Abstract
The objectives of this review article were to examine the dynamics of Varroa destructor infestation levels in Africanized honey bees (AHB) in Brazil, since this parasitic mite was first detected in 1977. Data from published research articles, conference proceedings, congress abstracts, and unpublished data obtained from academic researchers was included. Although mite infestations varied significantly along the years, there were no indications that varroa negatively impacted Brazilian apiculture. The mean infestation levels have remained around 4.5 mites per 100 adult bees, with a median of 3.8, during the last 45 years. Adult bee and worker brood infestation rates were found to be similar, though with some geographical variation, including a tendency for higher infestations in the southern regions of the country. Various researchers have suggested that the low infestation levels could be a consequence of the tropical and subtropical climate, honey bee hybridization, grooming and hygienic behaviors, honey bee and mite genetic factors, low nutritional stress, management practices, low migratory stress, and environmental conditions. The lack of a need for chemical treatment of varroa infestations facilitates apiary management and favors organic beekeeping throughout the country. However, though AHB colonies and beekeeping in Brazil thrive without the need for treatment measures, more research should be conducted to better assess the impact that the low varroa mite infestations have on AHB colony health and productivity.
Similar content being viewed by others
1 Introduction
Fifty years ago, the only major honey-producing areas not infested by varroa mites were the USA, Canada, Australia, and New Zealand (De Jong et al. 1982a). In 2020, only Australia was reportedly free of Varroa destructor infestation (Iwasaki et al. 2015; Roberts et al. 2017; Australian Government 2019; Brettell et al. 2020). Except for winter losses, this cosmopolitan mite is considered the main cause of honey bee colony decline for beekeeping in Europe and North America. Varroa destructor is also responsible for the transmission of viruses that cause diseases affecting honey bees at individual and colony levels (Genersch et al. 2010; Genersch and Aubert 2010).
Varroa was apparently introduced from Japan to Paraguay in 1971 (De Jong et al. 1982b; Traynor et al. 2020), arriving in Brazil in 1972 (De Jong et al. 1982b; Thoms et al. 2019), unwittingly transported by a beekeeper who was interested in the bees from Japan. The first infestation in Brazil was officially reported in 1977 (Morse and Gonçalves 1979), becoming a matter of concern for researchers and beekeepers.
Due to hybridization of existing populations of European honey bees (EHB) with imported African honey bees (Apis mellifera scutellata) beginning in 1957 (Kerr 1967), a new poly-hybrid Africanized honey bee (AHB) emerged (Gonçalves 1974). Compared to the EHB, this new bee had many advantages, including better adaptation to the environment and greater robustness, disease resistance, and productivity (De Jong 1996). Probably the most important advantage of the AHB is tolerance to infestation by V. destructor (De Jong et al. 1982b: Medina-Flores et al. 2014; Oddie et al. 2018).
Various factors favorable for AHB but adverse for varroa infestation in Brazil have been studied, such as climate and bee strain (Moretto et al. 1991a), grooming (Guzman-Novoa et al. 2012; Invernizzi et al. 2015), hygienic behavior (Wielewski et al. 2012; Spivak and Danka 2021), mite haplotype (Calderón et al. 2010), shorter capped brood cell duration (Moretto et al. 1995; Rosenkranz 1999), infested brood cell inspection and re-capping (Corrêa-Marques and De Jong 1989; Martin et al. 2020), smaller comb cell dimensions, and low mite fertility in AHB worker brood cells (Calderón et al. 2010; Maggi et al. 2010).
Iwasaki et al. (2015) noted that Australia’s varroa-free status is an opportunity for the research community to understand honey bee dynamics prior to varroa invasion. Likewise, the long-term low infestation levels in Brazil merit further study, as this situation provides an opportunity to investigate the interactions between managed honey bees and V. destructor without interference from chemical controls.
The objectives of this review article were to examine the dynamics of V. destructor infestation levels (VIL) in Brazil from its discovery in this country in 1977 to 2020, including variability of these levels across climate regions and on adult bees versus worker brood of AHBs in Brazilian apiculture.
2 Materials and methods
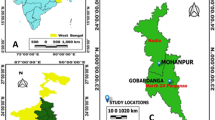
Data were retrieved from published papers (n = 26), congress abstracts (n = 32), and unpublished studies (n = 13) about V. destructor infestations in diverse locations throughout Brazil (Figure 1), totaling 361 data sources. A database was created with the following information: sampling year, latitude and longitude, Brazilian geographic region, and ontogenetic phase (adult bee or sealed brood). Data (Table S.M. 1 – Supplementary Material) were gathered and validated for analysis from information in publications and by contacting researchers (Figure 1).
GPS locations where Africanized honey bee samples were collected to investigate infestation by Varroa destructor (red dots) from previous research (published and unpublished data). Black lines = national and regional borders. Yellow lines = state borders. Source: Cartographic base: Google Maps, 2019. Modified by authors with survey data.
The method used to separate and count varroa mites from adult honey bees was noted when documents were assessed; the method developed by Stort et al. (1981) was the most common method used nationwide. In brief, mites are separated from honey bees in a solution of 70% ethanol, followed by a final visual inspection to confirm complete separation of the parasites. The infestation levels were calculated by dividing the number of mites by the number of honey bees in each sample.
Varroa mites in worker brood were examined according to Gonçalves et al. (1982), by counting the number of adult female mites in brood cells uncapped with forceps in initial studies. Methods proposed by Medina and Martin (1999) and by Dietemann et al. (2013) were also used in later studies.
Apiaries were located by Global Positioning System (GPS), highlighted on a map (Figure 1), and described in detail (Table S.M. 1—Supplementary Material) where the 361 samples were collected from. Apiary coordinates were usually cited in the studies included, and for those without such coordinates, the official GPS location of the municipality was considered.
Varroa infestation levels were compared by sampling year, Brazilian geographic region, and ontogenetic phase (adult bees or sealed brood). All honey bee samples were collected from stationary (non-migratory) apiaries.
PERMANOVA analysis used square root transformation on data in a similarity matrix based on Euclidean distances to test the interactions between independent, qualitative, and quantitative predictors, examining their effects on the dependent variable, VIL(%). Anderson-Darling was used to test the normality of the response variable. Kruskal-Wallis was used to examine differences between regions, and the Wilcoxon test was used to compare the two components of the ontogenetic phase. All the analyses were performed using R software (R Core Team 2021) with a significance level set at p < 0.05.
