Abstract
In the process of preparing inverse opal, the structure of inverse opal is affected by many factors, and the filling factor of inverse opal is difficult to directly test. In this paper, SnO2 inverse opal was prepared with the sol–gel method by cooperative opal template. The repetition times of the infiltrating precursor into the opal templates were investigated in detail. The band-gap positions of SnO2 inverse opal were tested. In order to prepare perfect inverse opal structure, the filling quantity of the precursor is greater, as the diameter of the PS microsphere of opal is bigger. The filling factor of air in inverse opal can be calculated with a formula derived from Bragg's law. For inverse opal, the filling factor of air in inverse opal gradually enlarges as the diameter of the void increases.
Export citation and abstract BibTeX RIS
Introduction
Since Yablonovitch [1] and John [2] theoretically showed the ability of three-dimensional periodic dielectric materials possessing photonic band gap, in 1987, photonic band-gap materials have become an important and popular field of research. Inverse opal structure, as an important three-dimensional photonic band-gap material, has promising applications in optical communication, photonic computing, switching, lasing, solar cells, etc [3–5].
One of the most important characteristics of opal and inverse opal is band-gap position, which can confine and control the propagation of light with minimal losses. Band-gap positions of opal and inverse opal can be described with Bragg's law [6, 7]:
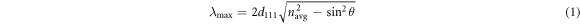
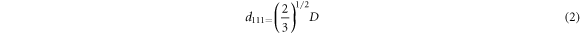
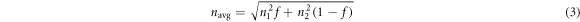
Where  is the wavelength of the band-gap position,
is the wavelength of the band-gap position,  is the lattice constant,
is the lattice constant,  is the average refractive index of the materials, and
is the average refractive index of the materials, and  is the angle between the incident light and the surface normal of the sample. As the surface of the sample is predominated by the (111) face, the lattice constant can be calculated with formula (2). Where
is the angle between the incident light and the surface normal of the sample. As the surface of the sample is predominated by the (111) face, the lattice constant can be calculated with formula (2). Where  is the diameter of the PS microspheres.
is the diameter of the PS microspheres.  can be calculated with formula (3). Where
can be calculated with formula (3). Where  and
and  are the refractive index of different materials,
are the refractive index of different materials,  is the volume fraction of the compositional material.
is the volume fraction of the compositional material.
In the past, inverse opal materials have been prepared with many methods [8–11]. In general, the preparation of inverse opal materials can be conducted in the following sequential steps: assembly of an opal template on microslide; infiltration and deposition of a matrix phase, or a precursor to a solid matrix phase; selective removal of the opal template to yield an inverse opal structure. In fact, in the process of preparing inverse opal, the filling factor of inverse opal is affected by the concentration of the precursor solution, capillary force, infiltration time, operation times, annealing temperature, heating rate of annealing, and so on. The framework of inverse opal cannot occupy all the residual space, and the true filling factor  of air in inverse opal is difficult to directly test. However, the true filling factor
of air in inverse opal is difficult to directly test. However, the true filling factor  of inverse opal is an important parameter for application in optical communication, and photonic computing. Therefore, it is necessary to research the filling factor
of inverse opal is an important parameter for application in optical communication, and photonic computing. Therefore, it is necessary to research the filling factor  of inverse opal.
of inverse opal.
