OPEN-ACCESS PEER-REVIEWED
RESEARCH ARTICLE
Eline Jurgens1, Edward J. Knaven1, Eva C.A. Hegeman1, Marjolein W.M. van Gemert1, Judith M.A. Emmen2, Ingrid Willemen2, Yvonne Mulder4, Linda IJsselstijn4, Ben M. de Rooij1,*, Theo H.M. Noij1,3
1Research group Analysis Techniques in Life Sciences, Avans University of Applied Sciences, Breda, the Netherlands. 2Amphia Hospital, Breda, the Netherlands. 3Charles River, ’s-Hertogenbosch, the Netherlands. 4Maasstad Hospital, Rotterdam, the Netherlands.
Journal of Applied Bioanalysis. Vol.5. No.2. pages 34-45 (2019)
Published 15 April 2019. https://doi.org/10.17145/jab.19.006 | (ISSN 2405-710X)
Download Supplemental Information
- Abstract
- Keywords
- Introduction
- Materials and methods
- Results and discussion
- Method development
- Method validation
- Figures and Tables
- 1,2-[2H2]-cortisol internal standard
- Saliva samples
- Estimation of the appropriate calibration range
- Validation of the analytical method
- Calibration function
- QC samples and acceptance of the run
- Recovery, within-run repeatability and within-laboratory precision
- Limit of detection and limit of quantification
- Analyte stability in saliva
- Matrix effects
- Conclusions
- Acknowledgment
- References
*Correspondence:
de Rooij BM. . Research Group Analysis Techniques in the Life Sciences, Avans University of Applied Sciences, Lovensdijkstraat 61-63, 4818 AJ, Breda, the Netherlands. Phone: +31 6 10231886.
Citation:
Jurgens E, Knaven EJ, Hegeman EC, van Gemert MW, Emmen JM, Willemen I, Mulder Y, IJsselstijn L, de Rooij BM*, Noij TH. Quantitative Profiling of Seven Steroids in Saliva using LC-MS/MS. J Appl Bioanal 5(2), 34-45 (2019).
Editor: Dr. Irene Panderi, National and Kapodistrian University of Athens, Athens, Greece.
Open-access and Copyright:
©2019 Jurgens E et al. This article is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Funding/Manuscript writing assistance:
The authors have financial support or funding to report and they declare that no writing assistance was utilized in the production of this article.
Competing interest:
The authors have declared that no competing interest exist.
Article history:
Received 25 November 2018, Revised 07 February 2019, Accepted 13 February 2019.
Abstract
Objectives
For the diagnosis and treatment of disorders like congenital adrenal hyperplasia, the determination of steroids in saliva might form a more patient-friendly method. Therefore a selective and sensitive LC-MS/MS method was developed for androstenedione, cortisol, cortisone, 11-deoxycortisol, 21-deoxycortisol, 17α-hydroxyprogesterone, and testosterone in saliva.
Methods
Saliva was extracted and concentrated. Steroids were determined by LC-MS/MS, using multiple reaction monitoring upon electrospray ionization.
Results
A method was developed to determine seven steroids in saliva in one single LC-MS/MS run. Limits of quantification were 0.05 ng/mL for androstenedione, 0.25 ng/mL for cortisol, 0.50 ng/mL for cortisone, 0.125 ng/mL for 11-deoxycortisol, 0.125 ng/mL for 21-deoxycortisol, 0.125 ng/mL for 17α-hydroxyprogesterone, and 0.05 ng/mL for testosterone. The within-run repeatability was < 5% and the within-laboratory precision was < 5% for all analytes except androstenedione, where the saliva used in the validation study contained androstenedione levels below the LOQ.
Conclusions
A sensitive and selective method was developed for the determination of a profile of seven steroids in saliva in one analytical run
Keywords
Steroids, profile, LC-MS/MS, congenital adrenal hyperplasia, saliva.
Introduction
A steroid profile can be used to discriminate between healthy individuals and individuals with an adrenal disorder, such as Cushing’s syndrome, Addison’s disease or congenital adrenal hyperplasia [1]. Congenital adrenal hyperplasia (CAH) is a group of autosomal recessive disorders in which the adrenal cortisol biosynthesis from cholesterol is impaired [2]. The most prevalent enzyme deficiency in CAH is 21-hydroxylase (>90%), followed by 11β-hydroxylase (5%), with the severity of the clinical presentation depending on the degree of the deficiency [3]. In many countries, CAH is included in the newborn screening program [4]. The Dutch newborn screening program results in the detection of CAH in 15 to 20 newborns every year [5].
Hormone replacement therapy aims to replace the missing glucocorticoids and mineralocorticoids [6]. Clinical diagnosis and monitoring of therapy are usually performed by repeated blood sampling. Analytical methods exist for the analysis of CAH relevant steroids in dried blood spots, plasma or serum [7-11]. Steroid panels consisting of 3 [7] up to 16 steroids [9] are described using liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). Usually, reversed-phase chromatography is applied using water/methanol [7-9] or water/acetonitrile [10,11] gradients. Ions are formed by Atmospheric Pressure Chemical Ionization (APCI) [7-9] or Electrospray Ionization (ESI) [10,11] and usually determined as positive ions, except aldosterone [9] and occasionally cortisol and cortisone [10].
In this study, saliva was chosen as a potential alternative matrix. Saliva can be collected by non-invasive means, which is more patient-friendly and offers the possibility of repeated sampling in cases where several venipunctures are impossible or undesirable, especially in newborns [6]. Moreover, salivary steroid hormone concentrations are thought to reflect serum free steroid concentrations, which are the biologically active forms. These advantages of saliva may make it feasible to better tailor cortisol replacement therapy in CAH patients. Saliva can be collected by passive drooling [3], direct spitting [6,12], or by using specific collection devices with swabs. The swabs are approved only for a limited number of steroids [3,13], therefore the direct spitting method was selected in this study.
Since most steroids are present in lower concentrations in saliva compared to blood [14 -17], there is a need for sensitive analytical methodology. LC-MS/MS methods exist concerning the quantification of steroids in saliva, however, some of these are focused on one or two steroids only, or they include steroids which are not related to CAH [6,12,14,15,18]. A radioactive immunoassay was used to investigate androstenedione and 17α-hydroxyprogesterone in saliva of CAH patients [6], while others apply derivatization with 2-hydrazino-1-methylpyridine prior to determination of steroids in saliva by LC-MS/MS (12). Different sets of steroids were determined in saliva using liquid chromatography – mass spectrometry (LC-MS) and applied for various groups of patients [12,14,15,18]. Sample pretreatment is usually performed by Solid Phase Extraction [12,14,18] or Liquid Liquid Extraction [15]. A set of 5 steroids was detected in relation to CAH in relatively large volume saliva samples (10 mL) by the application of Selective Ion Monitoring (SIM) of the most abundant ions [18]. In this research Electrospray Ionization combined with tandem mass spectrometry is applied in saliva for the determination of a panel of seven steroids which are useful for clinical diagnosis and monitoring of CAH therapy [2,3].