3 Results
Of all 361 samples analyzed (Table S.M. 1 – Supplementary Material), most were from apiaries located in the Northeast (26.6%), South (33.5%), and Southeast (32.7%) regions of Brazil; only a few studies were available from the Midwest (3.3%) and Northern (3.9%) regions (Figure 2).
As the primary objective of this research was to determine whether VIL in AHB in Brazil has changed over time, we treated it as the response variable. However, according to the Anderson-Darling test, VIL was not normally distributed, with gaps or insufficient samples in some years. There were significant effects on varroa infestation levels of year and region of the country but not of ontogenetic bee phase (Table I).
Based on PERMANOVA analysis, one of the factors with a significant influence on VIL in honey bee colonies was year (p < 0.0010). Differences among years and lack of data in some years were observed (Figure 3).
The VIL on AHB in the various Brazilian geographic regions varied significantly (p = 0.0060; Table I). The Southeast presented the highest VIL (5.5%), though not significantly different from the VIL recorded for the Midwest (5.0%), based on the Kruskal-Wallis test (Figure 4).
Varroa infestation levels in Africanized honey bee colonies plotted by Geographic Region of Brazil. Means indicated by white dots and medians by horizontal lines inside the box. Equal letters do not differ significantly according to the Kruskal-Wallis test; SE: Southeast; S: South; N: North; NE: Northeast; MW: Midwest.
When we evaluated the ontogenetic phase (p = 0.3700, Table I), 86.15% of the samples were from AHB adult workers (A_W), and 13.85% were from worker brood cells (W_B). There was no significant difference in VIL between these two phases (p = 0.3459) by the Wilcoxon test (Figure 5).
4 Discussion
Significant variation in V. destructor infestation levels was found in AHB in Brazil, considering the factors year and Brazilian geographic region. However, no colony losses have been reported due to varroa infestations (Maggi et al. 2016; Requier et al. 2018; Castilhos et al. 2019; Peixoto et al. 2021). Also, considerable fluctuation in VIL was found; in a few years, VIL was greater than 10%, which would be considered dangerous in other regions of the world. However, damage at the colony level was not reported, despite speculation that varroa infestation levels could increase over the years due to the introduction and subsequent dominance of a more virulent mitotype of the mite (Garrido et al. 2003; Carneiro et al. 2007).
The factor year had a moderate influence on the response variable VIL (R2 = 0.194; p = 0.001), but this may be due to variations in sample numbers and gaps in years reported, which led to limitations in data, even though missing data did not affect the validity of the PERMANOVA analysis (Figure 6).
The overall mean infestation level was 4.5% and median 3.8%, in this study, and VIL remained under 5% thanks to the confluence of many factors, according to published articles reviewed here, such as tropical and subtropical climate (Moretto et al. 1991a), honey bee Africanization (Moretto et al. 1991b; Guerra-Jr et al. 2000), grooming (Guzman-Novoa et al. 2012; Invernizzi et al. 2015) and hygienic behaviors (Spivak and Danka 2021), mite haplotype (Calderón et al. 2010), capped cell period (Moretto et al. 1995; Rosenkranz 1999; Martin et al. 2020), brood cell dimensions and the low fertility of the mite in AHB worker brood cells (Maggi et al. 2010), AHB queen fertility and reproduction (Calderón et al. 2010), honey bee and mite genetic factors (Mendoza et al. 2020; Beaurepaire et al. 2022), low nutrition stress (DeGrandi-Hoffman and Chen 2015), low migratory stress (Simone-Finstrom et al. 2016), and local apiary management practices (De Jong 1996; Castilhos et al. 2021).
Nevertheless, we must consider that beekeeping management is inherently highly diverse; the large number of small-scale beekeepers limits uniform implementation of standardized best management practices (Kulhanek et al. 2021). However, there is relatively little migratory beekeeping in Brazil, which could be an advantage in facing the varroa problem.
Varroa mites survive and reproduce in AHB colonies with much less damage to the bees than in EHB colonies; however, this does not mean that AHB are immune to the effects of the mite. Various studies have demonstrated that varroa significantly shortens the life span of AHB and reduces their size and weight (De Jong et al. 1982b; De Jong and De Jong 1983; Mattos and Chaud-Neto 2012; Koleoglu et al. 2018; Reyes-Quintana et al. 2019).
In Brazil, beekeepers do not normally use chemicals to treat varroa; instead, they rely on the ability of AHB to deal with this parasitic mite (De Jong 1996; Castilhos et al. 2021). With infestation levels remaining below 5 mites per 100 bees, beekeeping is conducted without chemically treating for varroa, which favors organic beekeeping throughout the country. Nevertheless, though there is no evidence that varroa infestations cause colonies to collapse in Brazil, more research should be conducted to better assess the impact that the low varroa mite infestations have on AHB colony health and productivity.
Availability of data and material
Data collected are available at the Supplementary Material (Table S. M. 1).
Code availability
Not applicable.
