3. Characterization of SPIONs
SPIONs were characterized to understand their chemical and structural properties. The absorption profile of the nanoparticles was checked in UV visible spectrum using UV–vis (Shimadzu). The size and chemical composition of the nanoparticles were checked through SEM and EDX. Further the size precision was measured through TEM (TEECNAI G2 Spirit Biotwin—120 kV). The confinement of the nanoparticle to the three coordinates as 3D images were noted by AFM (Bruker, Germany). The particulate nature of the SPIONs were recorded in the 2θ range of 20°–80° in a fixed-time mode at room temperature using Smartlab X-ray diffractometer (Rigaku, Japan) and the stability of the SPIONs was examined by zeta potential (Brookhaven Zeta-PALS).
2.4. Exposure study of SPIONs on plant model, Vigna radiata
2.4.1. Pretreatment of seeds
The seeds of Vigna radiata were surface sterilized with 2 % sodium hypochlorite solution and were soaked overnight in distilled water. The soaked seeds were again surface sterilized before use.
2.4.2. Treatment of seeds with SPIONs
The impact analysis of the SPOINs on Vigna radiata was carried out for a study period of 6 days. The SPIONs sample were diluted with distilled water for the hydroponic culturing to a working concentrations of 1 μg ml−1, 10 μg ml−1 and 20 μg ml−1. The seeds were allowed to germinate and grown in-vitro using culture tubes containing SPIONs with no other nutrient. Each culture tubes were introduced with pretreated seeds containing a M-shaped paper inserts (Whatmann filter paper) in order to keep them in contact with the SPIONs solution. The seed germination and growth were observed for the study concentrations in triplicates with distilled water as control.
2.4.3. Growth and biomass measurement
The germinated seeds were checked for their root and shoot length on the 3rd and 6th day. The total weight of the plants on the 6th day was measured to evaluate their biomass.
2.4.4. Qualitative phytochemical analysis
2 g of the plant was crushed with 15 ml of distilled water using mortar and pestle. The puree was filtered using Whatmann Filter paper. The filtrate was used to assess the presence of the following phytochemical test.
2.4.4.1. Test for carbohydrates
Few drops of Molisch's reagent was added to the extract and along with concentrated H2SO4. Formation of red color confirmed the presence of carbohydrates (Kumari et al 2016).
2.4.4.2. Test for proteins
The aqueous extract was added to 1 ml NaOH and 1% CuSO4 and appearance of violet color stood positive for proteins (Kumari et al 2016).
2.4.4.3. Test for lipids
2 ml of aqueous extract was added to 0.5 N KOH (prepared in ethanol) with few drops of phenolphthalein and heated in water bath for an hour. Soap formation confirmed the presence of lipids (Praveen and Nair 2014).
2.4.4.4. Test for tannins
The aqueous extract when added to 0.5 ml of 10 % FeCl3, formation of brownish blue color confirmed the presence of tannins (Edeoga et al 2005, Samrot et al 2010).
2.4.4.5. Test for saponins
The extract was mixed with distilled water and shaken vigorously forms honey comb froth confirmed the occurrence of saponins (Ezeonu and Ejikeme 2016).
2.4.4.6. Test for alkaloids
The acidic plant extract treated with 0.5 ml Dragendroff's reagent forms red color precipitates indicative of alkaloids (Samrot et al 2019).
2.4.4.7. Test for flavonoids
2 ml extract added to 0.5 ml NaOH formed a yellow colored solution, which disappeared with 70 % HCl indicated the presence of flavonoids. (Samrot et al 2019).
2.4.4.8. Test for phenols
The test solution turning blue color when 5% FeCl3 was added to the extract indicated the presence of phenols (Samrot et al 2019).
2.4.4.9. Test for cardiac glycosides
1 ml extract mixed with 0.5 ml of glacial acetic acid with few drops of 1% FeCl3 had brown ring at the interface indicated the presence of cardiac glycosides (Auwal et al 2014).
