Abstract
Introduction
Therapy with the SQ®-standardised grass sublingual immunotherapy (SLIT)-tablet has been shown to be efficacious and well-tolerated in a large clinical development programme. The objective of this study was to investigate patients’ satisfaction with treatment by the SQ® grass SLIT-tablet during routine application.
Methods
In the setting of a non-interventional, open-label, observational study, patients with allergic rhinoconjunctivitis were started on the SQ® grass SLIT-tablet 4–5 months before the grass pollen season and were followed until the end of the season. Treatment satisfaction was assessed by patients completing the Treatment Satisfaction Questionnaire for Medication (TSQM II, German version 1.4) before and after the grass pollen season. Compliance and changes in subjective well-being were also assessed.
Results
Data of 271 patients treated with the SQ® grass SLIT-tablet by 117 allergists in Germany were analysed. The TSQM score for global satisfaction was (mean ± standard deviation) 79.40 ± 19.98 after 6–9 months of therapy, with the highest scores for the dimensions side effects (92.89 ± 16.49) and convenience (88.19 ± 14.33), followed by effectiveness (70.74 ± 25.20). Treatment effect was assessed by the treating physician as very good or good in 83.1% of patients. The subjective well-being of the patients compared with the previous years was assessed as improved by 87.8% of the patients at the end of the study.
Conclusions
Global satisfaction measured with the TSQM in patients treated with the SQ® grass SLIT-tablet was high, with the highest scores in the dimensions side effects and convenience, followed by effectiveness, and increased between 3 months and 6–9 months of treatment initiation.
Funding
ALK-Abelló Arzneimittel GmbH
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allergic rhinoconjunctivitis is associated with impairments in how patients function in day-to-day life and may significantly limit the quality of life of the patient as well as affecting school learning performance and work productivity [1].
Allergy immunotherapy (AIT) is currently the only available treatment of allergic rhinoconjunctivitis that has an effect on the underlying chronic allergic disease and has, therefore, the potential of being of long-term benefit to the patient. AIT has been investigated in several double-blind, placebo-controlled trials [2–5]. While AIT was originally administered as subcutaneous injections, alternative applications via the oral mucosal route by sublingually applied drops and, most recently, by sublingual tablets have become available [6]. In contrary to AIT by subcutaneous injections (SCIT), for which the patient has to visit the clinic at regular intervals, AIT by sublingual application generally only requires that the patient receives the first administration in the physician’s office and then continues taking the medication at home usually visiting the clinic only to arrange follow-up prescriptions. Compliance and adherence to treatment are therefore important prerequisites for the effectiveness of both administration routes, but especially for the sublingual treatment mode as a home treatment [7]. The SQ®-standardised grass sublingual immunotherapy (SLIT)-tablet contains a fast-dissolving extract of Phleum pratense (timothy grass; GRAZAX®; ALK, Hørsholm, Denmark), which is indicated for the disease-modifying treatment of grass pollen-induced rhinitis and conjunctivitis in adults and children (5 years and older). The tolerability and efficacy of the tablet, including long-term efficacy and disease-modifying effect, has been demonstrated in several randomized controlled trials (RCTs) in Europe and USA [8–20], and tolerability in adults and children, health-related quality of life and feasibility of intra-seasonal start of treatment in real life have been documented in several non-interventional observational studies [21–25].
In other studies, treatment satisfaction with medication has been found to be associated with adherence and persistence to treatment [26]. The Treatment Satisfaction Questionnaire for Medication (TSQM) is a validated instrument including the three most common dimensions (i.e. medication effectiveness, side effects of use and convenience of use) as well as an overall satisfaction rating, representing the individual's judgement across all three specific treatment attributes as most predictive indicator of patient satisfaction and adherence across different types of medication and patient populations [27].
AIT applied as tablets over a 3-year period can be considered to be a long-lasting treatment of allergy. The TSQM is, therefore, a suitable tool to measure treatment satisfaction for this type of treatment and to compare it with results for treatments of other chronic diseases. Application of the TSQM in patients treated with AIT has currently been reported from only one study [28]. The aim of the non-interventional observational study reported here was to measure treatment satisfaction by using the TSQM in patients during routine treatment with the SQ® grass SLIT-tablet.
Methods
Study Design
The study was designed as a multi-centre, open, uncontrolled, observational study according to non-interventional post-marketing surveillance study guidelines included in the German drug law for non-interventional studies analysed by epidemiological methods after marketing authorisation. Centres were asked to record data on two to three patients in a consecutive order, dependent on the patient’s willingness to participate in the study, in order to avoid a selection bias. Physicians were asked to document all patients who were potentially eligible to be included in a patient log. The study period was between January 2008 and March 2009.
Ethics and Data Protection
According to German drug law, the relevant authorities must be notified of non-interventional post-marketing studies. If only data on treatment as part of the routine medical practice are recorded, these studies are not subject to approval by an independent ethics committee. In our study, patients were asked for their consent to participate. For recording and evaluation of data, patients were assigned a 3-digit patient number. Direct identification of the patients was restricted to the offices of those physicians participating in the study.