References
Australian Government – Department of Agriculture, Fisheries and Forestry (2019) Biosecurity and trade/ pests, diseases and weeds/ bees and bee pests and diseases/ honey bees, crop pollination and varroa mite frequently asked questions/ Which other countries have varroa mite? Available at: https://www.agriculture.gov.au/biosecurity-trade/pests-diseases-weeds/bees/honeybees-faqs#references. Accessed 20 Oct 2022
Bacha GL Jr, Felipe-Silva AS, Pereira PLL (2009) Rate of infestation by Varroa destructor in apiaries under geoprocessing. Arq Bras Med Vet Zootec 61(6):1471–1473. https://doi.org/10.1590/S0102-09352009000600034. (Supplementary Material, rows 55-57)
Banderó JL, Nascimento EGM, Celmer ST (2016) Ocorrência de Varroa destructor em apiários de Mato Grosso em 2015. In: Congresso Brasileiro de Apicultura 21. Fortaleza-CE. Available at: https://www.researchgate.net/publication/313811707. Accessed 25 Mar 2020. (Unpublished, Supplementary Material, row 269)
Barreto MR, Banderó JL (2020) Índice de infestação de Varroa destructor em Nova Ubiratã – MT. Universidade Federal do Mato Grosso – UFMT. (Unpublished, Supplementary Material, row 321)
Beaurepaire A, Arredondo D, Genchi-García ML, Castelli L, Reynaldi FJ, Antunez K, Invernizzi I, Mondet F, Le Conte Y, Dalmon A (2022) Genetic diversification of an invasive honey bee ectoparasite across sympatric and allopatric host populations. Infect Genet Evol 103:105340. https://doi.org/10.1016/j.meegid.2022.105340
Beelen R, Guimarães-Beelen PM, Lima EG (2011) Avaliação do índice de infestação do ácaro Varroa destructor em colônias de abelhas Apis mellifera no estado de Alagoas. Universidade Federal de Alagoas - UFAL. (Unpublished, Supplementary Material, rows 189-198)
Beelen R, Guimarães-Beelen PM, Lima EG, da Silva HM (2018) Hygienic behavior, varroa and nosema levels of Africanized honey bee colonies in Alagoas state, northeastern Brazil. In: Eurbee, 8. Ghent - Belgium. Universidade Federal de Alagoas - UFAL. (Unpublished. Supplementary Material, row 278)
Brettell LE, Riegler M, O’Brien C, Cook JM (2020) Occurrence of honey bee-associated pathogens in Varroa-free pollinator communities. J Inv Pathol 171:107344. https://doi.org/10.1016/j.jip.2020.107344
Bridi TR, Polesso AM, Abreu L, Bogus GM, Borsuk LC (2018) Incidência de Varroa destructor e Aethna tumida em colmeias de Apis mellifera na região oeste de Santa Catarina. In: Congresso Brasileiro de Apicultura, 22, p 211. Anais… Available at: https://docplayer.com.br/86183525. (Supplementary Material, rows 314-320)
Calderón RA, van Veen JW, Sommeijer MJ, Sanchez LA (2010) Reproductive biology of Varroa destructor in Africanized honey bees (Apis mellifera). Exp Appl Acarol. 50(4):281–297. https://doi.org/10.1007/s10493-009-9325-4
Cappelari FA, De Jong D (2010) Efeito do ácaro Varroa destructor sobre a abelha Apis mellifera. In: Encontro sobre abelhas, 9. Ribeirão Preto-SP, Brasil. Anais…, p 147–151. Available at: http://rge.fmrp.usp.br/encontro/documentos//09/09147.pdf. Accessed 01 Nov 2020. (Unpublished, Supplementary Material, rows 58-61)
Carneiro FE, Torres RR, Strapazzon R, Ramirez SA, Guerra JC Jr, Koling DF, Moretto G (2007) Changes in the reproductive ability of the mite Varroa destructor (Anderson and Trueman) in Africanized honey bees (Apis mellifera L.) (Hymenoptera: Apidae) colonies in southern Brazil. Neotrop. Entomol. 36(6):949–952. https://doi.org/10.1590/S1519-566X2007000600018
Carneiro FE, Barroso GV, Strapazzon R, Moretto G (2014) Reproductive ability and level of infestation of the Varroa destructor mite in Apis mellifera apiaries in Blumenau, State of Santa Catarina, Brazil. Acta Sci Biol Sci 36(1):109–112. https://doi.org/10.4025/actascibiolsci.v36i1.20366. (Supplementary Material, rows 230)
Castagnino GLB, Orsi RO, Funari SRC (2009) Éster de sacarose no controle do Varroa destructor em abelhas africanizadas. Acta Sci Anim Sci 31(3):287–293. https://doi.org/10.4025/actascianimsci.v31i3.4988. (Supplementary Material, row 52)
Castagnino GLB, Orsi RO (2012) Produtos naturais para controle do ácaro Varroa destructor em abelhas africanizadas. Pesq Agropec Bras 47(6):738–744. https://doi.org/10.1590/S0100-204X2012000600002. (Supplementary Material, rows 46-47)
Castagnino GLB, Pinto LFB, Carneiro MRL (2016) Correlação da infestação de Varroa destructor sobre o comportamento higiênico de abelhas Apis mellifera. Arch de Zootec 65(252):549–554. https://doi.org/10.21071/az.v65i252.1924. (Supplementary Material, row 245)
Castilhos D, Bergamo GC, Gramacho KP, Gonçalves LS (2019) Bee colony losses in Brazil: a 5-year online survey. Apidologie 50(3):263–272. https://doi.org/10.1007/s13592-019-00642-7
Castilhos D, Bergamo GC, Kastelik JP (2021) A survey of honey bee colony losses in Brazil in 2018-2019. Braz J Anim Environ Res 4(4):5017–5041. https://doi.org/10.34188/bjaerv4n4-016
Clementino DC, Galindo GM, Milfont MO (2016) Infestation rate of Varroa destructor in Apis mellifera colonies in the South Agreste of Pernambuco – Brazil. Rev Ver Agroeco Des Sust 11(3):177–181. https://doi.org/10.18378/rvads.v11i34289. (Supplementary Material, row 270)
Corrêa-Marques MH, De Jong D (1989) Uncapping of worker bee brood, a component of the hygienic behavior of Africanized honey bees against the mite Varroa jacobsoni Oudemans. Apidologie 29(3):283–389. https://doi.org/10.1051/apido:19980307
Correia-Oliveira ME, Trocoli LS, Souza PA, Sobrinho FC, Carvalho CAL (2016) Infestação do ácaro Varroa destructor em Apis mellifera no estado de Alagoas. In: Congresso Brasileiro de Apicultura, 21. (Unpublished. Supplementary Material, rows 246-249)
da Silva HM (2019) Índice de infestação do ácaro Varroa destructor e comportamento higiênico em colônias de abelhas Apis mellifera L. em Alagoas. 41 p. Trabalho de Conclusão de Curso em Zootecnia – Centro de Ciências Agrárias, Universidade Federal de Alagoas. Rio Largo, AL. Available at: http://www.repositorio.ufal.br/handle/riufal/7273. Accessed 20 Mar 2020. (Unpublished. Supplementary Material, rows 306-313)
da Silva HM, Lima LAA, da Silva ACF, Beelen R (2017) Avaliação do índice de infestação do ácaro Varroa destructor em Alagoas. In: Congresso Nordestino de Produção Animal, 12. Anais… UNIVASF, Petrolina, PE/ Juazeiro, BA, p. 86. Available at: http://www.cnpa2017.univasf.edu.br/files/anais_2017.pdf. Accessed 24 Mar 2021. (Unpublished. Supplementary Material, rows 280-281)
da Silva FEJ, de Souza EA, da Silva MR, de Medeiros VF, da Silveira-Neto AA (2015) Índice de infestação do ácaro Varroa destructor em colônias de abelhas Apis mellifera no município de Marcelino Vieira, RN. In: Reunião Annual da SBPC, 67. Anais… Available at: http://sbpcnet.org.br/livro/67ra/resumos/resumos/2649_1154ad 5690567f81ea7aeba7818c2e730.pdf. Accessed 25 Mar 2020. (Unpublished. Supplementary Material, row 240).