2.4.5. Prussian blue staining of plant parts
The root tips, the basal region of shoot and the leaf apex of the plants after 6 days treatment were subjected to Prussian blue staining to identify iron elements present in the tissues. Equal volumes of freshly prepared 2% Potassium ferrocyanide and 2% Hydrochloric acid were mixed together to get Prussian blue staining buffer and the above mentioned plant parts were soaked in staining buffer separately for 15 min (Wei et al 2015). After incubation, they were rinsed in distilled water and thin sections of the sample was cut using a blade & scalpel and fixed on a glass slide to observe under Light microscope at 10 X magnification.
2.4.6. ICP-OES analysis of Vigna radiata
SPIONs treated plants were rinsed in distilled water, dried and digested with 2 ml of nitric acid to obtain its acidic extract. The obtained extract was heated to 120 °C for 30 min and subjected for ICP–OES analysis to detect iron (Agilent ICP-OES VDV 51009) (Geisler-Lee et al 2012).
2.5. Exposure study of SPIONs on animal model, Eudrilus eugeniae
2.5.1. Collection and pre-treatment of earthworms
The earthworm, Eudrilus eugeniae were collected from SS vermicompost, Tambaram, Chennai and were kept in manure rich soil. Prior to the nanoparticle exposure, they were allowed to adapt to the soil environment for 2 days. The moisture content was maintained and the Earthworms were fed with powdered cow dung every 24 h (Samrot et al 2017, Samrot et al 2019d). The earthworms were pretreated as mentioned earlier (Samrot et al 2017).
2.5.2. Exposure of Eudrilus eugeniae with SPIONs
The pretreated earthworms were exposed to different concentrations of SPIONs (10 mg, 20 mg and 30 mg) added to soil, as per the same procedures followed in our earlier reports on gold nanoparticle by Samrot et al (2018c). A count of 20 earthworms was introduced to each of the study concentration. On the 10th day, the earthworms from each concentration was subjected to histology and ICP–OES analysis. Similar protocol was trialed for all the SPIONs concentration to observe the impact on reproduction for a period of 30 days (Samrot et al 2017). Phenotypic changes in appearance and behavioral patterns were observed for each set of earthworms and compared to the control.
2.5.3. Histology studies of Eudrilus eugeniae
On the 10th day, foregut, midgut and hindgut region of three earthworms from each concentration was cut using a surgical blade. Tissues were preserved in 10 % formaldehyde solution, dehydrated in ethanol and then fixed in wax. Sections were made using microtome and H & E staining was performed. Sections were observed under microscope and histological images were documented.
2.5.4. ICP-OES Analysis of Eudrilus eugeniae
2.6. Statistical analysis
All the experiments were performed in triplicates and the mean value is represented as results.
3. Results
3.1. Characterization of SPIONs
3.1.1. UV–vis
The absorbance property of the synthesized SPIONs can be understood on exposure to UV-Visible radiations. From the results, it is observed that the synthesized SPIONs show maximum absorbance near 420 nm followed by a blunt peak in the range of 450 nm–540 nm (figure 1). Kumar et al (2015) has stated that the absorption peak for Fe3O4 appeared at 492 nm while Samrot et al (2017) reported peak absorbance near 250–260 nm for SPIONs, these discrepancies in the absorption spectra could be due to the varying size and composition of SPIONs as a contributing factor.
Figure 1. UV–vis analysis of SPIONs.
Download figure:
Standard image High-resolution image3.1.2. SEM-EDX
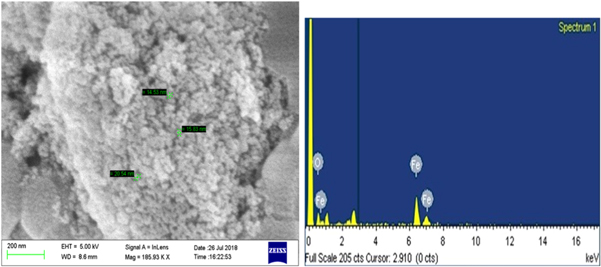
Using SEM, the topographical view was visualized at the magnification of 185.93 KX where the synthesized SPIONs were in the size range of 14.53 nm to 20.54 nm (figure 2). Samrot et al (2019c) showed in his earlier discussions the size of SPIONs produced using ammonia as reducing agent was around 25 nm, whereas the use of a different reducing agent in this case Cobalt chloride in NaOH did contribute to the change in size of the nanoparticle formation. EDX results affirmed the elemental composition of the synthesized SPIONs which showed the presence of Fe and O.
