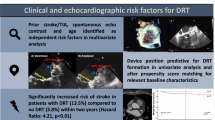
Abstract
Background
Device-related thrombus (DRT) after left atrial appendage closure (LAAC) is associated with adverse outcomes, i.e. ischemic stroke or systemic embolism (SE). Data on predictors of stroke/SE in the context of DRT are limited.
Aims
This study aimed to identify predisposing factors for stroke/SE in DRT patients. In addition, the temporal connection of stroke/SE to DRT diagnosis was analyzed.
Methods
The EUROC-DRT registry included 176 patients, in whom DRT after LAAC were diagnosed. Patients with symptomatic DRT, defined as stroke/SE in the context of DRT diagnosis, were compared against patients with non-symptomatic DRT. Baseline characteristics, anti-thrombotic regimens, device position, and timing of stroke/SE were compared.
Results
Stroke/SE occurred in 25/176 (14.2%) patients diagnosed with DRT (symptomatic DRT). Stroke/SE occurred after a median of 198 days (IQR 37–558) after LAAC. In 45.8% stroke/SE occurred within one month before/after DRT diagnosis (DRT-related stroke). Patients with symptomatic DRT had lower left ventricular ejection fractions (50.0 ± 9.1% vs. 54.2 ± 11.0%, p = 0.03) and higher rates of non-paroxysmal atrial fibrillation (84.0% vs. 64.9%, p = 0.06). Other baseline parameters and device positions were not different. Most ischemic events occurred among patients with single antiplatelet therapy (50%), however, stroke/SE was also observed under dual antiplatelet therapy (25%) or oral anticoagulation (20%).
Conclusion
Stroke/SE are documented in 14.2% and occur both in close temporal relation to the DRT finding and chronologically independently therefrom. Identification of risk factors remains cumbersome, putting all DRT patients at substantial risk for stroke/SE. Further studies are necessary to minimize the risk of DRT and ischemic events.
Graphical Abstract

Similar content being viewed by others
Background
Left atrial appendage closure (LAAC) is an established strategy for stroke prevention in patients with atrial fibrillation (AF) and contraindications against the standard treatment with oral anticoagulation (OAC) [1, 2]. Formation of device-related thrombus (DRT) has increasingly been considered as a relevant finding after LAAC and appears to be associated with impaired outcomes including increased rates of ischemic stroke and systemic embolism (SE) [3,4,5,6]. Previous studies found DRT to be related to multiple factors including patient and procedural characteristics (i.e. device position) as well as postprocedural antithrombotic regimen [4, 6,7,8]. Nonetheless, further data on DRT and its impact on ischemic events are warranted. In this matter it remains unclear whether DRT is directly causative for ischemic stroke or systemic embolism (SE) or rather a marker of increased thrombotic risk [7]. Also, little is known about the characteristics of stroke/SE in patients with DRT, such as the temporal correlation of the adverse event and the diagnosis of DRT as well as the LAAC procedure itself, respectively.
Therefore, this study sought to compare patients with symptomatic DRT, i.e. occurrence of stroke/SE in patients with DRT after LAAC, against patients with non-symptomatic DRT to assess stroke/SE risk in DRT patients.
Methods
Study population
The multicenter EUROC-DRT registry included a total of 176 patients, in whom DRT after LAAC was diagnosed during clinical follow-up (FU). Definition of DRT as used in this study has been described elsewhere [9]. In accordance with each participating center`s protocol, patients underwent regular clinical FUs after LAAC. In the case of DRT detection, patients were included in the registry. Informed consent was mandatory for all patients in each of the participating centers` registries, which were approved by the local ethics committees. All included patients received long-term clinical FU or telephone interviews to monitor the outcome. The group of patients with documented stroke/SE (including transient ischemic attack [TIA]) before or after the diagnosis of DRT was referred to as “symptomatic DRT” and compared with “non-symptomatic DRT”, meaning the group of patients with DRT but without stroke/SE. For additional analysis, patients with “symptomatic DRT” were further analyzed according to the temporal association of the thromboembolic event and the time of DRT diagnosis. Patients with stroke/SE occurring within a timeframe of one month before/after DRT diagnosis (as previously established [5]) were labeled “DRT-related stroke/SE” and compared with patients suffering a stroke/SE but beyond the given timeframe, labeled “incidental stroke/SE”. To assess risk factors for stroke/SE in patients with DRT, baseline characteristics, laboratory and echocardiographic parameters, postprocedural anticoagulation, device position and timing of stroke were compared between both groups and between DRT-related stroke/SE and all other patients.