da Silva LA, Guerra JCV Jr, Moretto G, De Jong D, Gonçalves LS (1992) Infestação de abelhas africanizadas Apis mellifera L. pelo ácaro Varroa jacobsoni. Anais… Congresso Brasileiro de Apicultura 9:156–159 (Unpublished, Supplementary Material, row 11-17)
da Silva SJR (2003) Infestação pelo ácaro Varroa destructor no estado de Roraima, Brasil em 2002-2003. Setor de Entomologia - Museu Integrado de Roraima – Universidade Federal de Roraima – UFRR. (Unpublished. Supplementary Material, rows 42-45)
da Silva SJR (2016) Infestação pelo ácaro Varroa destructor no município de Cantá, estado de Roraima, Brasil em 2016. Setor de Entomologia - Museu Integrado de Roraima – Universidade Federal de Roraima – UFRR. (Unpublished. Supplementary Material, rows 282-283)
da Silva SJR, Casadio GML (1999) Infestação de colônias de Apis Mellifera L. por Varroa jacobsoni Oudemans na aldeia do Contão, RR, Brasil. Bol Mus Int Roraima 5(1):13–18. https://doi.org/10.24979/bolmirr.v5i01.734. (Supplementary Material, row 40)
DeGrandi-Hoffman G, Chen Y (2015) Nutrition, immunity, and viral infections in honey bees. Curr. Opin. Insect Sci. 10:170–176. https://doi.org/10.1016/j.cois.2015.05.007
De Jong D (1996) Africanized honey bees in Brazil, forty years of adaptation and success. Bee World 77(2):67–70. https://doi.org/10.1080/0005772X.1996.11099289
De Jong D, De Andrea-Roma D, Gonçalves LS (1982) A comparative analysis of shaking solutions for the detection of Varroa jacobsoni on adult honey bees. Apidologie 13(3):297–306. https://doi.org/10.1051/apido:19820308. (Supplementary Material, row 6)
De Jong D, De Jong PH (1983) Longevity of Africanized honey bees (Hymenoptera: Apidae) infested by Varroa jacobsoni (Parasitiformes: Varroidae). J Econ Entom 76(4):766–768. https://doi.org/10.1093/jee/76.4.766
De Jong D, Gonçalves LS (1981) The varroa problem in Brazil. Am Bee J 121:186–189
De Jong D, Morse RA, Eickwort GC (1982) Mite pests of honey bees. Ann Rev Entomol 27(1):229–252. https://doi.org/10.1146/annurev.en.27.010182.001305
Del Rey MC, de Araújo EAB, Castagnino GLB, Pinto LFB, de Carvalho GGP, Leite VM (2010) Taxa de infestação do Varroa destructor no apiário experimental da UFBA. In: Reunião Anual da Sociedade Brasileira de Zootecnia, 47, n. 4629. Anais… Salvador, BA – Brasil. Available at: http://sbz.org.br/new/pt/documentos%2D%2Danais-das-reunioes. Accessed 01 Mar 2021. (Unpublished, Supplementary Material, row 80)
Dietemann V, Ellis JD, Neumann P (2013) The Coloss Beebook Volume II, Standard methods for Apis mellifera pest and pathogen research, Introduction. J Apic Res 52(4):1–4. https://doi.org/10.3896/IBRA.1.52.4.16
Engels W, Gonçalves LS, Steiner J, Buriolla AH, Issa MRC (1986) Varroa-befall von Carnica-völkern in Tropenklima. Apidologie 17(3):203–216. https://doi.org/10.1051/apido:19860302. (Supplementary Material, row 7)
Estevan S, Abreu L, Bogus GM, Borsuk LC (2018) Ocorrência de Varroa destructor e Aethina tumida em colmeias de Apis mellifera no município de Trindade do Sul-RS. In: Congresso Brasileiro de Apicultura, 22. p 207. Anais… Available at: https://docplayer.com.br/86183525. (Unpublished.Supplementary Material, row 300)
Fogaça MJ, Morais AL, de Paula R, Modro AFH, Maia E (2012) Varroa destructor mite infestation in honey bee hives in Novo Horizonte do Oeste - Rondônia. In: Congresso sobre recursos naturais da Amazonia Ocidental. Rolim de Moura - RO Rev Bras Cie Am 1(1):59–63. Available at: https://www.periodicos.unir.br/index.php/rolimdemoura/ article/view/536/587. (Supplementary Material, row 203)
Freire DS, Costa PSC, Santos AB (2018) Avaliação do índice de infestação pelo ácaro Varroa destructor em colônias do apiário da Universidade Estadual do Sudoeste da Bahia, campus Vitória da Conquista-BA. In: Congresso Brasileiro de Apicultura, 22. p. 202. Anais… Available at: https://docplayer.com.br/86183525. (Unpublished. Supplementary Material, row 299)
Garrido C, Rosenkranz P, Paxton RJ, Gonçalves LS (2003) Temporal changes in Varroa destructor fertility and haplotype in Brazil. Apidologie 34(6):535–541. https://doi.org/10.1051/apido:2003041
Genersch E, Aubert M (2010) Emerging and re-emerging viruses of the honey bee (Apis mellifera L.). Vet Res 41(6):54. https://doi.org/10.1051/vetres/2010027
Genersch E, von der Ohe W, Kaatz H, Schroeder A, Otten C et al (2010) The German bee monitoring project: a long-term study to understand periodically high winter losses of honey bee colonies. Apidologie 41(3):332–352. https://doi.org/10.1051/apido/2010014
Gomes RVRS, Albuquerque JGSS, Gramacho KP (2018) Nível de infestação com varroa em colônias de abelhas africanizadas em área canavieira. In: Congresso Brasileiro de Apicultura, 22, p 204. Anais… Available at: https://docplayer.com.br/86183525. (Unpublished. Supplementary Material, row 298)
Gomes RVRS, Barros TA, Cabral RS, Costa JR (2020) Índices infestação de Varroa destructor no Sertão, Agreste e Zona da Mata de Pernambuco. Universidade Federal Rural de Pernambuco - UFRPE. (Unpublished, Supplementary Material, rows 322-324)
Gonçalves LS (1974) The Introduction of the African bees (Apis mellifera adansonii) into Brazil and some comments on their spread in South America. Am Bee J 114(11):411–419
Gonçalves LS, De Jong D, Nogueira RH (1982) Infestation of feral honey bee colonies in Brazil by Varroa jacobsoni. Am Bee J 122(4):249–251 (Supplementary Material, rows 2-5)