Figure 2. SEM-EDX analysis of SPIONs.
Download figure:
Standard image High-resolution image3.1.3. TEM
TEM images revealed the aggregation of synthesized SPIONs with particles appearing roughly spherical shaped in the size range between 15 nm – 20 nm. These results on size and distribution supplemented with the SEM results (figure 3). The smaller size of SPIONs were evenly reported where Justin et al (2018) stated SPIONs of size 15 nm and Samrot et al (2019c) detailed the SPIONs produced of 20 nm in size when NaOH was used as the reducing agent.
Figure 3. TEM analysis of SPIONs.
Download figure:
Standard image High-resolution image3.1.4. AFM
The 3D illustrative image of the synthesized SPIONs was seen with the help of AFM which recorded the size to be around 15 nm, which further supported the TEM and SEM results (figure 4).
Figure 4. AFM analysis of SPIONs.
Download figure:
Standard image High-resolution image3.1.5. Zeta potential analysis
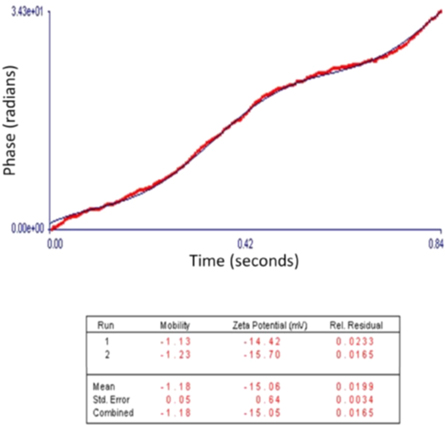
The particle stability detected through the charge distribution on the surface of the synthesized SPIONs exhibited the zeta potentials as −15 mV (figure 5). The values such as these indicate that the particles are stable with negative surface charge. Nanoparticles are said to be stable when their zeta potential values are greater than +25 mV or less than −25 mV(Justin et al 2018).
Figure 5. Zeta potential analysis of SPIONs.
Download figure:
Standard image High-resolution image3.1.6. XPS
From the obtained records of XPS, the binding energy of Fe was recognized at 710 eV and the binding energy of CO and O was at 285.0 eV and 533.0 eV respectively (figure 6), which was showing it to be Fe3O4. Samrot et al (2018d) has also reported the characteristics of Fe3O4 as the binding energy at 711.8 eV, FeO at 709.6 eV and Fe at 706.7 eV. By fitting the XPS data in Shirley background subtraction using the CASAPEAK software, the highresolution spectrum of C 1s, O 1s, Fe 2p3/2 and Fe 2p1/2 were represented.
Figure 6. XPS analysis of SPIONs. High resolution spectrum of (a) Carbon, (b) Iron, (c) Oxygen and (d) XPS survey spectrum.
Download figure:
Standard image High-resolution image3.1.7. XRD
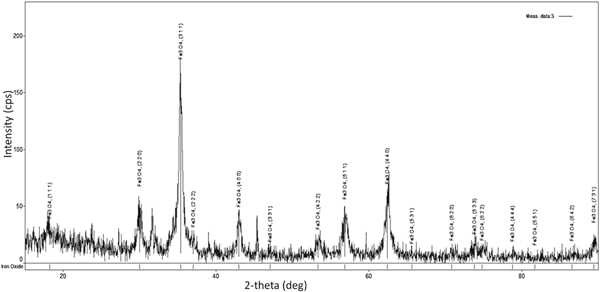
The XRD diffractogram with sharp peaks signify high crystalline nature of the synthesized particle. The diffraction peaks of 2θ with the planes at (220), (311),(400), (511), and (440) figure 7 corresponds to cubic spinel arrangement of Fe3O4 (JCPDS 85-1436). This is more comparable with the XRD data of SPIONs in our earlier report (Samrot et al 2018d).
Figure 7. XRD analysis of SPIONs.
Download figure:
Standard image High-resolution imageUsing the below Debye–Scherrer equation, the crystallite size was calculated.