Echocardiographic assessment
Risk factor analysis included echocardiographic parameters as well as device position after LAAC. For this matter, the assessment included baseline transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) with an evaluation of left ventricular ejection fraction, presence of spontaneous echocardiographic contrast (SEC) (°I-°III), left atrial and ventricular volumes. Post-procedurally, 2-dimensional TEE focused on device position applying a standardized protocol as previously prescribed [7]. Evaluation of device position included assessment for complete occlusion (i.e., residual peri-device flow < 3 mm), implantation depth (measured towards the mitral annulus and along the left upper pulmonary vein [LUPV] ridge). Ostial position was achieved if LUPV ridge length was < 10 mm. Implanted occluders such as the AMPLATZER ACP and Amulet (Abbott Laboratories, Chicago, IL, USA), LAmbre (Lifetech Scientific, Shenzhen, China) and Ultraseal (Cardia Inc, Eagan, MN, USA) devices, featuring a proximal disc covering the LAA ostium, were categorized as pacifier occluders. Non-pacifier occluders included the WATCHMAN (Boston Scientific Inc, Marlborough, MA, USA), Wavecrest (Biosense Webster Inc, Irvine, CA, USA) and Occlutech LAA occluder (Occlutech International AB; Helsingborg, Sweden).
Statistical analysis
Categorical variables are presented as frequencies with percentages included. χ2 analysis was performed for additional analysis. Continuous variables are presented as mean ± standard deviation. For comparison of central tendencies of two or more groups, Mann–Whitney U or Kruskal–Wallis analyses were performed, respectively. All statistical analyses were performed with SPSS software version 25.0.0.1 (IBM Corporation, Somers, NY). Statistical significance was assumed when the null hypothesis could be rejected at p < 0.05.
Results
Dynamics of symptomatic DRT
Out of the 176 included patients with DRT in the EUROC-DRT registry, stroke/SE occurred in 14.2% (25/176) patients. Hereby, the median maximum FU after LAAC was 682 (Interquartile range [IQR] 368–1175) days, 671 (IQR 355–1037) days in patients with symptomatic and 682 (IQR 366–1231) days in non-symptomatic DRT (p = 0.55). DRT were detected after a median of 93 (IQR 51–166) days after LAAC (Table 1). Exact dates of stroke/SE were available in 24/25 patients and occurred after a median of 198 (IQR 37–558) days after LAAC. In relation to DRT diagnosis, stroke/SE, therefore, occurred after a median of 27 (IQR − 7–464) days after DRT detection with 45.8% (11/24) of cases occurring within one month before/after DRT diagnosis was made (DRT-related stroke/SE) (Fig. 1). Data on anti-thrombotic regimen at the time of stroke/SE was available in 80.0% (20/25) of patients. Hereof, vitamin K antagonists (VKA) were administered in one patient (5.0%), direct oral anticoagulation (DOAC) in 4 patients (20.0%). Mainly, patients were on single antiplatelet therapy (SAPT) (n = 10, 50.0%) or dual antiplatelet therapy (DAPT) (n = 5, 25.0%). Of note, data on anti-thrombotic regimens at the time of DRT diagnosis and treatment strategies have been published elsewhere [7].
With regards to DRT, characteristics and its impact on stroke/SE, DRT were mainly located centrally on the occluder (47.3%) or along the LUPV ridge transition zone (41.9%) (Fig. 2). The remaining DRT were found on the occluder at the mitral valve side (10.9%). No difference was seen between symptomatic and non-symptomatic patients in regard to DRT position on the occluder (p = 0.64). DRT size measured vertically and horizontally were numerically larger in symptomatic DRT patients but missed significance (p = 0.22 and = 0.51, respectively).