The aim of this study was to develop and validate a sensitive and specific LC-MS/MS method for the quantification of seven steroids in a single run; androstenedione, cortisol, cortisone, 11-deoxycortisol, 21-deoxycortisol, 17α-hydroxyprogesterone, and testosterone in saliva. Validation parameters were based on the Clinical and Laboratory Standards Institute EP15-A2 protocol and extended due to recommendations in multiple reviews [19-22]. Parameters included limits of quantification and detection (LOQ and LOD), precision, reproducibility, recovery, specificity, stability at different temperatures and matrix effects.
Materials and methods
Chemicals and materials
Reference compounds and internal standards androstenedione, 2,3,4-[13C3]-androstenedione , cortisol, cortisone, 2,2,4,6,6,12,12-[2H7]-cortisone, 11-deoxycortisol (1 mg/mL in methanol), 2,2,4,6,6-[2H5]-11-deoxycortisol, 21-deoxycortisol (100 µg/mL in methanol), 2,2,4,6,6,21,21,21-[2H8]-21-deoxycortisol, 17α-hydroxyprogesterone (17α-OHP), 2,3,4-[13C3]-17α-hydroxyprogesterone, testosterone, and 2,3,4-[13C3]-testosterone were purchased from Sigma-Aldrich (Zwijndrecht, the Netherlands). 1,2-[2H2]-cortisol was purchased from Buchem (Apeldoorn, the Netherlands).
Purity for all stable isotope labelled internal standards was >98%. Acetonitrile (ACN) and methanol (both UPLC-MS grade) were purchased from Boom (Meppel, the Netherlands), formic acid LC-MS grade was purchased from Sigma-Aldrich (Zwijndrecht, the Netherlands), and ethyl acetate HPLC grade was purchased from Macron ChromAR (Deventer, the Netherlands). Water (Milli-Q) was produced using a Millipore Advantage A10 station.
Sample preparation
Saliva samples of varying volumes were centrifuged for 15 minutes at 3000 rpm in a Heraeus Sepatech Megafuge 1.0, after which 500 µL of the supernatant was transferred to another tube and frozen at -20°C, if not used immediately. After thawing, samples were mixed, followed by addition of 10 µL of an internal standard (IS) mixture containing all seven IS at a concentration of 100 ng/mL in 80:20 water:ACN. Saliva was spiked with 11-deoxycortisol, 21-deoxycortisol, 17α-hydroxyprogesterone or testosterone whenever endogenous concentrations were below the LOQ. Liquid-liquid extraction was performed using 5 mL of ethyl acetate for each 500 µL saliva aliquot. The samples were centrifuged shortly for phase separation and the organic layer was transferred and evaporated at 30 °C (pressurized air), followed by reconstitution in 500 µL 80:20 water:ACN.
Instrumental analysis
A 1200 series HPLC from Agilent Technologies (Santa Clara, USA) was used. Separation was performed on an ACE UltraCore SuperC18 column (L=100 mm, I.D. = 2.1 mm, particle size 2.5 µm, pore size of 25 Å, Advanced Chromatography Technologies Ltd., Aberdeen, Scotland).
A flow rate of 0.25 mL/min was applied to the column using a gradient of phase A (0.1 % formic acid, FA) and phase B (0.1 % FA in acetonitrile). The gradient was initiated with 20% B increasing to 40% B over 5.3 min. which was retained up to 12 minutes, followed by a direct change to 20% B at 12.01 min. and equilibration (Total run time 16.5 min.). Column temperature was maintained at 30 °C. The injection volume was 50 µL.
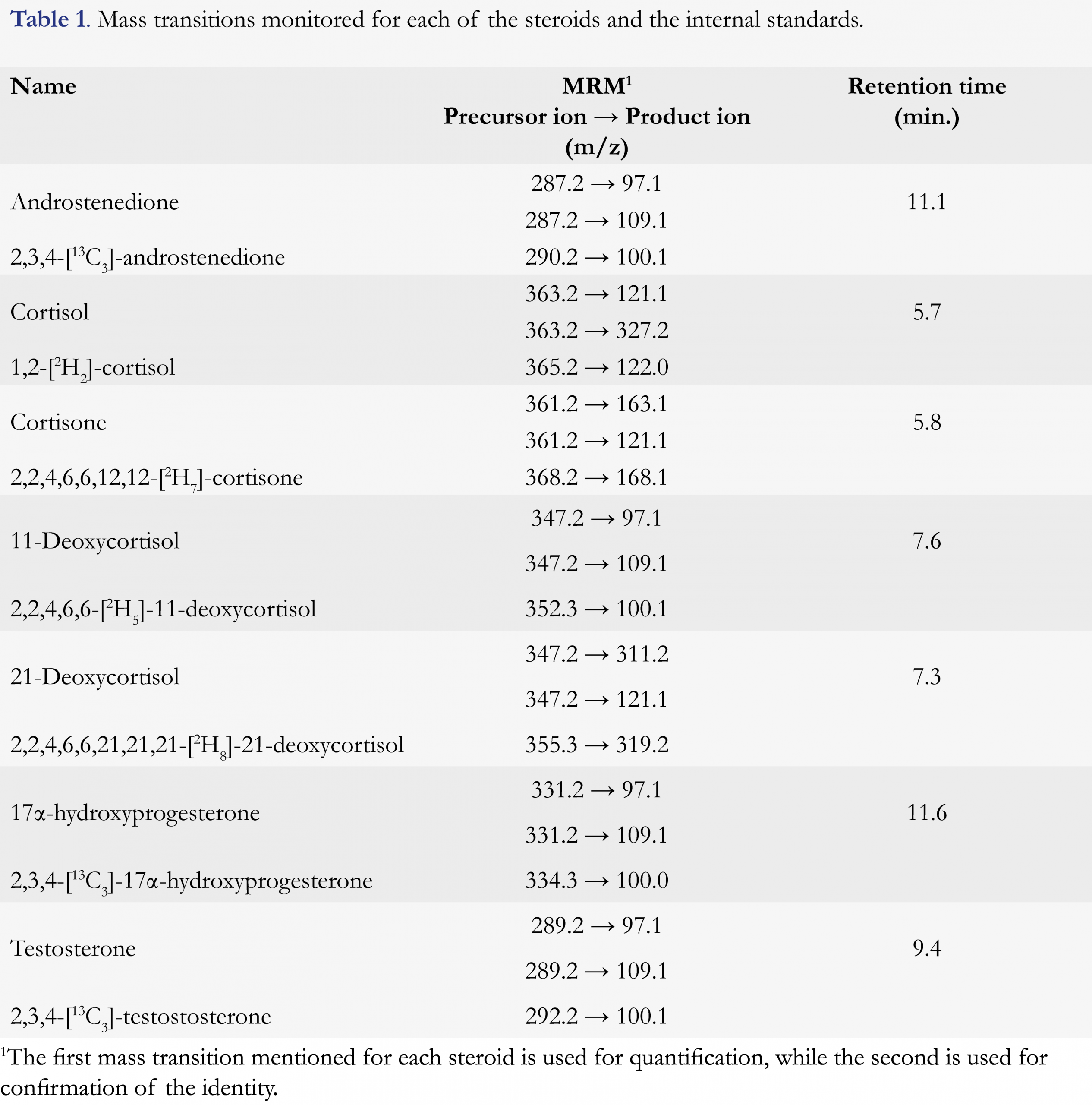
The mass spectrometer was a 6460 triple quadrupole mass spectrometer (Agilent Technologies), equipped with an electrospray Ionization source (ESI, Agilent Jet Stream Technology), operated in positive mode. The main source settings were: electron multiplier voltage 400 V, capillary voltage 3500 V, nozzle voltage 0 V, gas temperature 350 °C, gas flow 5 L/min, nebulizer pressure 60 psi, sheath gas heater 350 °C and sheath gas flow 11 L/min. Analytes were detected in dynamic multiple reaction monitoring (dMRM) with a cycle time of 1000 ms. Mass transitions were optimized by Agilent’s Optimizer software and the optimum mass transitions for steroids and the respective internal standards are presented in Table 1.