Guerra JCV Jr, Gonçalves LS, De Jong D (2000) Africanized honey bees (Apis mellifera L.) are more efficient at removing worker brood artificially infested with the parasitic mite Varroa jacobsoni Oudemans than are Italian bees or Italian/Africanized hybrids. Genet Mol Biol 23(1):89–92. https://doi.org/10.1590/S1415-47572000000100016
Guimarães-Cestaro L, Serrão JE, Alves MLTMF, Message D, Teixeira ÉW (2017) A scientific note on occurrence of pathogens in colonies of honey bee Apis mellifera in Vale do Ribeira. Brazil. Apidologie 48(3):384–386. https://doi.org/10.1007/s13592-016-0481-3. (Supplementary Material, rows 210-229)
Guzman-Novoa E, Emsen B, Unger P, Espinosa-Montaño LG, Petukhova T (2012) Genotypic variability and relationships between mite infestation levels, mite damage, grooming intensity, and removal of Varroa destructor mites in selected strains of worker honey bees (Apis mellifera L.). J Inv Path 110(3):314–320. https://doi.org/10.1016/j.jip.2012.03.020
Invernizzi C, Zefferino I, Santos E, Sánchez L, Mendoza Y (2015) Multilevel assessment of grooming behavior against Varroa destructor in Italian and Africanized honey bees. J Apic Res 54(4):321–327. https://doi.org/10.1080/00218839.2016.1159055
Issa MRC, Morais MM, Pereira RA, De Jong D, Gonçalves LS (2008) Menor infestação e reprodução do ácaro Varroa destructor em colmeias de abelhas africanizadas (Apis mellifera L.) com alto grau de comportamento higiênico. In: Encontro sobre abelhas, 8. Ribeirão Preto-SP, Brasil. Anais…, p 693. Available at: http://rge.fmrp.usp.br/encontro/documentos/08/08693.pdf. Accessed 28 Feb 2021. (Unpublished, Supplementary Material, rows 53-54)
Issa MRC, Morais MM, Pereira RA, De Jong D, Gonçalves LS (2012) Variation in the incidence of the mite Varroa destructor in brood cells and adult workers in Africanized honey bees (Apis mellifera L.) with a high degree of hygienic behavior in winter and in summer. In: Encontro sobre abelhas, 10. Ribeirão Preto-SP, Brasil. Anais…, p. 257. Available at: http://rge.fmrp.usp.br/encontro/documentos/10/10257.pdf. Accessed 28 Feb 2021. (Unpublished, Supplementary Material, rows 201-202)
Iwasaki JM, Barratt BI, Lord JM, Mercer AR, Dickinson KJ (2015) The New Zealand experience of varroa invasion highlights research opportunities for Australia. Ambio 44:694–704. https://doi.org/10.1007/s13280-015-0679-z
Kerr WE (1967) The history of the introduction of African bees to Brazil. South African Bee J 39(2):3–5
Koleoglu G, Goodwin PH, Reyes-Quintana M, Hamiduzzaman MM, Guzman-Novoa E (2018) Varroa destructor parasitism reduces hemocyte concentrations and prophenol oxidase gene expression in bees from two populations. Parasitol Res 117(4):1175–1183. https://doi.org/10.1007/s00436-018-5796-8
Kulhanek K, Steinhauer N, Wilkes J, Wilson M, Spivak M et al (2021) Survey-derived best management practices for backyard beekeepers improve colony health and reduce mortality. PLoS ONE 16(1):e0245490. https://doi.org/10.1371/journal.pone.0245490
Lopes ATC, Dantas LFCF, Fontes FM, Araújo-Neto ER, Morais LS, Cunha AFS, Gramacho KP (2016) Nosemosis and varroatosis of Africanized honey bees Apis mellifera in the Brazilian Northeast. In: Anais… Congresso Brasileiro de Apicultura, 21. Fortaleza - CE. (Unpublished, Supplementary Material, rows 262-268)
Maciel SVSA, Pires ASM, Pires GP, Oliveira IPP, Pinto LFB, Castagnino GLB (2018) Levantamento de enfermidades apícolas no apiário da UFBA de Entre Rios. In: Congresso Brasileiro de Zootecnia, 28 - Goiania - GO. Available at: https://docplayer.com.br/86183525. Accessed 10 Mar 2021. (Unpublished, Supplementary Material, rows 250-251)
Maggi M, Damiani N, Ruffinengo S, De Jong D, Principal J, Eguaras M (2010) Brood cell size of Apis mellifera modifies the reproductive behavior of Varroa destructor. Exp. Appl. Acarol. 50:269–279. https://doi.org/10.1007/s10493-009-9314-7
Maggi M, Antúnez K, Invernizzi C, Aldea P, Vargas M et al (2016) Honeybee health in South America. Apidologie 47:835–854. https://doi.org/10.1007/s13592-016-0445-7
Martin SJ, Hawkins GP, Brettell LE, Reece N, Correia-Oliveira ME, Allsopp MH (2020) Varroa destructor reproduction and cell re-capping in mite resistant Apis mellifera populations. Apidologie 51(3):369–381. https://doi.org/10.1007/s13592-019-00721-9
Mattos IM, Chaud-Neto J (2012) Analysis of mortality in Africanized honey bee colonies with high levels of infestation by Varroa destructor. Sociobiology 59(2):369–380. https://doi.org/10.13102/sociobiology.v59i2.601
Medina LM, Martin S (1999) A comparative study of Varroa jacobsoni reproduction in worker cells of honey bees (Apis mellifera) in England and Africanized bees in Yucatan. Mexico. Exp Appl Acarol 23:659–667. https://doi.org/10.1023/A:1006275525463
Medina-Flores CA, Guzmán-Novoa E, Hamiduzzaman M, Aréchiga-Flores CF, López-Carlos MA (2014) Africanized honey bees (Apis mellifera) have low infestation levels of the mite Varroa destructor in different ecological regions in Mexico. Genet Mol Res 13(3):7282–7293. https://doi.org/10.4238/2014.February.21.10
Mendes RB, Serra LS, Chagas-Sobrinho F, de Jesus JS, das Neves VSL, Leoni LN, Correia-Oliveira ME, de Carvalho CAL (2017) Incidence of the Varroa destructor mite in Apis mellifera in the Atlantic Forest area. In: Congresso Nordestino de Produção Animal, 12. Anais… UNIVASF, Petrolina, PE/Juazeiro, BA. p 50–52. Available at: https://docplayer.com.br/86183525. (Unpublished. Supplementary Material, row 294)