Baseline characteristics
Patients with symptomatic DRT were younger (73.9 ± 8.2 vs. 76.4 ± 8.4 years, p = 0.15) and trended to be rather male (80.0% vs. 62.9%, p = 0.10) than patients with non-symptomatic DRT (Table 2). Non-paroxysmal AF was more common than paroxysmal AF in the overall group of patients suffering from DRT (67.6%). This finding was even more pronounced in symptomatic DRT, with non-paroxysmal AF being present in 84.0% (21/25) and paroxysmal AF in 16.0% (4/25) of patients in this group (p = 0.06). Additional characteristics potentially attributing to an increased stroke risk, such as arterial hypertension (96.0% vs. 83.4%, p = 0.10), diabetes mellitus (36.0% vs. 21.9%, p = 0.12) and previous stroke/TIA (64.0% vs. 47.0%, p = 0.12) were documented more often in patients with symptomatic DRT. Of note, established risk scores showed no difference between patients with symptomatic and non-symptomatic DRT.
In baseline echocardiography, patients with symptomatic DRT featured a significantly lower ejection fraction compared to patients with non-symptomatic DRT (50.0 ± 9.1% vs. 54.2 ± 11.0%, p = 0.03). Further echocardiographic assessment including atrial and ventricular volumes showed no significant differences.
Occluder type and position
In total, pacifier occluders were implanted in 63.1% (111/176) and non-pacifier occluders in 36.9% (65/176) of patients with DRT after LAAC. Symptomatic DRT were registered equally frequently with both occluder types, with 13.5% (15/111) in pacifier occluders and with 15.4% (10/65) in non-pacifier occluders (p = 0.73) (Fig. 3). Further information on implanted occluders are given in Supplemental Table I. Amplatzer (58.0%) and Watchman occluder (33.5%) were mainly implanted and were accountable for all documented DRT. Other occluders were only implanted in a few cases, therefore, no DRT were detected in these patients. The rate of complete occlusion of the LAA (defined as residual peridevice flow < 3 mm) was overall satisfying (85.6%) and did not differ between both groups either (p = 0.39). In the overall collective, an ostial coverage of the LAA ostium was achieved roughly in a third of patients, while in patients with symptomatic DRT this rate was numerically lower than in patients with non-symptomatic DRT without achieving statistical significance (28.6% vs. 36.0%, p = 0.59). In this matter, no difference was seen concerning the implantation depth along the LUPV (p = 0.40) or along the mitral side of the LAA (p = 0.71).
DRT-related stroke/SE vs. incidental stroke/SE
Symptomatic DRT were further distinguished into the temporally connected occurrence of stroke/SE (within one month before/after DRT diagnosis = DRT-related stroke) and into incidental stroke/SE (beyond one month before/after DRT diagnosis) (Supplemental Table II). Patients with temporally connected stroke/SE trended to feature an overall decreased risk for stroke compared to patients with incidental stroke/SE: Patients were numerically younger (72.3 ± 9.7 vs. 75.8 ± 6.6 years, p = 0.39), had lower incidences of diabetes mellitus (27.3% ± 46.2%, p = 0.34) and prior strokes (45.5% ± 76.9%, p = 0.11). In addition, established risk scores, such as the ATRIA and CHA2DS2-VASC-Score, were unanimously lower in patients with temporally connected stroke/SE. In non-pacifier occluders, DRT-related stroke/SE occurred numerically more often than incidental stroke/SE while in pacifier occluders, stroke/SE occurred more likely after a timespan of one month before/after DRT diagnosis (incidental). Additional analysis compared DRT-related stroke/SE with incidental stroke/SE and non-symptomatic DRT (Supplemental Table III), which found no significant differences.
Discussion
Device-related thrombosis has been increasingly recognized as a relevant complication after LAAC and is linked to an increased rate of adverse events such as stroke or systemic embolism. Although the mechanism and risk factors of DRT have been described before, the relevance of DRT and its implications on patients’ outcome as well as potential treatment regimen remain poorly understood. In this matter, it remains of interest to understand DRT dynamics and its behavior to become symptomatic, i.e. cause stroke/SE.