Data were processed using Masshunter QQQ Quantitative Analysis for LC-MS version B.07.00, build 7.0.457.0 (2008, Agilent). Calibration graphs were prepared by linear least squares regression with a weighing factor of 1/x. Microsoft Excel 2013 was used for additional data evaluation.
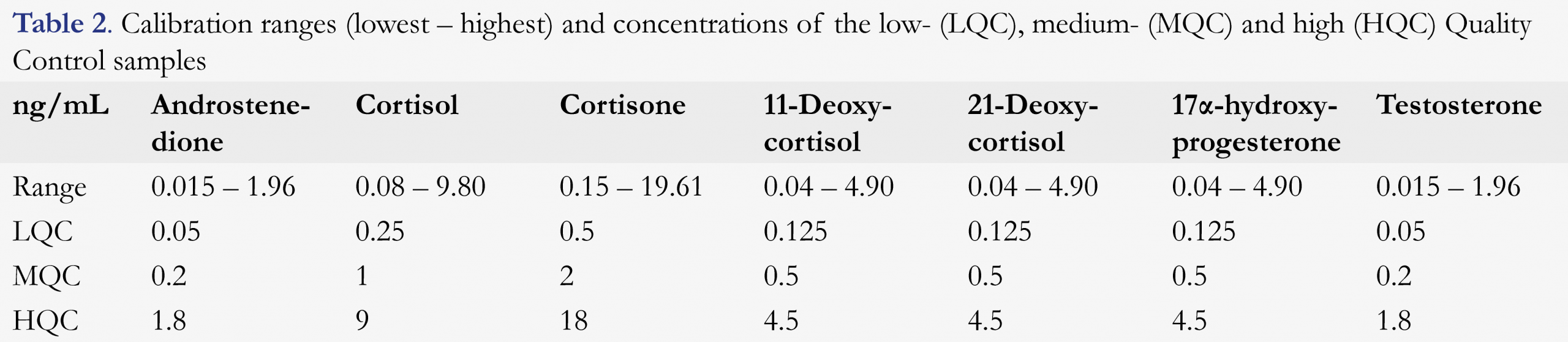
Calibrators and quality control samples
Calibrators were prepared by serial dilution in 80:20 water:ACN. The concentration ranges are given in Table 2. Aliquots (500 µL) were transferred to 10 mL polypropylene tubes and stored at -20 °C. Quality controls (QCs) consisted of 80:20 water:ACN containing all seven steroids at low (LQC), medium (MQC) or high concentrations (HQC) as presented in Table 2. All QCs were stored at -20 °C in 500 µL aliquots. Before analysis, internal standards were added (10 µL) of a solution of 100 ng/mL of each stable isotope labeled IS. On each day, two zero samples were included, which consisted of a 80:20 water:ACN mixture including 10 µL of internal standards. Blanks consisted of a 80:20 water:ACN mixture.
Sample collection
Considering the circadian rhythm of salivary steroids, saliva samples were collected as early as possible (between 9:00 – 11:00 a.m.) by direct spitting in 10 mL polypropylene tubes. Saliva donors (>20 years) were asked not to consume a large meal or brush their teeth within 60 minutes prior to sample collection and not to drink any caffeinated beverages within 15 minutes prior to sample collection. The donors rinsed their mouth with water at least 10 minutes prior to sample collection [22]. Prior to saliva donation, the donors gave written informed consent to participate in this study.
Method performance
The validation was based on the Clinical and Laboratory Standards Institute EP15-A2 protocol [23]. Saliva samples from two donors (A and B) were analyzed daily in triplicate for five days. Each run also included calibrators, blanks, zero samples, and quality control samples (two for each level LQC, MQC and HQC).
The saliva used in the validation study (A and B) was spiked with 11-deoxycortisol, 21-deoxycortisol and 17α-hydroxyprogesterone at concentrations of 0.6 ng/mL (A) and 1.8 ng/mL (B). The saliva (B) was additionally spiked with testosterone (0.1 ng/mL).
The linearity of the method was accepted if R2 was ≥ 0.99. The limit of detection (LOD) was calculated using the signal-to-noise ratio (SNR) of the qualifier of the lowest calibrator for each of the steroids, calculating the corresponding concentration for SNR = 3,0.
The limit of quantification (LOQ) was evaluated at the level of the LQC (in 80:20 water:ACN) with 10 scans per peak. For the LOQ, the acceptance criterion was 85 – 115%. The acceptance criterion for the MQCs and HQCs was 90 – 110%. One QC per day per steroid was allowed to exceed the acceptance criteria, without rejection of the run.