Mendoza Y, Tomasco IH, Antúnez K, Castelli L, Branchiccela B, Santos E, Invernizzi C (2020) Unraveling honey bee-Varroa destructor interaction: multiple factors involved in differential resistance between two Uruguayan populations. Vet Scie 7(3):116. https://doi.org/10.3390/vetsci7030116
Mercês CC, Correia-Oliveira ME, Mendes RB, Santos EB, Carvalho CAL (2017a) Ocorrência de varroatose em abelhas Apis mellifera em Santo Amaro, BA. In: Semana de atualização em Ciências Agrárias, Ambientais e Biológicas, 2. Anais… Cruz das Almas -BA. p.144. Available at: https://www2.ufrb.edu.br/iisecab/images/Anais_2_SECAB_2017 .pdf. Accessed 28 Feb 2020. (Unpublished. Supplementary Material, row 277)
Mercês CC, Correia-Oliveira ME, Mendes RB, Santos EB, Carvalho CAL (2017b) Occurrrence of ectoparasite on honey bee Apis mellifera in Velho Chico, BA. In: Simpósio de Agricultura Ecológica da Bahia. Anais… Cruz das Almas-BA. Dias, F.P.M (ed.), p 125–128. Available at: https://sea.ufr.edu.br/SEA/article/view/544/pdf. Accessed 28 Feb 2020. (Unpublished. Supplementary Material, row 284)
Moreira SBLC, Queiroz GS, de Castro HA, de Souza EA, Pereira DS, de Holanda-Neto JP (2017) Varroa destructor mite infestation in colonies of Africanized honey bees (Apis mellifera L.) in the potiguar semiarid region, Northeastern Brazil. Rev Ver Agroec Des Sust 12(1):143–149. https://doi.org/10.18378/rvads.v12i1.4845. (Supplementary Material, rows 238-239)
Moreira SBLC, Queiroz GS, Pereira DS, de Holanda-Neto JP (2015) Avaliação da taxa de infestação pelo ácaro Varroa destructor em abelhas operárias de Apis mellifera em um apiário do município de Marcelino Vieira, RN. In: Congresso Baiano de Apicultura e Meliponicultura, 7. Anais… Ilhéus, Bahia p. 29. Available at: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/126641/1/Congresso5.pdf. (Unpublished, Supplementary Material, row 242)
Moretto G, Gonçalves LS, De Jong D, Bichuette MZ (1991) The effects of climate and bee race on Varroa jacobsoni Oud infestations in Brazil. Apidologie 22(3):197–203. https://doi.org/10.1051/apido:19910303. (Supplementary Material, rows 8-10)
Moretto G, Gonçalves LS, Jong D, D. (1991) Africanized bees are more efficient at removing Varroa jacobsoni. Preliminary data. Am Bee J 131(7):434
Moretto G, Pillati A, De Jong D, Goncalves LS, Cassini FI (1995) Reduction of varroa infestations in the state of Santa Catarina, in southern Brazil. Am Bee J 135(7):498–500 (Supplementary Material. Rows 18-38)
Morse RA, Gonçalves LS (1979) Varroa disease, a threat to world beekeeping. Glean. Bee Cult. 107(4), 179-181. Available at: https://agris.fao.org/agris-search/search.do?recordID =US201301386532. Accessed 18 Mar 2020
Nascimento RJT (2014) Infestação de Varroa destructor e sua relação com comportamento higiênico em colônias de abelhas africanizadas no Campo das Vertentes-MG. Trabalho de Conclusão de Curso – TCC. Universidade Federal de São João del-Rey - UFSJ. Available at: https://www.ufsj.edu.br/portal2-repositorio/File/cozoo/TCC/2014-1/TCC_Roberto JuniorTexeiraNascimento.pdf. Accessed 25 Feb 2021. (Unpublished, Supplementary Material, rows 204-209)
Neves VSL, Santos EB, Mercês CC, Correia-Oliveira ME, Carvalho CAL (2017) Beekeeping challenges: Apis mellifera versus Varroa destructor. In: In: Simpósio de Agricultura Ecológica da Bahia. Anais… Cruz das Almas-BA. Dias, F.P.M (ed.), p 180–184. Available at: https://sea.ufr.edu.br/SEA/article/view/544/ pdf. Accessed 28 Feb 2020. (Unpublished. Supplementary Material, rows 285-293)
Nunes HTS, Gomes PTO (2016) Análise do comportamento higiênico e da infestação de ácaros Varroa destructor das colônias do apiário didático do IFRN - Campus Pau dos Ferros. Trabalho de Conclusão de Curso - IFRN. (Unpublished. Supplementary Material, row 279)
Oddie M, Büchler R, Dahle B, Kovacic M, Le Conte Y, Locke B, de Miranda JR, Mondet F, Neumann P (2018) Rapid parallel evolution overcomes global honey bee parasite. Sci Rep 8:7704. https://doi.org/10.1038/s41598-018-26001-7
Olinto FA (2014) Comportamento higiênico e identificação de patógenos em colmeias de Apis mellifera L. africanizadas no Sertão Paraibano. Sistemoteca - Biblioteca Digital da Universidade Federal de Campina Grande, Pombal, PB - Brasil. 62 p. Available at: http://dspace.sti.ufcg.edu.br:8080/jspui/handle/riufcg/676. Accessed 25 Mar 2020. (Unpublished, Supplementary Material, rows 233-237)
Oliveira-Filho AA, Castagnino GLB, Pinto LFB, Biasi C, Campos AAS (2017) Varroa destructor infestation rate in the off-season in the UFBA experimental apiary. In: Congresso Nordestino de Produção Animal, 12. Anais… UNIVASF, Petrolina, PE/ Juazeiro, BA. p 20–21. Available at: http://www.cnpa2017.univasf.edu.br/files/anais_ 2017.pdf. Accessed 18 Mar 2020. (Unpublished. Supplementary Material, row 295)
Pegoraro A, Marques EM, Neto AC, Costa EC (2000) Natural infestation of Apis mellifera scutellata by Varroa jacobsoni. Arch Vet Sci 5(1):89–93. https://doi.org/10.5380/avs.v5i1.3891. (Supplementary Material, row 39)
Peixoto CM, Mercês CC, Correia-Oliveira ME, Carvalho CAL (2017) Ocorrência de Varroa destructor em colônias de Apis mellifera em Viçosa - MG. In: Semana de atualização em Ciências Agrárias, Ambientais e Biológicas, 2. Anais… Cruz das Almas-BA. p. 109. Available at: https://www2.ufrb.edu.br/iisecab/images/Anais_2_SECAB_2017 .pdf. Accessed 28 Feb 2020. (Unpublished. Supplementary Material, rows 271-276)