In this study, stroke or systemic embolism occurred in approximately 14% of patients, in whom DRT were documented at one point after LAAC. This result confirms previously published studies by Alkhouli et al. [10] and Simard et al. [6], which found increased rates of 13.2% and 16.9%, respectively. Similar rates were also observed in the initial PROTECT-AF study, which described stroke in patients with DRT in 15% of cases (3/20) [2]. These findings clearly exceed the rates of stroke in patients without DRT after LAAC, which were found to be 3.8% in our own EUROC-DRT registry [11] and 3.6% in the study by Simard et al. Given the incidence of DRT after LAAC, which ranges between 3 and 4% [2, 3, 5, 6], stroke/SE after DRT presents a numerically relevant finding. Notwithstanding, detection rates of DRT are likely underestimated, as imaging follow-ups are not routinely conducted in all patients and depend on each center’s individual protocol. As previously shown, late DRT occurs in a relevant portion of patients [7], however, imaging FUs are mainly conducted within the first months after LAAC. Therefore, the rate of DRT-associated stroke/SE could also be underestimated.
Out of all symptomatic DRT in this study, stroke/SE became apparent before DRT diagnosis in approximately 45%, hereof approximately 80% within ten days before DRT diagnosis. It is likely that the embolic event initiated further imaging diagnostics, which then detected DRT as a possible cause of stroke. A temporal relation (stroke/SE within one month before/after DRT diagnosis) was seen in 45% of cases, which supports the results by Dukkipati et al. [5]. Additionally, in 42% of cases, stroke/SE occurred within a time period of 90 days after LAAC (hereof most DRT were diagnosed shortly afterwards), which is considered to be prone to DRT formation, as endocardialization of the implanted occluder surface is still incomplete [12]. This temporal relation provides support to the thesis that DRT may be directly causative of DRT, as thrombogenic formation could potentially (partially) embolize and become symptomatic.
Interestingly, and in contrast to the just given argumentation, stroke/SE occurred independently from DRT diagnosis (> 6 months before/after DRT diagnosis) in about 40% of cases (10/24). These “time-staggered” cases of symptomatic DRT may support a fundamentally opposite understanding that DRT are not directly causative but rather present a “marker”, hinting at an overall increased thrombogenic state of the patient.
This study also aimed to evaluate how symptomatic DRT differ from non-symptomatic DRT. While established risk factors for stroke/SE, such as older age, the incidence of arterial hypertension, diabetes mellitus, non-paroxysmal AF and history of stroke/TIA trended to be increased in patients with symptomatic DRT, only baseline left ventricular ejection fraction (p = 0.03) appeared to be predictive in univariate analysis. Of interest, device position, which has been addressed and identified as a relevant predictor for DRT formation [6, 11], did not influence the incidence of thromboembolic events in these DRT patients. Furthermore, the position of the DRT on the occluder surface as well as its size had no predictive value in our analysis. In our study, stroke/SE occurred similarly often in the pacifier and non-pacifier occluders. However, higher rates of DRT have been described in the non-pacifier occluder Watchman [13] compared to pacifier occluders [14,15,16]. In line with the higher rate of DRT, the incidence of ischemic events has been described to be non-inferior in pacifier occluders compared to non-pacifier occluders [17]. This corroborates randomized comparisons, documenting a higher closure rate with pacifier concluders (not found in our data) as a possible reason [17, 18]. Concluding from these findings, based on patient and procedural characteristics, it appears difficult to predict, which DRT become symptomatic and which remain non-symptomatic. This however imposes an issue of uncertainty, as no adequate consensus on DRT management and standardized treatment regimen exists. Therefore, intensified echocardiographic follow-ups and initiation of medical treatment should be considered in all patients with proof of DRT. Therefore, we advise to conduct follow-up TEE in all patients after three and six months after LAAC during the phase of endothelialisation. Depending on the risk for DRT formation, further TEE follow-ups should be routinely conducted, as late DRT are also observed. In the case of DRT diagnosis, TEE follow-ups should be intensified until DRT resolution is achieved. However, to rule out the reformation of DRT, further TEE follow-ups and modification of therapy should be considered.