Method recovery was determined by spiking saliva at LQC and MQC concentrations. Calculated concentrations were compared to theoretical concentrations after blank subtraction. Analyte stability in saliva was evaluated after a 5-day incubation period at 22 °C, at 3-8 °C and at -20 °C. Saliva from a volunteer was spiked with 10 ng/mL of cortisone, 5 ng/mL of cortisol, 2.5 ng/mL of 17α-OHP, 11-deoxycortisol and 21-deoxycortisol, and 1 ng/mL of testosterone and androstenedione. Triplicate samples were analyzed followed by a comparison of the before- and after-incubation concentrations. Matrix effects were evaluated for all steroids. Blank saliva was extracted and the remains were evaporated to dryness, followed by addition of calibrators with a low, medium and high concentration. These calibrators were analyzed simultaneously without the addition of the saliva matrix. The steroid concentrations in pure solvent were then compared to the steroid concentrations in solvent + evaporated saliva matrix after blank subtraction.
Specificity of the method was determined by analysis of relevant potential interferences including aldosterone, prednisolone, 21-hydroxyprogesterone, corticosterone, 11α-hydroxyprogesterone, 11β-hydroxyprogesterone, β-estradiol-6-one, epitestosterone, estriol, dehydroepiandrosterone (DHEA) and fenofibrate.
Results and discussion
Method development
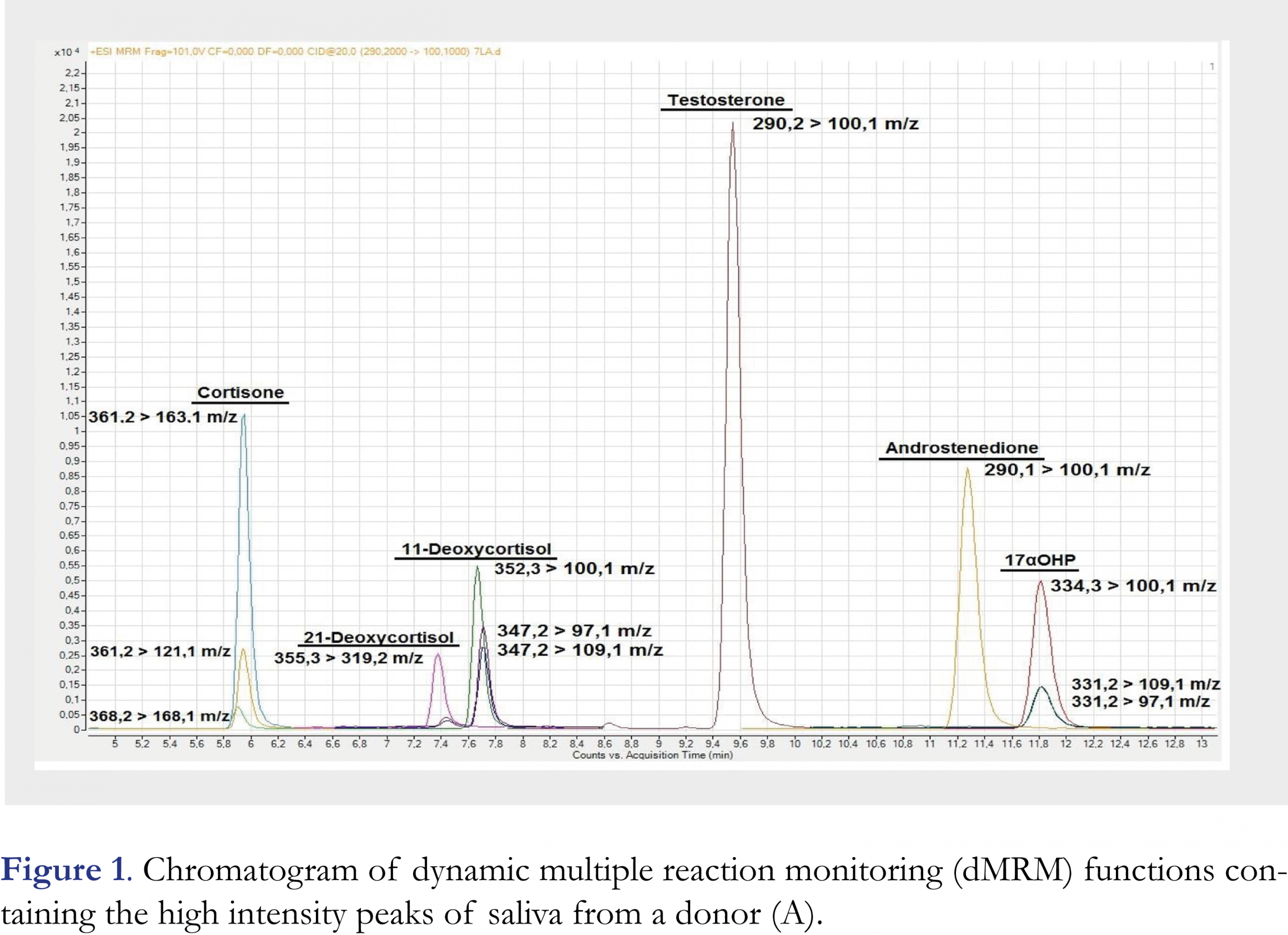
Initially, isocratic elution with methanol as modifier was used to separate all seven steroids, including the critical pair of the isobaric compounds 11-deoxycortisol and 21-deoxycortisol. However, full scans of the individual steroids revealed the significant formation of [M+Na]+ ions, as well as [M+H]+ ions. Supplemental Figure 1 shows the full scan MS result of a 100 ng/mL androstenedione solution in 50:50 methanol:water, which demonstrates the significance of [M+Na]+ formation. The [M+H]+:[M+Na]+ ratio differed for each of the steroid (range 1.3 to 8.6), and was dependent on injection volume and the source and purity of the methanol applied. Ma and Kim [24] described adduct formation specific for the analysis of steroids and demonstrated how the use of ACN instead of methanol had overcome [M+Na]+ formation. Therefore, methanol was replaced by ACN. For this modifier, gradient elution was necessary for separation of the steroids including the critical pair of isobaric, 11-deoxycortisol and 21-deoxycortisol. Cortisone and cortisol were not baseline separated, but dMRM was used to distinguish them.