As far as we know, in the work of Willen Vos et al [12], the true filling factor  of TiO2 inverse opal was tested with small-angle x-ray scattering (SAXS) and calculated with the Beer–Lambert law. However, in their result, the filling factor
of TiO2 inverse opal was tested with small-angle x-ray scattering (SAXS) and calculated with the Beer–Lambert law. However, in their result, the filling factor  was not consistent with the observed result in the scanning electron microscopy (SEM) images. In Yang et al [13], the filling factor
was not consistent with the observed result in the scanning electron microscopy (SEM) images. In Yang et al [13], the filling factor  of ZnO inverse opal was investigated and calculated by changing the ethanol/water ratio of the precursor on one opal template. In Zhao et al [14], the filling factor
of ZnO inverse opal was investigated and calculated by changing the ethanol/water ratio of the precursor on one opal template. In Zhao et al [14], the filling factor  of inverse opal was investigated by changing the liquid surface dropping velocity on a 580 nm opal template. In Ruda et al [15], the filling factor
of inverse opal was investigated by changing the liquid surface dropping velocity on a 580 nm opal template. In Ruda et al [15], the filling factor  of ferroelectric inverse opal film was introduced by using sol–gel infiltration on one opal template, but the detailed calculation process was not explained. In Zhou et al [16–18] and Jianbei et al [19], the
of ferroelectric inverse opal film was introduced by using sol–gel infiltration on one opal template, but the detailed calculation process was not explained. In Zhou et al [16–18] and Jianbei et al [19], the  was investigated and calculated by changing the incident angle, then the filling factor
was investigated and calculated by changing the incident angle, then the filling factor  of inverse opal was calculated with formula (3).
of inverse opal was calculated with formula (3).
However, in our opinion, we do not need to calculate the  If the
If the 

 are fixed, the filling factor
are fixed, the filling factor  of air in inverse opal can be calculated with the following formula (4) which is derived from formulas (1) and (3).
of air in inverse opal can be calculated with the following formula (4) which is derived from formulas (1) and (3).
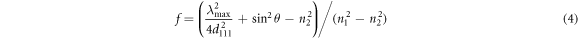
As an important semiconductor material, SnO2 with the optical band gap between the energy band and conduction band of 3.7 eV [20] is applied in chemistry electrodes, solar cells, gas sensors, etc. SnO2 inverse opal material has been prepared by the opal template cooperating with the sol–gel method [21–24]. However, the filling factor  of air in SnO2 inverse opal has not been investigated and calculated. In this paper, the filling factor
of air in SnO2 inverse opal has not been investigated and calculated. In this paper, the filling factor  of air in SnO2 inverse opal was investigated and calculated with formula (4).
of air in SnO2 inverse opal was investigated and calculated with formula (4).
Materials and methods
PS microspheres with a diameter of about 280 nm and a standard deviation of less than 5% were prepared by emulsifier-free emulsion polymerization technology [25]. SnCl4 · 5H2O, oxalic acid and ethanol are AR and were bought from Sinopharm Chemical Reagent Co. Ltd. All chemicals used in the experimental section were used as received without further purification.
Preparation of SnO2 precursor solution
10 g SnCl4 · 5H2O was dissolved in an oxalic acid solution which was mixed with 5.13 g oxalic acid and 300 ml ethanol. In order to try to remove the Cl− in the oxalic acid solution and form a clathrate of Sn4+ and oxalic acid, and to concentrate the precursor solution of SnO2, the mixed solution was stirred at 30 °C to a 200 ml transparent solution.
Preparation of SnO2 inverse opal
SnO2 inverse opal was prepared in the following steps: First, opal templates were prepared with 220, 340, 390, 425 and 530 nm monodisperse PS microspheres with a vertical self-assembling method assisted by a peristaltic pump [26, 27]. Second, the opal templates were heated at 80 °C for 15 min to enhance the mechanical stability. Third, the opal templates were infiltrated into the SnO2 precursor solution for one minute, then placed into a 55 °C oven for one hour. The repetition time for the above operations is dependent on the diameter of the PS microsphere. Finally, the opal templates with the SnO2 precursor were sintered in an oven with a rising rate of 50 °C h−1 from room temperature to 500 °C and maintained for 2 h to remove the opal templates, then naturally cooled to room temperature. In order to completely remove the PS microspheres and avoid carbonization of the PS microspheres, the door of the oven should be opened a little for the PS microspheres to react with oxygen in the air.
Characterization
The surface morphology of opal templates and SnO2 inverse opal was characterized with an S-4800 II field-emission scanning electron microscope (FESEM) at 15 kV. The inner structure of the opal templates and SnO2 inverse opal was characterized with a Philips Tecnai 12 transmission electron microscope (TEM). The reflecting spectra of the opal templates and SnO2 inverse opal were tested with an Ocean Optics Maya 2000 Pro Spectrometer and a COIC MA2001 microscope in the light of a halogen tungsten lamp (wavelength range: 400–1100 nm). The impinging angle  between the incident light and normal of the substrate is 0 in this work.
between the incident light and normal of the substrate is 0 in this work.