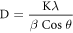
where K is known as constant which is equal to 0.90, θ represents the Bragg angle, λ represents the wavelength of the incident x-ray used (λ = 0.1540 nm), β is Full Width at Half Maximum (FWHM) of the sharp peak recorded from the XRD analysis. The high intensity sharp peaks were considered in order to calculate the average crystallite size. It was found to be 11.97 nm. The maximum diffraction peak at plane (311) with FWHM of 0.521° corresponded to crystallite size of 15.98 nm. This further supports the results of AFM (15 nm) which is a 3D imaging. Thus the size of each crystal or grain of SPIONs was understood from XRD.
3.2. Impact of SPIONs on plant growth
3.2.1. Root and shoot measurement
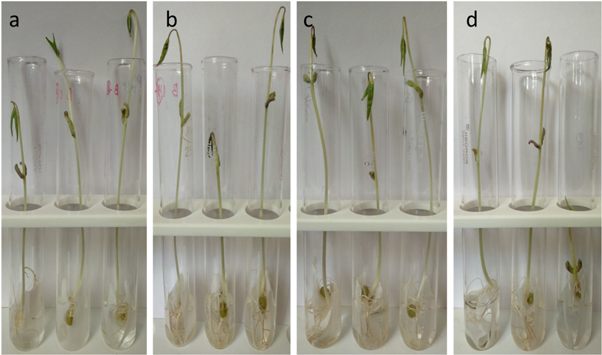
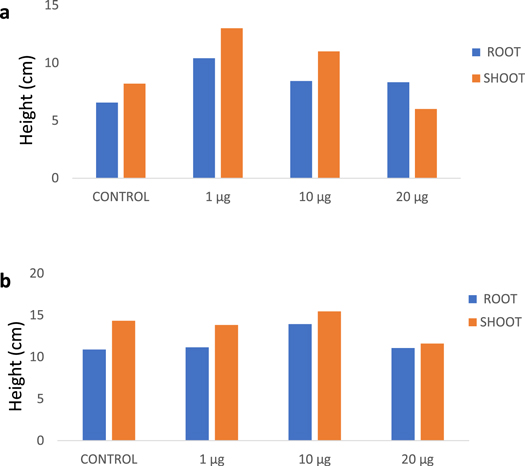
The effect of SPIONs on root and shoot development was examined by measuring their growth in centimeter (cm). Vigna radiata plants were measured on 3rd and 6th day. The minimal concentration (1 μg ml−1 and 10 μg ml−1) exposure has led to biostimulation in treated plants showing increased growth in root and shoot compared to control, expressing a maximum growth of 13:10.4 (shoot : root) by 1 μg ml−1 treated plants (figures 8 and 10(a)). And also, optimistic impact as numerous rootlets development was evident with 1 μg ml−1 and 10 μg ml−1 treated Vigna radiata which is on par with the results of Shankramma et al (2016) who stated an enhanced growth in iron oxide treated Solanum lycopersicum owing to biomineralization and has also identified the deposition of iron oxides on the root hairs, root tips and nodal regions of treated plants. While the prolonged exposure (4–6 days) at the minimal concentration (1 μg ml−1 and 10 μg ml−1) did not influence the growth of treated plants effectively as compared to the control which showed progressive growth (figures 9 and 10(b)). However, there was reduction in root development in 20 μg ml−1 exposed groups. Similar report has been discussed by Bombin et al (2015), where they studied the iron oxide nanoparticles (Fe2O3) with different charge coatings on Arabidopsis thaliana to hinder the root and shoot growth with reduced pollen viability, distressing its reproducing ability. Conversely, Iannone et al (2016) has exposed citric acid coated Fe3O4 nanoparticles on Triticum aestivum under hydroponic condition and reported null effect of Fe3O4 on plant growth and germination but reported an increased activity of antioxidant enzymes. Lin and Xing (2007) experimented with zinc oxide nanoparticle in higher plants and concluded with termination of root elongation in radish, grape and ryegrass.
Figure 8. Growth of Vigna radiata on Day 3 (a) CONTROL (b) 1 μg ml−1 (c) 10 μg ml−1 (d) 20 μg ml−1.
Download figure:
Standard image High-resolution imageFigure 9. Growth of Vigna radiata on Day 6 (a) CONTROL (b) 1 μg ml−1 (c) 10 μg ml−1 (d) 20 μg ml−1.