Method validation
Figures and Tables
[Click to enlarge]
1,2-[2H2]-cortisol internal standard
The internal standard used for cortisol was 1,2-[2H2]-cortisol. Natural isotope ions of cortisol were insignificant at low concentrations but contributed to the peak area of the IS at higher concentrations, starting from 3 ng/mL. To limit the significance of the natural isotopes of cortisol, the concentration of the IS was increased from 0.59 ng/mL to 1.96 ng/mL, resulting in a linear range up to a concentration of 10 ng/mL. Several recommendations are given in the literature to use internal standards with three or more isotopic labels [19,20], which is strongly supported by our findings
Saliva samples
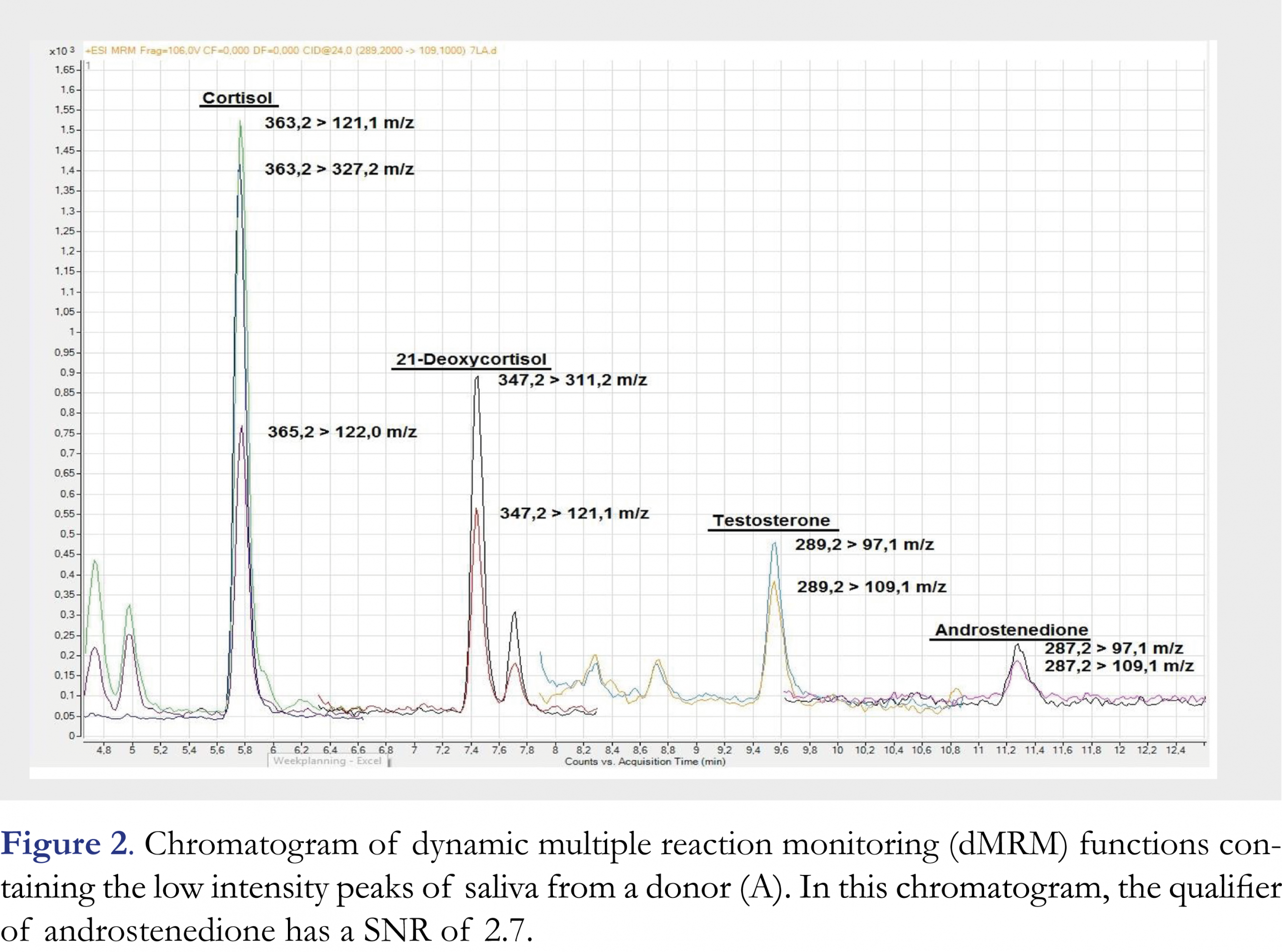
The chromatogram of saliva (sample A) is presented in Figures 1 and 2. High (Figure 1) and low (Figure 2) intensity peaks are split into two figures. All peaks are baseline separated except for cortisone and cortisol, which are separated based on their different mass-to-charge ratios.
Estimation of the appropriate calibration range
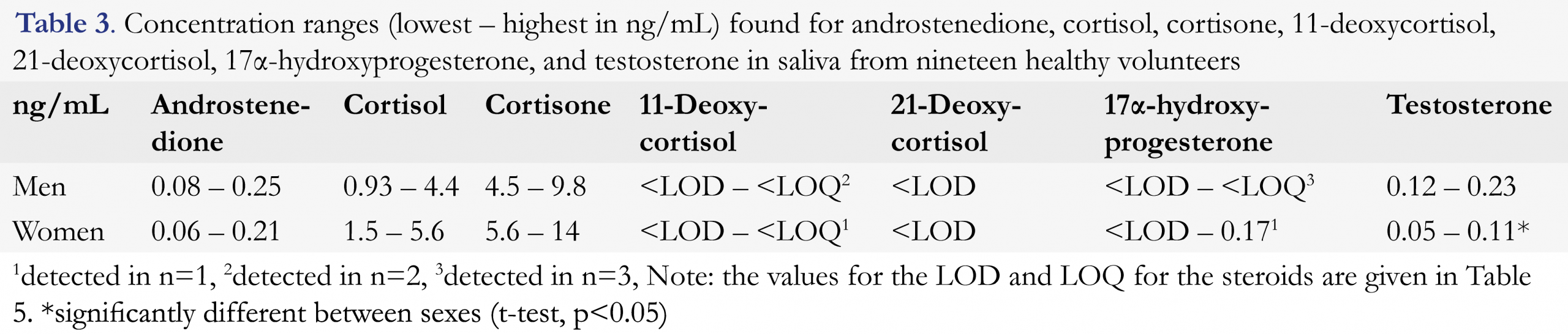
To obtain estimates of the concentration ranges of the seven steroids in saliva, nineteen healthy volunteers donated saliva. The established ranges are presented in Table 3. Since the method was further developed after this experiment, the results should be interpreted as indicative.