Results and discussion
Opal templates made of different diameter PS microspheres have been reported in many papers. Figure 1 shows the SEM and TEM images of the opal templates. At macro-scale, the opal templates are a long-range ordered structure; at micro-scale, the PS microspheres in the opal template arrange as well-ordered hexagonal close-packed structure. Figures 1(a) and (b) show the SEM and TEM images of the 220 nm PS microsphere opal template. Figures 1(c) and (d) show the SEM and TEM images of the 390 nm PS microsphere opal template. They all show the well-ordered hexagonal close-packed structure of PS microspheres.
Figure 1. SEM and TEM images of 220 and 390 nm PS microsphere opal: (a), (b) 220 nm, (c), (d) 390 nm.
Download figure:
Standard image High-resolution imageSince PS microsphere is an organic compound, the precursor solution of SnO2 was made up of SnCl4 and the organic compound oxalic acid and ethanol. The strong interaction between the precursor solution of SnO2 and polystyrene spheres tends to result in 'surface-templated' structures. F.2, 3, 4 all show the 'surface-templated' structures.
In the process of preparing SnO2 inverse opal, the repetition time of the operation is very important. Figure 2 shows the SEM images of SnO2 inverse opal which were prepared by infiltrating the precursor into the 220 nm PS microsphere opal with different time.
Figure 2. SEM images of SnO2 inverse opal were prepared by infiltrating the precursor into 220 nm PS microsphere opal: (A), (B) one time, (C), (D) two times, (E), (F) three times.
Download figure:
Standard image High-resolution imageFigures 2(A) and (B) are the SEM images which were prepared once (one time). In A, it can be seen that the top of the SnO2 inverse opal is not a long-range ordered structure. In B, circle 1 represents SnO2 occupying the space of the adjacent two PS microspheres, circle 2 represents SnO2 occupying the space of the adjacent three PS microspheres, circle 3 exhibits the skeleton of SnO2 formed around one PS microsphere. The skeleton was not completely formed, but the sublayer holes can be observed from the top holes.
Figures 2(C) and (D) are the SEM images which were prepared twice (two times). In C, it can be seen that the top of the SnO2 inverse opal is a long-range ordered structure, although there are some defects e.g. circle 1, rectangle 2 existing. The circle 1 in D represents the perfect skeleton of SnO2 formed around one PS microsphere, although a few of the skeletons are broken e.g. circle 2, 3, 4 existing, most of the skeletons in C and D are unbroken and ordered. Of course, we can consider that the defects such as circle 1 in C and circle 4 in D arise from the polystyrene template itself, such as PS microsphere vacancy.
Figures 2(E) and (F) are the SEM images which were prepared three times. F is the magnifying part of E. It is obvious that the top of the SnO2 inverse opal is covered with SnO2, and there is no interconnected exit hole. But in the crossing section, many long-range ordered holes exist, and inverse opal structure exists under the top of the SnO2.
From figure 2, for the 220 nm PS microsphere opal, it can be seen that the infiltrating precursor for one time or three times cannot form perfect inverse opal structure, but the two times is appropriate.
Figure 3 shows the SEM images of the SnO2 inverse opal which were prepared by infiltrating the precursor into the 390 nm PS microsphere opal with different time. Figures 3(A) and (B) are the SEM images which were prepared one time. In A, except circle 1, few perfect skeletons were formed at the top of the SnO2 inverse opal. The most structure is shown as circle 2 and 3 which represent SnO2 occupying the space of the adjacent three PS microspheres. If SnO2 does not completely fill the spaces around the PS microspheres, different structures will be formed. In B, it shows different structures, such as circle 1, 3, 4 and rectangle 2. Figures 3(C) and (D) are the SEM images which were prepared two times. D is the magnified part of C. In C and D, most perfect skeletons on the top of the SnO2 inverse opal were formed, although a few broken skeletons exist e.g. in circle 1 and 2. Figures 3(E) and (F) are the SEM images which were prepared three times. F is the magnified part of E. It is obvious that perfect skeletons on the top of the SnO2 inverse opal were formed.