As previously shown [6, 7], re-initiation of intensified antithrombotic treatment results in satisfying rates of DRT resolution, therefore the risk of stroke/SE from DRT should be carefully weighed against the risk of bleeding or intracranial hemorrhage [19]. Given the broad spectrum of available treatment regimen physicians should be encouraged to attempt medical treatment for DRT resolution. In addition, the optimal preventive post-LAAC antithrombotic treatment remains to be defined. As most centers start DAPT for 3–6 months after LAAC and eventually switch to single antiplatelet therapy, novel approaches, such as low-dose-DOAC may prove to be a feasible option. In the randomized ADRIFT study, low-dose rivaroxaban was superior to dual antiplatelet therapy to control thrombin generation while few DRT were observed only in the DAPT group [20]. Also, Cepas-Guillen et al. were able to demonstrate a superior outcome of long-term-low-dose Apixaban (2.5 mg b.i.d) treatment with reduced risk of bleeding and a combined endpoint of stroke/SE/DRT in comparison to SAPT and DAPT [21]
In summary, derived from the findings above and complementing studies, stroke or systemic embolism is a common complication in patients with a 3–fivefold increased risk in comparison to patients without DRT after LAAC. Timing of stroke/SE suggests a potential link to the formation of DRT, as stroke/SE trend to occur during the initial phase of occluder endocardialization and trend to feature a temporal relation to DRT diagnosis. As no risk factors for DRT becoming symptomatic can be derived from the results above, further randomized, prospective studies are warranted. Until then, as no standardized clinical implications on DRT management exist, the diagnosis of DRT should always demand attention and the evaluation of medical treatment, to prevent thromboembolic events.
Limitations
The major limitation of this study is its retrospective character. All included patients with DRT were collected by the individual centers, which all followed the individual screening and follow-up protocols. Clinical data, echocardiographic FU and information on outcomes were not available in all patients. Of note, whether DRT were present in patients during the time of stroke/SE were not available in all patients, which could lead to a misinterpretation of the provided results. Also, data on antithrombotic medication and change to medical therapy after discharge, during stroke/SE are not documented in all patients. Assessment of device position was not conducted by a single core lab and therefore could be influenced by subjective data assessment. In addition, the clinical outcome of stroke/SE in DRT patients is unknown, although this information is crucial for understanding the clinical importance and impact of DRT-related stroke/SE.
Abbreviations
- AF:
-
Atrial fibrillation
- DOAC:
-
Direct oral anticoagulation
- DRT:
-
Device-related thrombosis
- DAPT:
-
Dual antiplatelet therapy
- FU:
-
Follow-up
- IQR:
-
Interquartile range
- LA:
-
Left atrium
- LAA:
-
Left atrial appendage
- LAAC:
-
Left atrial appendage closure
- LUPV:
-
Left upper pulmonary vein
- LV:
-
Left ventricle
- OAC:
-
Oral anticoagulation
- SAPT:
-
Single antiplatelet therapy
- SE:
-
Systemic embolism
- SEC:
-
Spontaneous echocardiographic contrast
- TIA:
-
Transient ischemic attack
- TTE:
-
Transthoracic echocardiography
- TEE:
-
Transesophageal echocardiography
- VKA:
-
Vitamin K antagonist
References