Peixoto CM, Correia-Oliveira ME, Lima-Silva F, Ramos CECO, Carvalho CAL (2021) Varroa destructor in Apis mellifera colonies in Brazil. J. Apic. Res. https://doi.org/10.1080/00218839.2021.1960746 (Supplementary Material, rows 325-361)
Piccirillo GA, De Jong D (2004) Old honey bee brood combs are more infested by the mite Varroa destructor than are new brood combs. Apidologie 35(4):359–364. https://doi.org/10.1051/apido:2004022. (Supplementary Material, row 41)
Pinho VR, Souza DC, Silva SBA, Ribeiro LS, Samel-Rocha J (2018) Incidência do ácaro Varroa destructor associada a dietas em colônias de Apis mellifera. In: Congresso Brasileiro de Apicultura, 22, p 212. Anais… Available at: https://docplayer.com.br/86183525. (Unpublished. Supplementary Material, row 301)
Pinto FA, Puker A, Message D, Barreto LMRC (2011) Varroa destructor in Juquitiba, Vale do Ribeira, Southeastern Brazil: seasonal effects on the infestation rate of ectoparasitic mites in honey bees. Sociobiology 57(3):511–518. Available at: https://www.locus.ufv.br/bitstream/123456789/19823/1/artigo.pdf. (Supplementary Material, rows 62-63)
Pinto FA, Puker A, Message D, Barreto LMRC (2012) The ectoparasite mite Varroa destructor (Anderson and Trueman) in southeastern Brazil apiaries: effects of the hygienic behavior of Africanized honey bees on infestation rates. Arq Bras Med Vet Zootec 64(5):1194–1199. https://doi.org/10.1590/S0102-09352012000500017. (Supplementary Material, rows 107-108)
Pinto FA, Puker A, Message D, Barreto LMRC (2015) Infestation rate of the mite Varroa destructor in commercial apiaries of the Vale do Paraíba and Serra da Mantiqueira, southeastern Brazil. Arq Bras Med Vet Zoot 67(2):631–635. https://doi.org/10.1590/1678.7264. (Supplementary Material, rows 64-79)
Queiroz GS, Moreira SBLC, Paiva CS, Pereira DS, Holanda-Neto JP (2015) Avaliação do índice de infestação de ácaro Varroa destructor em apiário do município de Encanto, RN. In: Congresso Baiano de Apicultura e Meliponicultura, 7. Anais… p. 56. Ilhéus, Bahia. Available at: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/126648/1 /Congresso7.pdf. (Unpublished, Supplementary Material, row 241)
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Requier F, Antúnez K, Morales CA, Sánchez PA, Castilhos D et al (2018) Trends in beekeeping and honey bee colony losses in Latin America. J Apic Res 57(5):657–662. https://doi.org/10.1080/00218839.2018.1494919
Reyes-Quintana M, Espinosa-Montaño LG, Prieto-Merlos D, Koleoglu G, Petukhova T, Correa-Benítez A, Guzman-Novoa E (2019) Impact of Varroa destructor and deformed wing virus on emergence, cellular immunity, wing integrity and survivorship of Africanized honey bees in Mexico. J Inv Pathol 164:43–48. https://doi.org/10.1016/j.jip.2019.04.009
Roberts JMK, Anderson DL, Durr PA (2017) Absence of deformed wing virus and Varroa destructor in Australia provides unique perspectives on honey bee viral landscapes and colony losses. Sci Rep 7:6925. https://doi.org/10.1038/s41598-017-07290-w
Rosenkranz P (1999) Honey bee (Apis mellifera L.) tolerance to Varroa jacobsoni Oud. in South America. Apidologie 30(2–3):159–172. https://doi.org/10.1051/apido:19990206
Ruela TH, Silveira RD, Rodrigues GA, Soares DAM, Matioli AL (2014) Ocorrência do ácaro Varroa destructor em colmeias de abelhas Apis mellifera no município de Diamantina, MG. In: Congresso Brasileiro de Apicultura, 20. Anais… Belém, PA. p 206. Available at: http://www.semabelhasemalimento.com.br/wp-content/ uploads/2015/03/anais-conbrapi-2014.pdf. (Unpublished, Supplementary Material, rows 243-244)
Ruela TH (2016) Ocorrência do ácaro Varroa destructor em colmeias de abelhas Apis mellifera no município de Diamantina - MG. Trabalho de conclusão de curso de Zootecnia da Universidade Federal dos Vales do Jequitinhonha e Mucurí - UFVJM. (Unpublished, Supplementary Material, rows 252-259)
Sambrana IR, dos Reis VDA (2017a) Infestação pelo ácaro Varroa destructor em abelhas africanizadas adultas e pupas de 2016 a 2017 em Ladário-MS. Pesquisa para Empresa Brasileira de Pesquisas Agropecuárias. (Unpublished, Supplementary Material, rows 260-261)
Sambrana IR, dos Reis VDA (2017b) Infestação pelo ácaro Varroa destructor em abelhas africanizadas adultas e pupas de 2016 a 2017 em Ladário-MS. In: Congresso Brasileiro de Apicultura 22. p. 205-206. Anais… Available at: https://docplayer.com.br/86183525. (Unpublished, Supplementary Material, rows 296-297)
Santos LG, Alves MLTMF, Message D, Pinto FA, Silva MVGB, Teixeira EW (2014) Honey bee heath in apiaries in the Vale do Paraiba, São Paulo State, southeastern Brazil. Sociobiology 61(3):307–312. https://doi.org/10.13102/sociobiology.v6i3.307-312. (Supplementary Material, rows 81-106)
Schafaschek TP,Stefaniak LC, Bolzani R, Arioli CJ (2010) Infestação de ácaro Varroa destructor em colméias de Apis mellifera na Região do Alto Vale do Rio do Peixe no Estado de Santa Catarina, em Diferentes estações do ano. In: Anais... Congresso Brasileiro de Apicultura, 18. Cuiabá, MT. Available at: https://biblioteca.epagri.sc.gov.br/consulta/ SCHAFASCHEK.Atual=1. (Unpublished, Supplementary Material, rows 109-143)