All steroids were successfully quantified in saliva from the volunteers except for 21-deoxycortisol which was not detected in any of the saliva samples. 11-deoxycortisol and, 17α-hydroxyprogesterone (17α-OHP) levels were very low and only occurred in a few samples. Based on these results, appropriate calibration ranges and LQC, MQC and HQC concentrations were selected for cortisol, cortisone, testosterone and androstenedione.There were no significant differences between the two sexes, except for testosterone which was significantly higher in males (average 0.16 ng/mL) than females (0.08 ng/mL, Student’s t-test, p < 0.05), which was to be expected [25]. The observed values fall within the concentration ranges described using LC-MS/MS (cortisol 0.20 – 8.50 ng/mL [1, 26], cortisone 3.06 – 15.64 ng/mL [26], testosterone (in both sexes) 0.01 – 0.11 ng/mL [12, 27, 28], androstenedione 0.08 – 1.22 ng/mL [27, 28]. RIA-based salivary reference ranges are reported for 17α-OHP, but cannot be compared to LC-MS/MS data since assay interference is a well-recognized problem [28]. To our knowledge, there are no reports of reference ranges for 21-deoxycortisol and 11-deoxycortisol in healthy subjects in saliva.
Validation of the analytical method
Compound identification
Steroidal structures are very similar and interferences have been reported [29]. Therefore, in LC-MS/MS-based methods the retention times, mass transitions and MRM ratios are used for identification. The retention times were within 1% deviation of the average retention times for all steroids and were therefore considered stable.
For the MRM ratio, different requirements are suggested in the literature, Honour suggests an acceptable deviation of 15% [20] whereas Rauh suggests 30% [28], and Methlie suggests 40% [30]. In this study, all deviations of the MRM ratios of saliva measurements were below 20%, except for four measurements at lower concentrations where variation in MRM ratios was below 30%.
Potential interferences were selected based on molecular weight using the KEGG Steroid biosynthesis pathway, drugs which are commonly used and commercial availability [29, 31]. Interferences were analyzed at 100 ng/mL in water. Supplementary Table 1 shows the chromatographic results for all interferences tested in this study. Some of the potential interferences analyzed did not yield a response, showing the selectivity of the dMRM. In dMRM mass transitions are only determined at specific time frames where the analyte of interest is expected to elute.
Baseline separation was achieved for all interferences except for corticosterone, which had an identical retention time as 21-deoxycortisol. The presence of corticosterone in a saliva sample can be identified by the MRM ratio, which is 0.55 for 21-deoxycortisol and 2.0 for corticosterone.
Calibration function
Calibration curves were constructed using the ratio between the peak area of one MRM (used as quantifier) and the peak area of the corresponding IS, as a function against the concentration. A weighing of 1/x was applied to all calibration graphs to emphasize the effect of the lower calibrators.
A few calibrators could not be quantified accurately due to low SNRs. Those calibrators were rejected from the calibrator set since accurate quantification could not be guaranteed. The lowest calibrator was rejected from the calibrator set of 21-deoxycortisol (0.038 ng/mL) and androstenedione (0.015 ng/mL), resulting in a calibrator set of 7 calibrators. A contamination with a high concentration of 11-deoxycortisol was present in calibrator 3 (0.306 ng/mL), resulting in the rejection of this calibrator from the calibrator set. All calibration graphs were linear with R2 > 0.998. All calibration functions are given in Supplementary Tables 2a-g.
QC samples and acceptance of the run
A total of ten LQCs, ten MQCs and ten HQCs were quantified for five days. The concentrations prepared are given in Table 2. All runs were accepted after evaluation of the QC’s. All LQC’s were within 15% of the nominal value, except for 21-deoxycortisol on day 4 (17.3%) and 6 (15.3%), and 11-deoxycortisol on day 6 (15.4%). All MQC and HQC were within 10% of the nominal value, except MQC of 11-deoxycortisol on day 7 (11.4%).
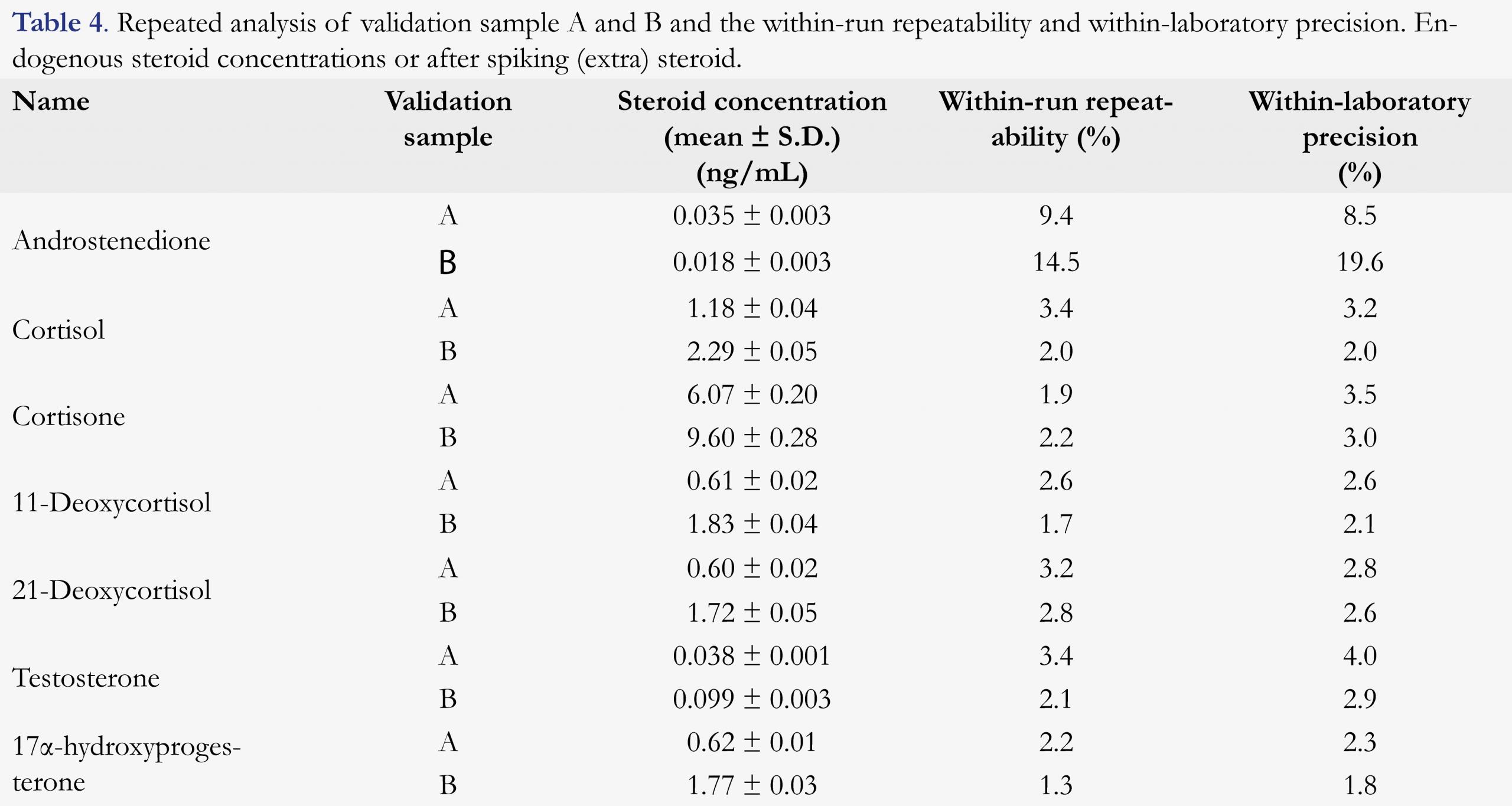
Recovery, within-run repeatability and within-laboratory precision
Recovery of the extraction procedure varied between 82%-123%. The within-run repeatability and within-laboratory precision were investigated by the repeated analysis of two collected saliva samples (A and B), measured in triplicate over five days. Part of the steroids was spiked into these samples. Within-run repeatability and within-laboratory precision were calculated as described in EP15 [23]. Table 4 shows the average steroid concentrations quantified in two saliva samples. The within-run repeatability and within-laboratory precision found are within 10%, except for androstenedione in sample B (14.5 and 19.6 %) which appeared around the limit of detection.