Tzikas A, Shakir S, Gafoor S, Omran H, Berti S, Santoro G, Kefer J, Landmesser U, Nielsen-Kudsk JE, Cruz-Gonzalez I, Sievert H, Tichelbacker T, Kanagaratnam P, Nietlispach F, Aminian A, Kasch F, Freixa X, Danna P, Rezzaghi M, Vermeersch P, Stock F, Stolcova M, Costa MA, Ibrahim R, Schillinger W, Meier B, Park J-W (2016) Left atrial appendage occlusion for stroke prevention in atrial fibrillation: Multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention 11:1170–1179
Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S (2011) Safety of percutaneous left atrial appendage closure: results from the watchman left atrial appendage system for embolic protection in patients with AF (PROTECT AF) clinical trial and the continued access registry. Circulation 123:417–424
Fauchier L, Cinaud A, Brigadeau F, Lepillier A, Pierre B, Abbey S, Fatemi M, Franceschi F, Guedeney P, Jacon P, Paziaud O, Venier S, Deharo JC, Gras D, Klug D, Mansourati J, Montalescot G, Piot O, Defaye P (2018) Device-related thrombosis after percutaneous left atrial appendage occlusion for atrial fibrillation. J Am Coll Cardiol 71:1528–1536
Sedaghat A, Nickenig G, Schrickel JW, Ince H, Schmidt B, Protopopov AV, Betts TR, Gori T, Sievert H, Mazzone P, Grygier M, Wald C, Vireca E, Allocco D, Boersma LVA (2021) Incidence, predictors and outcomes of device-related thrombus after left atrial appendage closure with the WATCHMAN device-Insights from the EWOLUTION real world registry. Catheter Cardiovasc Interv. https://doi.org/10.1002/ccd.29458
Dukkipati SR, Kar S, Holmes DR, Doshi SK, Swarup V, Gibson DN, Maini B, Gordon NT, Main ML, Reddy VY (2018) Device-related thrombus after left atrial appendage closure: incidence, predictors, and outcomes. Circulation 138:874–885
Simard T, Jung RG, Lehenbauer K, Piayda K, Pracoń R, Jackson GG, Flores-Umanzor E, Faroux L, Korsholm K, Chun JKR, Chen S, Maarse M, Montrella K, Chaker Z, Spoon JN, Pastormerlo LE, Meincke F, Sawant AC, Moldovan CM, Qintar M, Aktas MK, Branca L, Radinovic A, Ram P, El-Zein RS, Flautt T, Ding WY, Sayegh B, Benito-González T, Lee O-H, Badejoko SO, Paitazoglou C, Karim N, Zaghloul AM, Agrawal H, Kaplan RM, Alli O, Ahmed A, Suradi HS, Knight BP, Alla VM, Panaich SS, Wong T, Bergmann MW, Chothia R, Kim J-S, de Pérez PA, Bazaz R, Gupta D, Valderrabano M, Sanchez CE, El Chami MF, Mazzone P, Adamo M, Ling F, Wang DD, O’Neill W, Wojakowski W, Pershad A, Berti S, Spoon D, Kawsara A, Jabbour G, Boersma LVA, Schmidt B, Nielsen-Kudsk JE, Rodés-Cabau J, Freixa X, Ellis CR, Fauchier L, Demkow M, Sievert H, Main ML, Hibbert B, Holmes DR, Alkhouli M (2021) Predictors of device-related thrombus following percutaneous left atrial appendage occlusion. J Am Coll Cardiol 78:297–313
Sedaghat A, Vij V, Al-Kassou B, Gloekler S, Galea R, Fürholz M, Meier B, Valgimigli M, O’Hara G, Arzamendi D, Agudelo V, Asmarats L, Freixa X, Flores-Umanzor E, De Backer O, Søndergaard L, Nombela-Franco L, McInerney A, Korsholm K, Nielsen-Kudsk JE, Afzal S, Zeus T, Operhalski F, Schmidt B, Montalescot G, Guedeney P, Iriart X, Miton N, Saw J, Gilhofer T, Fauchier L, Veliqi E, Meincke F, Petri N, Nordbeck P, Rycerz S, Ognerubov D, Merkulov E, Cruz-González I, Gonzalez-Ferreiro R, Bhatt DL, Laricchia A, Mangieri A, Omran H, Schrickel JW, Rodes-Cabau J, Nickenig G (2021) Device-related thrombus after left atrial appendage closure: data on thrombus characteristics, treatment strategies, and clinical outcomes from the EUROC-DRT-registry. Circ Cardiovasc Interv. https://doi.org/10.1161/CIRCINTERVENTIONS.120.010195
Pracon R, Bangalore S, Dzielinska Z, Konka M, Kepka C, Kruk M, Kaczmarska-Dyrda E, Petryka-Mazurkiewicz J, Bujak S, Solecki M, Pskit A, Dabrowska A, Sieradzki B, Plonski A, Ruzyllo W, Witkowski A, Demkow M (2018) Device thrombosis after percutaneous left atrial appendage occlusion is related to patient and procedural characteristics but not to duration of postimplantation dual antiplatelet therapy. Circ Cardiovasc Interv 11:1–7