Download figure:
Standard image High-resolution imageFigure 10. Root and shoot measurement of Vigna radiata (a) on 3rd day (b) on 6th day.
Download figure:
Standard image High-resolution image3.2.2. Biomass evaluation
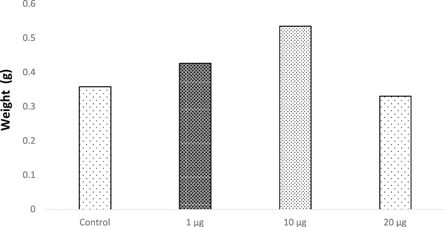
The plantlets of Vigna radiata were checked for their biomass content soon after the treatment with SPIONs and it was found that the plants treated with 1 μg ml−1 and 10 μg ml−1 of SPIONs showed maximum biomass of 0.426 g/plant and 0.53 g/plant respectively when compared to the control (0.358 g ml−1) (figure 11). This could be due to the growth stimulation factor of SPIONs in treated Vigna radiata. Wang et al (2015) reported that nano-ferric oxides at 20 mg l−1 on Citrullus lanatus showed increased soluble protein and chlorophyll contents. It could possibly infer that iron oxide nanoparticles at low concentrations are likely to promote the quality of the treated plants conversely the effect is stumpy at higher concentrations (20 μg ml−1) on treated Vigna radiata plantlets. From the observations and results, it can be deduced that the quality of the plants were affected by the presence of SPIONs. Ghodake et al (2011) also stated a comparable result where cobalt and zinc oxide nanoparticles have inhibited the root elongation in Allium cepa through bioaccumulation causing severe hazardous effect at cellular and chromosomal levels. Although Rui et al (2016) have supported that the use of iron oxide nanoparticles as iron fertilizers on Arachis hypogaea, a crop sensitive to iron deficiency had evidently showed increased root length, plant height, higher biomass, phytohormone contents and antioxidant enzyme activity, the prolonged exposure and long term usage may lead to risk associated with biomagnification, which are needed to be studied in field conditions.
Figure 11. Biomass of Vigna radiata.
Download figure:
Standard image High-resolution image3.2.3. Phytochemical screening
The phytochemical analysis of treated Vigna radiata indicated the presence of carbohydrates, protein, lipids, flavonoids, saponins, glycosides and the absence of phenols, tannins and alkaloids were observed (table 1).
Table 1. Phytochemicals screening in Vigna radiata.
| Phytochemicals | Control | 1 μg ml−1 | 10 μg ml−1 | 20 μg ml−1 |
|---|---|---|---|---|
| Carbohydrates | + | + | + | + |
| Protein | + | + | + | + |
| Lipids | + | + | + | + |
| Flavonoids | + | + | + | + |
| Tannins | − | − | − | − |
| Saponins | + | + | + | + |
| Glycosides | + | + | + | + |
| Phenols | − | − | − | − |
| Alkaloids | − | − | − | − |
'+' indicates presence.'−' indicates absence.
3.2.4. Prussian blue staining of plant tissues
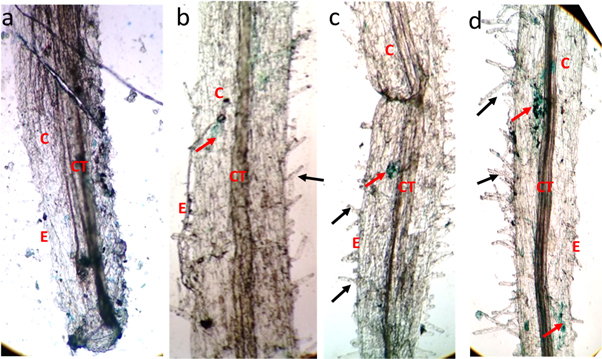
Accumulation of iron oxide particles was observed in all the test groups, where higher concentrations were found in the cortex of roots and stem (figures 12 and 13(b)–(d)), iron oxide particles were even found inside the leaves (figure 14). Prussian blue staining has been used to identify the uptake of iron by plants (Rios et al 2016). It is obvious from the SPIONs accumulated in tissues of plants might pave way for bioaccumulation.
Figure 12. Microscopic imaging of Prussian blue stained root (a) Control (b) 1 μg ml−1 (c) 10 μg ml−1 (d) 20 μg ml−1. E—epidermis, CT—connective tissue, C—cortex, black arrow—root hair, red arrow—iron oxide presence.