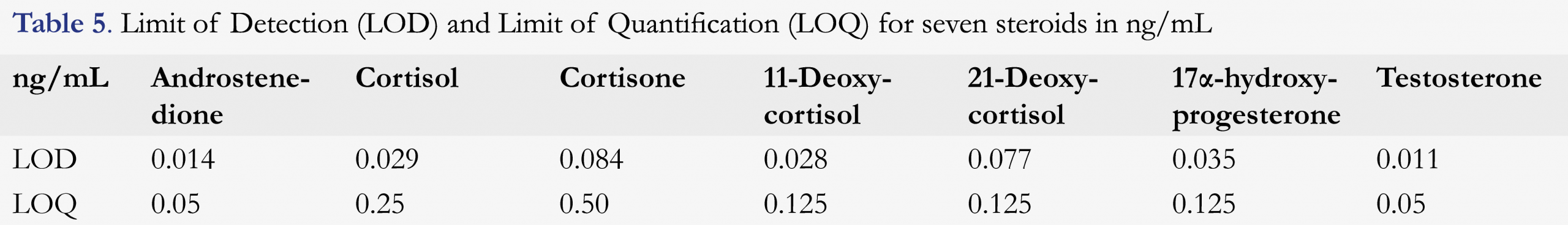
Limit of detection and limit of quantification
The LOD is determined by calculating the theoretical concentration at which the SNR = 3.0, using the lowest measured SNR of all LQC measurements. The LOQ is presented as the concentration of the LQC sample. Large differences in SNR were identified when quantifying the lowest calibrator for each steroid. On the first validation day, the SNR was much higher than on all other days. Table 5 shows the LOD and LOQ for each steroid. Results for the calibrator with the lowest concentration were used for cortisol, cortisone, 11-deoxycortisol, 17α-OHP, and testosterone. The lowest calibrator was rejected for androstenedione and 21-deoxycortisol due to SNR levels below 3.
Analyte stability in saliva
All steroids were stable during storage for five days at 22 °C, 4 8 °C and -20 °C with a 5% variability except for 21-deoxycortisol and 17α-OHP. 21-Deoxycortisol concentrations were stable when stored at -20 °C, but increased with 14 -18% when stored either at 4-8 °C or 22 °C. The 17α-OHP concentration decreased with 39% following storage at 22 °C, whereas concentrations remained stable when stored at 4 – 8 °C and -20 °C. In conclusion, saliva samples should always be stored at -20 °C.
Matrix effects
Calibrators with low (calibrator 2), medium (calibrator 4) and high (calibrator 6) concentration were analyzed with and without the addition of evaporated saliva extract. Matrix effects are limited to 10% at high and medium concentrations. The matrix effect of cortisone at low and medium concentrations is significant due to the endogenous concentration (7.52 ng/mL) spiked with 0.61 and 2.45 ng/mL. A similar trend is observed for cortisol at low concentrations due to the endogenous concentration (1.30 ng/mL) spiked with 0.30 ng/mL. The use of internal standards corrects for matrix effects variations.
Conclusions
An LC-MS/MS method was developed for the determination of a steroid profile in saliva in one analytical run. The profile consists of androstenedione, cortisol, cortisone, 11-deoxycortisol, 21-deoxycortisol, 17α-hydroxyprogesterone (17α-OHP) and testosterone and might be relevant for individuals with an adrenal disorder such as congenital adrenal hyperplasia (CAH) [1]. The method used a reasonable amount of saliva (0,5 mL) and the concept of dynamic-MRM guaranteed good selectivity and sensitivity for cortisol, cortisone, 11-deoxycortisol, 21-deoxycortisol, 17α-OHP and, testosterone, and was validated successfully for these steroids. Calibrators were used to assess linearity and to determine the LOD and LOQ. Salivary samples were used to determine within-run repeatability and within-laboratory precision. Androstenedione was present in very low concentrations (around LOD) in saliva samples and could therefore not be validated completely in this study. No interferences were found to co-elute except for corticosterone, which could not be chromatographically separated from 21-deoxycortisol. However, the presence of corticosterone was identified using the MRM ratio, since both compounds have identical mass transitions.
This method has the potential to be used for the determination of a steroid profile in saliva from CAH patients and would potentially lead to a more patient-friendly method for diagnostic or patient management purposes.
Acknowledgment
This project was funded by Nationaal Regieorgaan Praktijkgericht Onderzoek-SIA (NRPO-SIA), RAAK-Publiek grant number: 2013-15-2P in collaboration with Dr. J.M.A. Emmen from Amphia hospital, Dr. R.M.J. Hoedemakers from Jeroen Bosch hospital, Dr. A. Boer from Catharina hospital, Dr. Ir. L. IJsselstijn from Maasstad hospital, Dr. L.S.M. Boesten from IJsselland hospital, Dr. Ir. M.J.M. de Groot and Dr. B.S. Jakobs from St. Elisabeth hospital and Prof. Dr. G.J. de Jong from Utrecht University.