Aminian A, Schmidt B, Mazzone P, Berti S, Fischer S, Montorfano M, Lam SCC, Lund J, Asch FM, Gage R, Cruz-Gonzalez I, Omran H, Tarantini G, Nielsen-Kudsk JE (2019) Incidence, characterization, and clinical impact of device-related thrombus following left atrial appendage occlusion in the prospective global AMPLATZER amulet observational study. JACC Cardiovasc Interv 12:1003–1014
Alkhouli M, Busu T, Shah K, Osman M, Alqahtani F, Raybuck B (2018) Incidence and clinical impact of device-related thrombus following percutaneous left atrial appendage occlusion: a meta-analysis. JACC Clin Electrophysiol 4:1629–1637
Vij V, Piayda K, Nelles D, Gloekler S, Galea R, Fürholz M, Meier B, Valgimigli M, O’Hara G, Arzamendi D, Agudelo V, Asmarats L, Freixa X, Flores-Umanzor E, De Backer O, Sondergaard L, Nombela-Franco L, McInerney A, Korsholm K, Nielsen-Kudsk JE, Afzal S, Zeus T, Operhalski F, Schmidt B, Montalescot G, Guedeney P, Iriart X, Miton N, Saw J, Gilhofer T, Fauchier L, Veliqi E, Meincke F, Petri N, Nordbeck P, Ognerubov D, Merkulov E, Cruz-González I, Gonzalez-Ferreiro R, Bhatt DL, Laricchia A, Mangieri A, Omran H, Schrickel JW, Rodes-Cabau J, Sievert H, Nickenig G, Sedaghat A (2022) Clinical and echocardiographic risk factors for device-related thrombus after left atrial appendage closure: an analysis from the multicenter EUROC-DRT registry. Clin Res Cardiol. https://doi.org/10.1007/s00392-022-02065-4
Schwartz RS, Holmes DR, Van Tassel RA, Hauser R, Henry TD, Mooney M, Matthews R, Doshi S, Jones RM, Virmani R (2010) Left atrial appendage obliteration: Mechanisms of healing and intracardiac integration. JACC Cardiovasc Interv 3:870–877
Yoo DH, Gibson DN, Funk J, Pangborn K, Janczyk G, Price MJ (2021) Treatment and outcomes of device-related thrombus after transcatheter left atrial appendage closure. Circ Cardiovasc Interv 14:e010889
Rashid HN, Layland J (2021) Association between device-related thrombus and the neo-appendage with left-atrial appendage occlusion devices. Eur Heart J 42:1047–1048
Hildick-Smith D, Landmesser U, Camm AJ, Diener H-C, Paul V, Schmidt B, Settergren M, Teiger E, Nielsen-Kudsk JE, Tondo C (2020) Left atrial appendage occlusion with the Amplatzer™ Amulet™ device: full results of the prospective global observational study. Eur Heart J 41:2894–2901
Ali M, Rigopoulos AG, Mammadov M, Torky A, Auer A, Matiakis M, Abate E, Bakogiannis C, Tzikas S, Bigalke B, Sedding D, Noutsias M (2020) Systematic review on left atrial appendage closure with the LAmbre device in patients with non-valvular atrial fibrillation. BMC Cardiovasc Disord 20:1–13
Lakkireddy D, Thaler D, Ellis CR, Swarup V, Sondergaard L, Carroll J, Gold MR, Hermiller J, Diener HC, Schmidt B, MacDonald L, Mansour M, Maini B, O’Brien L, Windecker S (2021) Amplatzer amulet left atrial appendage occluder versus watchman device for stroke prophylaxis (Amulet IDE): a randomized. Controlled Trial Circulation 144:1543–1552
Galea R, De Marco F, Meneveau N, Aminian A, Anselme F, Gräni C, Huber AT, Teiger E, Iriart X, BabongoBosombo F, Heg D, Franzone A, Vranckx P, Fischer U, Pedrazzini G, Bedogni F, Räber L, Valgimigli M (2022) Amulet or watchman device for percutaneous left atrial appendage closure: primary results of the SWISS-APERO randomized clinical trial. Circulation 145:724–738
Garot P, Cormier B, Horvilleur J (2019) Device-related thrombus after left atrial appendage closure. Interv Cardiol Rev 14:42–44