Schafaschek TP, Arioli CJ, Bolzani R, Stefaniak LC, Piccoli C, Milani V, Frühauf M (2012) Monitoramento da infestação por ácaro Varroa destructor em colônias de Abelhas Apis mellifera Africanizadas no Estado de Santa Catarina. In: Anais… Congresso Brasileiro de Apicultura, 19. Gramado, RS. São Paulo: Mensagem Doce 116, 72. Available at: https://www.apacame.org.br/mensagemdoce/116/sanidade6.htm
Schafaschek TP, Arioli CJ, Dalbó MA, Milani V, Piccoli C, Brancher A, Majolo F (2013) Índice de infestação por Varroa destructor (Anderson & Trueman) (Mesostigmata: Varroidae) em colônias de Apis mellifera (Himenoptera: Apidae) no Estado de Santa Catarina. In: Anais…Congresso Internacional de Zootecnia, 23. Associação Brasileira de Zootecnia. Foz do Iguaçu, PR. CD-ROM. (Unpublished, Supplementary Material, rows 150-185)
Schafaschek TP, Hickel ER, de Oliveira CAL, de Toledo VAA (2019) Infestation and reproduction of Varroa destructor and hygienic behavior in colonies of Apis mellifera with queens for different genetic origins. Sociobiology 66(3):448–456. https://doi.org/10.13102/sociobiology.v66i3.3444. (Supplementary Material, rows 231-232)
Silva-Neto HB, Bendini JN, Santos e Silva FA, Souza DC (2011) Infestation levels of Varroa destructor in Africanized honey bees removed from metropolitan area, Teresina, PI, Brazil. In: Apimondia 42, Buenos Aires, Argentina. (Unpublished, Supplementary Material, row 186)
Silva-Neto HB, Souza DC (2011) Levantamento das Enfermidades Apícolas e do Índice de Infestação do Ácaro Varroa destructor em Abelhas Africanizadas na Região do Semiárido Piauiense. In: Encontro de Iniciação Científica da Universidade Federal do Piauí. Programa e Resumos. Teresina, PI, Brasil, CD-ROM, 2011. (Unpublished, Supplementary Material, rows 187-188)
Simone-Finstrom M, Li-Byarlay H, Huang M, Strand MK, Rueppell O, Tarpy DR (2016) Migratory management and environmental conditions affect lifespan and oxidative stress in honey bees. Sci Rep 6:32023. https://doi.org/10.1038/srep32023
Spivak M, Danka RG (2021) Perspectives on hygienic behavior in Apis mellifera and other social insects. Apidologie 52:1–16. https://doi.org/10.1007/s13592-020-00784-z
Stort AC, Gonçalves LS, Malaspina O, Moura-Duarte FA (1981) Study on sineacar effectiveness in controlling Varroa jacobsoni. Apidologie 12(3):289–297. https://doi.org/10.1007/s13592-020-00784-z
Teixeira EW, Chen Y, Message D, Pettis J, Evans JD (2008) Virus infections in Brazilian honey bees. J. Inv. Path. 99(1):117–119. https://doi.org/10.1016/j.jip.2008.03.014
Thoms CA, Nelson KC, Kubas A, Steinhauer N, Wilson ME, vanEngelsdorp D (2019) Beekeeper stewardship, colony loss, and Varroa destructor management. Ambio 48(10):1209–1218. https://doi.org/10.1007/s13280-018-1130-z
Torres RNS, Barreto MR (2013) Incidência de Varroa destructor (Anderson & Trueman) em criação de abelhas com ferrão na região de Sinop, Mato Grosso, Brasil. EntomoBrasilis 6(1):30–33. https://doi.org/10.12741/ebrasilis.v6i1.254. (Supplementary Material, rows 199-200)
Traynor KS, Mondet F, de Miranda JR, Techer M, Kowallik V, Oddie MAY, Chantawannakul P, McAfee A (2020) Varroa destructor: a complex parasite, crippling honey bees worldwide. Trends Parasitol. 36(7):592–606. https://doi.org/10.1016/j.pt.2020.04.004
Turcatto AP, Issa MRC, Morais MM, Almeida R (2012) Infestação pelo ácaro Varroa destructor em operárias adultas e em células de cria de abelhas africanizadas Apis mellifera na região de Franca, SP. EntomoBrasilis 5(3):198–203. https://doi.org/10.12741/ebrasilis.v5i3.195. (Supplementary Material, rows 48-51)
Vinhal-Silva N, Cunha SA, Souza PO, Vitena TMS, Rizzardo RAG (2018) Infestação de abelhas melíferas por varroa na Amazônia Legal. In: Congresso Brasileiro de Apicultura, 22, p 201. Anais… Available at: https://docplayer.com.br/86183525. (Unpublished. Supplementary Material, row 302-305)
Wielewski P, de Toledo VAA, Martins EN, Costa-Maia FM, Faquinello P, Lino-Lourenço DA, Ruvolo-Takasusuki MCC, de Oliveira CAL, Sereia MJ (2012) Relationship between hygienic behavior and Varroa destructor mites in colonies producing honey or royal jelly. Sociobiology 59(1):251–274. https://doi.org/10.13102/sociobiology.v59i1.682
Acknowledgements
Special thanks are due to Anderson Puker, Carlos Alfredo Lopes de Carvalho, David De Jong, Érica Weinstein Teixeira, John Kastelic, Maria Emilene Correia-Oliveira, Roger Beelen, and Tânia Patrícia Schafaschek for their continuous support of this research.
Author information
Authors and Affiliations
Contributions
DC collected the data and structured the variables. GCB performed the R statistical analyses. DDJ and JK carefully edited the text. Other authors uploaded the databases. All authors participated in writing and manuscript review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This is an observational study. The Ethics Commission of UFERSA/RN, Brazil, has confirmed that no ethical approval is required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Manuscript editor: Yves Le Conte
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 141 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Castilhos, D., Polesso, A.M., da Silva, A.C.F. et al. Varroa destructor infestation levels in Africanized honey bee colonies in Brazil from 1977 when first detected to 2020. Apidologie 54, 5 (2023). https://doi.org/10.1007/s13592-022-00984-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-022-00984-9