References
1. Inder WJ, Dimeski G, Russell A. Measurement of salivary cortisol in 2012 – laboratory techniques and clinical indications. Clin Endocrinol 77, 645–651 (2012). [Crossref]
2. Speiser PW, et al. Congenital Adrenal Hyperplasia due to steroid 21-hydroxylase deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol 95(9), 4133-4160 (2010). [CrossRef]
3. Dauber A, Kellogg M, Majzoub JA. Monitoring of therapy in Congenital Adrenal Hyperplasia. Clin Chem 56 (8), 1245-1251 (2010). [CrossRef]
4. Loeber JG. et al. Newborn screening programmes in Europe; arguments and efforts regarding harmonization. Part 1 – From blood spot to screening result. J Inherit Metab Dis 35, 603-611 (2012). [CrossRef]
5. National Institute for Public Health and the Environment (In Dutch: Rijksinstituut voor Volksgezondheid en Milieu RIVM). Flyer concerning CAH for parents of patients. https://www.rivm.nl/Documenten_en_publicaties/Algemeen_Actueel/Brochures/Preventie_Zorg_Ziekte/Hielprik/Informatiebladen_ziektes/Adrenogenitaal_syndroom_AGS/Download/Adrenogenitaal_syndroom_AGS.org
6. De Groot MJM, et al. Salivary morning androstenedione and 17α-OH progesterone levels in childhood and puberty in patients with classic Congenital Adrenal Hyperplasia. Clin Chem Lab Med 53(3), 1-8 (2015). [CrossRef]
7. Dhillon K, et al. An automated method on analysis of blood steroids using liquid chromatography tandem mass spectrometry: Application to population screening for Congenital Adrenal Hyperplasia in newborns. Clin Chim Acta 412, 2076–2084 (2011). [CrossRef]
8. Hicks RA, et al. Precursor-to-product ratios reflect biochemical phenotype in Congenital Adrenal Hyperplasia. Metabolomics 10(1) 123–131 (2014). [CrossRef]
9. Fiet J, et al. A liquid chromatography/tandem mass spectometry profile of 16 serum steroids, including 21-deoxycortisol and 21-deoxycorticosterone, for management of congenital adrenal hyperplasia. J Endocr Soc 1(3) 186–201 (2017).
10. Márta Z, et al. Simultaneous determination of thirteen different steroid hormones using micro UHPLC-MS/MS with on-line SPE system. J Pharm Biomed Anal 150, 258–267 (2018). [CrossRef]
11. Janzen N, et al. Rapid steroid hormone quantification for congenital adrenal hyperplasia (CAH) in dried blood spots using UPLC liquid chromatography-tandem mass spectrometry. Steroids 76, 1437-1442 (2011). [CrossRef]
12. Shibayama Y, et al. Simultaneous determination of salivary testosterone and dehydroepiandrosterone using LC-MS/MS: Method development and evaluation of applicability for diagnosis and medication for late-onset hypogonadism. J Chrom B 877, 2615-2623 (2009). [CrossRef]
13. Gröschl M, et al. Evaluation of saliva collection devices for the analysis of steroids, peptides and therapeutic drugs. J Pharm Biomed Anal 47, 478–486 (2008). [CrossRef]
14. McWhinney BC, Briscoe SE, Ungerer JPJ, Pretorius CJ, Measurement of cortisol, cortisone, prednisolone, dexamethasone and 11-deoxycortisol with ultra high performance liquid chromatography–tandem mass spectrometry: Application for plasma, plasma ultrafiltrate, urine and saliva in a routine laboratory. J Chrom B 878, 2863-2869 (2010). [CrossRef]
15. Matsui F, et al. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay for simultaneous measurement of salivary testosterone and cortisol in healthy men for utilization in the diagnosis of late-onset hypogonadism in males. Endocrine J 56(9) 1083-1093 (2009). [CrossRef]
16. Baxendale PM, Jacobs HS, James VHT. Plasma and salivary androstenedione and dihydrotestosterone in women with hyperandrogenism. Clin Endo. 18(5) 447-457 (1983). [CrossRef]
17. Lewis JG. Steroid analysis in saliva: an overview. Clin Biochem Rev 27, 139-146 (2006).
18. Shindo M, et al. Identification of 17-hydroxyprogesterone and other steroid hormones in saliva from a normal child and patients with congenital adrenal hyperplasia by plasmaspray liquid chromatography/mass spectrometry. Biomed. Chromotogr 4 (4), 171-174 (1990). [CrossRef]
19. Kushnir MM, et al. Liquid chromatography tandem mass spectrometry for analysis of steroids in clinical laboratories. Clin Biochem 44, 77-88 (2011). [CrossRef]
20. Honour JW. Development and validation of a quantitative assay based on tandem mass spectrometry. Ann Clin Biochem 48, 97-111 (2011). [CrossRef]
21. Wudy SA, Schuler G, Sánchez-Guijo A, Hartmann MF. The art of measuring steroids: principles and practice of current hormonal steroid analysis. J Steroid Biochem Mol Biol 179, 88–103 (2018). [CrossRef]
22. SalivaBio Salimetrics: Saliva collection and handling advice. https://www.salimetrics.com/assets/documents/Saliva_Collection_Handbook.pdf
23. Clinical and Laboratory Standards Institute, User Verification of Performance for Precision and Trueness; Approved Guideline—Second Edition, CLSI document EP15-A2 [ISBN 1-56238-574-7], 25:17.
24. Ma Y, Kim H. Determination of steroids by liquid chromatography/mass spectrometry. J Am Soc Mass Spectrom 8, 1010-1020 (1997). [CrossRef]
25. Miller JN, Miller JC. Statistics and chemometrics for analytical chemistry, sixth ed, Pearson, Harlow (2010).
26. Fustinoni S, Polledri E, Mercadente R. High-throughput determination of cortisol, cortisone, and melatonin in oral fluid by on-line turbulent flow liquid chromatography interfaced with liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 27, 1450–1460 (2013). [CrossRef]
27. Turpeinen U, Hämäläinen E, Haanpää M, Dunkel L. Determination of salivary testosterone and androstendione by liquid chromagraphy-tandem mass spectrometry. Clin Chim Acta 413(5-6), 594–599 (2012). [CrossRef]
28. Rauh M, Gröschl M, Rascher W, Dörr HG. Automated, fast and sensitive quantification of 17α-hydroxyprogesterone, androstenedione and testosterone by tandem mass spectrometry with on-line extraction. Steroids, 71, 450-458 (2006). [CrossRef]
29. Meikle AW, et al. Pseudo-Cushing syndrome caused by fenofibrate interference with urinary cortisol assayed by high-performance liquid chromatography, J Clin Endocrinol Metab 88(8), 3521-3524 (2003). [CrossRef]
30. Methlie P, et al. Multisteroid LC–MS/MS assay for glucocorticoids and androgens and its application in Addison’s disease, Endocrine Connections 2, 125-136 (2013). [CrossRef]
31. Kyoto Encyclopedia of Genes and Genomes Pathways: Steroid hormone biosynthesis pathway scheme, http://www.genome.jp/kegg-bin/show_pathway?map00140
All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License