Duthoit G, Silvain J, Marijon E, Ducrocq G, Lepillier A, Frere C, Dimby SF, Popovic B, Lellouche N, Martin-Toutain I, Spaulding C, Brochet E, Attias D, Mansourati J, Lorgis L, Klug D, Zannad N, Hauguel-Moreau M, Braik N, Deltour S, Ceccaldi A, Wang H, Hammoudi N, Brugier D, Vicaut E, Juliard JM, Montalescot G (2020) Reduced rivaroxaban dose versus dual antiplatelet therapy after left atrial appendage closure: ADRIFT a randomized pilot study. Circ Cardiovasc Interv 13:e008481
Cepas-Guillen PL, Flores-Umanzor E, Regueiro A, Brugaletta S, Ibañez C, Sanchis L, Sitges M, Rodés-Cabau J, Sabaté M, Freixa X (2021) Low dose of direct oral anticoagulants after left atrial appendage occlusion. J Cardiovasc Dev Dis. 8:142
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Alexander Sedaghat has received travel grants from Abbott and Boston Scientific and is a proctor for Lifetech. Lars Sondergaard has received consultant fees and institutional research grants from Abbott and Boston Scientific, and is a shareholder in Eclipse Medical. Dr Cruz-Gonzalez is a proctor for Abbott, Boston Scientific and Lifetech and was funded by ISCIII (PI19/00658) and co-funded by ERDF, "A way to make Europe". Jens Erik Nielsen-Kudsk is a proctor and consultant for Abbott and Boston Scientific. Dabit Arzamendi is a proctor for Abbott and Boston Scientific. Xavier Freixa is a proctor for Abbott, Boston Scientific and Lifetech. Antonio Mangieri is part of the advisory board of Boston Scientific and received an institutional grant from Boston Scientific. Dr. Nombela-Franco has served as a proctor of Abbott Vascular and received speaker honoraria from Boston Scientific and Abbott Vascular. Dr. Meier has received consultant fees from Abbott. Xavier Iriart is a proctor for Abbott and Boston Scientific. Dr. Bhatt discloses the following relationships—Advisory Board: Angiowave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; Board of Directors: Angiowave (stock options), Boston VA Research Institute, Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock), Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Consultant: Broadview Ventures, Hims; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial); Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women's Hospital who assigned to Lexicon; neither I nor Brigham and Women's Hospital receive any income from this patent); Research Funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, Cleerly, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, 89Bio; Royalties: Elsevier (Editor, Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Takeda. Georg Nickenig has received honoraria for lectures or advisory boards from Abbott, AstraZeneca, Bayer, Berlin Chemie, Biosensus, Biotronic, BMS, Boehringer Ingelheim, Cardiovalve, Daiichi Sankyo, Edwards, Medtronic, Novartis, Pfizer, Sanofi Aventis and is a shareholder of Beren, and Cardiovalve. Georg Nickenig has also participated in clinical trials for Abbott, AstraZeneca, Bayer, Berlin Chemie, Biosensus, Biotronic, BMS, Boehringer Ingelheim, Cardiovalve, Daiichi Sankyo, Edwards, Medtronic, Novartis, Pfizer, Sanofi Aventis. Georg Nickenig has received research fundings from DFG, BMBF, EU, Abbott, Bayer, BMS, Boehringer Ingelheim, Edwards, Medtronic, Novartis, Pfizer. DOI G. Montalescot: research funds for the Institution or fees from Abbott, Amgen, AstraZeneca, Axis, Bayer, BMS, Boehringer-Ingelheim, Boston-Scientific, Cell Prothera, CSL Behring, Idorsia, Leo-Pharma, Lilly, Medtronic, Novartis, Pfizer, Quantum Genomics, Sanofi, Terumo.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vij, V., Cruz-González, I., Galea, R. et al. Symptomatic vs. non-symptomatic device-related thrombus after LAAC: a sub-analysis from the multicenter EUROC-DRT registry. Clin Res Cardiol 112, 1790–1799 (2023). https://doi.org/10.1007/s00392-023-02237-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-023-02237-w