Figure 3. SEM images of the SnO2 inverse opal were prepared by infiltrating precursor into the 390 nm PS microsphere opal: (A), (B) one time, (C), (D) two times, (E), (F) three times.
Download figure:
Standard image High-resolution imageFrom figure 3, for the 390 nm PS microsphere opal, it can be seen that infiltrating precursor one or two times is not enough to form perfect inverse opal structure, but three times is appropriate.
Figure 4 shows the SEM images of the SnO2 inverse opal which were prepared by infiltrating precursor into the 530 nm PS microsphere opal three times. It is obvious that a perfect skeleton was seldom formed.
Figure 4. SEM images of the SnO2 inverse opal were prepared by infiltrating the precursor three times into the 530 nm PS microsphere opal.
Download figure:
Standard image High-resolution imageFrom figures 2, 3 and 4, we can conclude that, in order to prepare a perfect skeleton on the top of the SnO2 inverse opal, in the same operational process, the diameter of the PS microsphere is bigger, and the repetition time of the infiltrating precursor is greater.
Figure 5 shows the TEM images of the SnO2 inverse opal. A and B were prepared by infiltrating the precursor into the 220 nm PS microsphere opal two times, C and D were prepared by infiltrating the precursor into the 390 nm PS microsphere opal three times. All of the TEM images show that the SnO2 inverse opal is long-range ordered and symmetrical.
Figure 5. TEM images of the SnO2 inverse opal were prepared by infiltrating the precursor into the opal template: (A), (B) 220 nm PS microsphere opal two times, (C), (D) 390 nm PS microsphere opal three times.
Download figure:
Standard image High-resolution imageFigure 6 shows the electron diffraction pattern of the SnO2 inverse opal based on the 390 nm PS microsphere opal and XRD patterns of the SnO2 inverse opal. The testing results of the lattice distance of the electron diffraction pattern and labeling the peak positions show that the crystal form of the SnO2 inverse opal is tetragonal phase, which corresponds with JCPDS41-1445. The  of the tetragonal phase is 2.006 [28].
of the tetragonal phase is 2.006 [28].
Figure 6. Electron diffraction pattern of the SnO2 inverse opal based on the 390 nm PS microsphere opal and XRD pattern of the SnO2 inverse opal.
Download figure:
Standard image High-resolution imageFigure 7. Reflecting spectra of opal and SnO2 inverse opal. Black is the 340 nm PS microsphere opal and the corresponding SnO2 inverse opal, red is the 390 nm PS microsphere opal and the corresponding SnO2 inverse opal, green is the 425 nm PS microsphere opal and the corresponding SnO2 inverse opal.
Download figure:
Standard image High-resolution imageOptical property is the most important characteristic of opal and inverse opal. In theory, band-gap positions of opal and inverse opal can be calculated with formulas (1), (2) and (3). The calculating  of opal is shown in table 1. However, for the SnO2 inverse opal, the filling factor
of opal is shown in table 1. However, for the SnO2 inverse opal, the filling factor  of air is not fixed since it is affected by the operation of precursor infiltration and calcination, and the
of air is not fixed since it is affected by the operation of precursor infiltration and calcination, and the  of the SnO2 inverse opal cannot be calculated.
of the SnO2 inverse opal cannot be calculated.
Table 1. Relative data of PS microsphere opal and SnO2 inverse opal
| Sample | D (nm) | Calculating 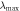 (nm) (nm) |
Testing 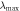 (nm) (nm) |
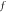
|

|
|---|---|---|---|---|---|
| Opal | 340 | 793 | 791 | 0.74 | — |
| Inverse opal | 225 | — | 437 | 0.86 | 0.14 |
| Opal | 390 | 909 | 920 | 0.74 | — |
| Inverse opal | 265 | — | 500 | 0.89 | 0.11 |
| Opal | 425 | 1014 | 1015 | 0.74 | — |
| Inverse opal | 295 | — | 535 | 0.92 | 0.08 |
For opal, D is the diameter of the PS microsphere; for inverse opal, D is the distance of the adjacent big void center.