Download figure:
Standard image High-resolution imageFigure 13. C.S of Prussian blue stained stem (a) Control (b) 1 μg ml−1 (c) 10 μg ml−1 (d) 20 μg ml−1. E—epidermis, C—Cortex, CT—conductive tissue, P—pith black arrow—abnormality in pith, red arrow—iron oxide presence.
Download figure:
Standard image High-resolution imageFigure 14. Microscopic image of Prussian blue stained leaves (a) Control (b) 1 μg ml−1 (c) 10 μg ml−1 (d) 20 μg ml−1 red arrow—iron oxide presence.
Download figure:
Standard image High-resolution image3.2.5. ICP-OES of Vigna radiata
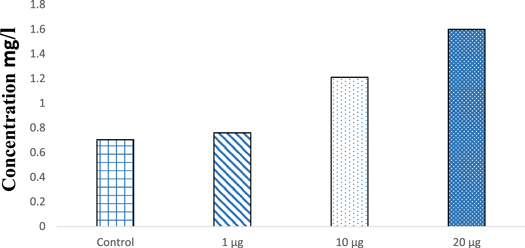
The incorporation of iron oxide into the plant tissues were further confirmed by ICP-OES. The study detected the highest iron content of 1.59 mg l−1 in 20 μg ml−1 treated plants, followed by 10 μg ml−1 having 1.21 mg l−1 and 1 μg ml−1 with 0.76 mg l−1 while the control having 0.705 mg l−1 of Fe (figure 15). The presence of Fe in control must be the natural mineral composition in the seeds. Raju et al (2016) have also used Vigna radiata as the plant model to study the effect of iron oxide nanoparticles and detected the uptake of iron oxide nanoparticles by the plant tissue through ICP-MS analysis.
Figure 15. ICP-OES of SPIONs treated plant tissue.
Download figure:
Standard image High-resolution image3.3. Impact of SPIONs on Eudrilus eugeniae
3.3.1. Effect on phenotypic character and reproduction
Due to the accumulation of SPIONs in the epithelium of earthworms, the color of the Earthworm was found to get darker with increasing concentrations but no mortality was observed in the Earthworms, Samrot et al (2017, 2018b) also reported similar observations when earthworms were exposed to magnetite and gold nanoparticles. The accumulation of nanoparticles has also affected the reproduction rate of earthworms. The reproduction rate was highly affected as the concentration increased table 2. Gold nanoparticles and silver nanoparticles were also found to reduce the number of earthworms after exposure. (Samrot et al 2018c, 2018e).
Table 2. Population study of earthworms before and after the SPIONs exposure.
| Concentration | Control | 10 mg | 20 mg | 30 mg | ||||
|---|---|---|---|---|---|---|---|---|
| No. of earthworms | Before | After | Before | After | Before | After | Before | After |
| 20 | 53 | 20 | 46 | 20 | 45 | 20 | 40 | |
3.3.2. Histology studies
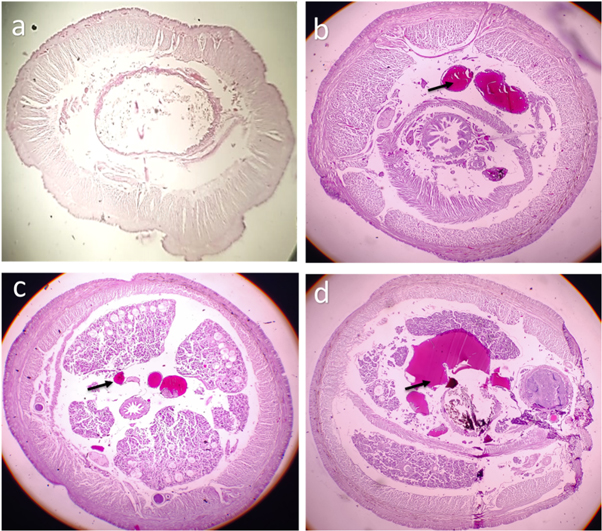
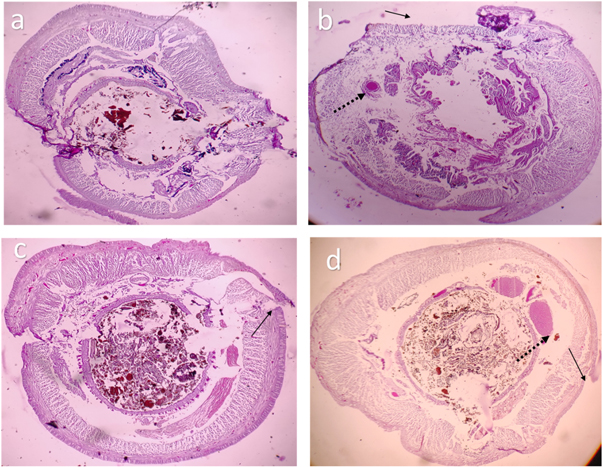
The histology images have revealed adverse effects of SPIONs caused on the tissues of earthworms. Impact of SPIONs in the gut region was evidenced by disintegration of the gut and lipofuscin deposits (figure 16). The outer structure of earthworm revealed drastic changes (figure 17). As the concentration of the Earthworms increased, erosion in circular and longitudinal muscle was observed (figures 16–18). And a complete destruction of gizzard area was also seen (figure 18(d)). Similar effects were observed in earthworms exposed to iron oxide, silver and gold nanoparticles causing disintegration of gut and other adverse effects in the Earthworms (Schlich et al 2013, Samrot et al 2018c, Samrot et al 2018e, ). The effect of carbon nanotubes along with sodium pentachlorophenol caused DNA and membrane damage on earthworms (Yang et al 2017).