The test results of the reflecting spectrum are shown in figure 7. The randomly selected positions of the opal have almost the same peak position and are very close to the calculating  in table 1, which demonstrates the uniform structure of opal. The randomly selected positions of the SnO2 inverse opal have almost the same peak position, which also demonstrates uniform structure.
in table 1, which demonstrates the uniform structure of opal. The randomly selected positions of the SnO2 inverse opal have almost the same peak position, which also demonstrates uniform structure.
In this paper, according to the geometry, for opal, the filling factor  of colloid microspheres is 74% [9].
of colloid microspheres is 74% [9].  is the refractive index of PS microspheres,
is the refractive index of PS microspheres,  is the refractive index of air. For inverse opal, in theory, the filling factor
is the refractive index of air. For inverse opal, in theory, the filling factor  of air is also 74%,
of air is also 74%,  is the refractive index of air, the framework of inverse opal occupies the residual space, and
is the refractive index of air, the framework of inverse opal occupies the residual space, and  is the refractive index of SnO2.
is the refractive index of SnO2.
According to the formula (4) and testing λmax, the true filling factor  of air in SnO2 inverse opal was calculated and shown in table 1. It can be seen that, for inverse opal, in the same operational process, the filling factor
of air in SnO2 inverse opal was calculated and shown in table 1. It can be seen that, for inverse opal, in the same operational process, the filling factor  of air in SnO2 inverse opal gradually enlarges as the diameter of the void increases, while the filling factor of SnO2 decreases. In order to achieve perfect inverse opal structure, the filling quantity of the precursor should be appropriate. In other words, the diameter of the PS microsphere of opal should be bigger, and the filling quantity of precursor should be greater. On the other hand, in the process of preparing inverse opal, the filling factor of inverse opal is affected by the concentration of the precursor solution, capillary force, infiltration time, operational times, annealing temperature, heating rate of annealing, and so on, so the framework of inverse opal cannot occupy all the residual space of opal. So the true filling factor
of air in SnO2 inverse opal gradually enlarges as the diameter of the void increases, while the filling factor of SnO2 decreases. In order to achieve perfect inverse opal structure, the filling quantity of the precursor should be appropriate. In other words, the diameter of the PS microsphere of opal should be bigger, and the filling quantity of precursor should be greater. On the other hand, in the process of preparing inverse opal, the filling factor of inverse opal is affected by the concentration of the precursor solution, capillary force, infiltration time, operational times, annealing temperature, heating rate of annealing, and so on, so the framework of inverse opal cannot occupy all the residual space of opal. So the true filling factor  of air in SnO2 inverse opal is always higher than the original opal.
of air in SnO2 inverse opal is always higher than the original opal.
Conclusion
In summary, SnO2 inverse opal was prepared with an opal template cooperating with a sol–gel method. The repetition times of the infiltrating precursor into the opal templates were investigated in detail. In order to prepare perfect inverse opal structure, the diameter of the PS microsphere of opal should be bigger, and the filling quantity of the precursor should be greater. The filling factor  of air in inverse opal can be calculated with the formula (4) derived from Bragg's law. For inverse opal, the filling factor
of air in inverse opal can be calculated with the formula (4) derived from Bragg's law. For inverse opal, the filling factor  of air is bigger as the diameter of the void increases.
of air is bigger as the diameter of the void increases.
Acknowledgments
This study was financially supported by the Foundation of Key Laboratory for Palygorskite Science and Applied Technology of Jiangsu Province (Project No. HPK201507), the Foundation of Jiangsu Provincial Engineering Laboratory for Advanced Materials of Salt Chemical Industry (No. SF201407) and the Huaiyin Institute of Technology Science Foundation (No. HGC1402).