Figure 16. Histology image of earthworm foregut-SPIONs (a) control (b) exposed to 10 μg SPIONs (c) exposed to 20 μg SPIONs (d) exposed to 30 μg SPIONs. black arrows denote lipofucsin deposits.
Download figure:
Standard image High-resolution imageFigure 17. Histology image of earthworm midgut-SPIONs (a) control (b) exposed to 10 μg SPIONs (c) exposed to 20 μg SPIONs (d) exposed to 30 μg SPIONs. black arrows denote erosion, dotted black arrows denote lipofucsin deposits.
Download figure:
Standard image High-resolution imageFigure 18. Histology image of earthworm hindgut-SPIONs (a) control (b) exposed to 10 μg SPIONs (c) exposed to 20 μg SPIONs (d) exposed to 30 μg SPIONs. black arrows denote erosion, dotted black arrows denote lipofucsin deposits.
Download figure:
Standard image High-resolution image3.3.3. ICP-OES of Eudrilus eugeniae
Accumulation of SPIONs into the treated earthworms can be witnessed with increase in concentration of nanoparticle. Accumulation of SPIONs in the control earthworms may be due to the nanoparticles present in the soil naturally (Samrot et al 2017). But the increased accumulation with increase in concentration (figure 19) explains that the effects noted are due to the nanoparticle ingestion.
Figure 19. ICP-OES analysis of earthworms.
Download figure:
Standard image High-resolution image4. Conclusion
In this study, SPIONs were produced by coprecipitation method in the presence of cobalt chloride. The nanoparticle size was found to be between 15 nm and 20 nm with crystalline nature and negative surface charge. On assessing the impact of synthesized SPIONs on Vigna radiata, it was found to increase the growth in treated plants at 1 μg ml−1 and 10 μg ml−1 concentration. High biomass and enormous rootlets formation were evident with 1 μg ml−1 and 10 μg ml−1 treated plants than control. The subcellular accumulation of SPIONs was visualized by Prussian blue staining. The successful uptake of SPIONs into the treated plant tissues have also been witnessed through ICP-OES analysis. Though there was growth stimulating effect initially, the prolonged exposure has rendered undesirable influence on the treated plants. In Eudrilus eugeniae, SPIONs exposure affected the appearance and the reproduction rate of treated earthworm with the increase in concentration. The disintegration of gut and lipofuscin deposits were noticed through histological studies and the accumulation of SPIONs into the Earthworms were detected through ICP-OES. Thus, nanoparticles are said to cause toxic effects to the environment and hence proper disposal methods are required. Mostly the iron oxides are not stable at higher temperatures and so they can be disposed by incineration.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.