All procedures in this study were in accordance with the 1964 Helsinki declaration (and its amendments). Since only data on treatment as part of the routine medical practice were recorded, the study was not subject to approval by an independent ethics committee. Written informed consent was not required because only data as part of the routine medical practice were recorded.
Patients and Treatment
Data on adult patients with a diagnosis of grass pollen-induced rhinitis and/or conjunctivitis with or without asthma with clinically relevant symptoms and no contraindications to a prescription of the SQ® grass SLIT-tablet were eligible to be documented in this study.
The study included adult patients who started their AIT with the SQ® grass SLIT-tablet (GRAZAX®, Phleum pratense 75,000 SQ-T/2800 BAU, ALK-Abelló) before the grass pollen season (GPS) 2008 (GPS1). Treatment was observed until the end of the first GPS with the grass SLIT-tablet (GPS2). To be included in the study, patients had to give their consent and be candidates for AIT with the SQ® grass SLIT-tablet according to normal clinical practice and the indications and contraindications described in the Summary of Product Characteristics (SmPC) [29]. Indications were grass pollen-induced rhinitis and conjunctivitis in adults with clinically relevant symptoms and diagnosed with a positive skin prick test and/or specific immunoglobulin E test to grass pollen. Contraindications were hypersensitivity to any of the excipients (fish gelatin, mannitol, sodium hydroxide), malignancy or systemic diseases affecting the immune system (e.g. autoimmune diseases, immune complex diseases or immune deficiency diseases), inflammatory conditions in the oral cavity with severe symptoms, such as oral lichen planus with ulcerations or severe oral mycosis, and uncontrolled or severe asthma [in adults: FEV1 (amount of air exhaled in first second) <70% of predicted value after adequate pharmacologic treatment].
Determination of Treatment Satisfaction
Patients’ satisfaction during routine treatment with the SQ® grass SLIT-tablet was determined using the TSQM II, German version 1.4 as patient reported outcome [27]. The TSQM comprises the dimensions effectiveness (3 items), side effects (5 items), convenience (3 items) and a global satisfaction rating (3 items). For each dimension the calculated TSQM scores can range from 0 (totally dissatisfied) to 100 (completely satisfied). Patients completed the TSQM after 3 months of treatment (TSQM1) and after 6–9 months of treatment (i.e. after the first GPS with treatment, TSQM2).
Assessments
The time schedule and the major observations of the study are shown in Fig. 1. At visit 1 (V1), when the patient was enrolled in the study, demographic data were recorded and data collected on the patient’s allergy history, including age at first appearance of symptoms, clinical manifestation of the allergy (rhinitis/conjunctivitis/asthma/atopic dermatitis), other allergies, the diagnostic tests performed and previous treatment by AIT, if applicable. The symptoms and medication use in the previous GPS (GPS1) were recorded retrospectively as nasal, ocular, bronchial and skin symptoms assessed on a scale of 0 to 3 (no/mild/moderate/severe symptoms), and the different types of symptomatic medication (topical nasal and eye drops/oral antihistamines/oral corticosteroids/bronchial β-sympathomimetics/bronchial steroids/other, to be specified) were recorded. The severity of the allergic disease was graded according to Clinical Global Impressions (CGI) on a 7-step scale [30]. Patients were asked about their satisfaction with the symptomatic medication in the previous GPS (GPS1, very satisfied/satisfied/unsatisfied/very unsatisfied), and concomitant treatments, AIT or other medication due to concomitant diseases were recorded. The patient received the first treatment with the SQ® grass SLIT-tablet at V1, and adverse reactions were observed during the following 30 min while the patient remained in the physician`s office; reactions were classified as either “tolerable” or “intolerable”. Tolerable reactions were defined as mild reactions at the application site specified in the SmPC and, if no treatment by medication was needed, not followed further in the case report form (CRF).
Study diagram. Treatment was started after the previous grass pollen season (GPS1) from January 2008 onwards with the first intake of the SQ® grass sublingual immunotherapy (SLIT)-tablet taking place in the clinic (V1). Patients were followed up at the 3-month visit (V2) when they came to the physician`s office for the follow-up prescription. The Treatment Satisfaction Questionnaire for Medication (TSQM) was completed by the patient at V2 (TSQM1) and at the final visit (V3 or V4) after GPS 2008 (GPS2) (TSQM2), depending on when the first administration had been performed. CGI Clinical Global Impressions
The patients subsequently came to the clinic every 3 months for follow-up prescriptions of 100 SQ® grass SLIT-tablets (V2–V4). The patients were requested to complete the TSQM at V2 (TSQM1) and at the final visit of the study after GPS2 (V3 or V4) after 6–9 months of treatment with the SQ® grass SLIT-tablet (TSQM2). At visits V2, V3 and V4 the physician asked the patient to provide additional information regarding compliance, convenience and satisfaction with the effect of treatment and recorded answers to the following questions:
-
1.
“Did the patient show up as scheduled for the follow-up prescription? (yes/no).
-
2.
“Did the patient take the tablets regularly in the past interval?” (yes/no). If the answer was no: “How often has the therapy been interrupted (sometimes/frequently)?”. The reasons for interruptions had to be specified (forgotten to take the tablet/side effects/other reasons).
-
3.
“How easy and convenient did the patient consider the intake of the tablets?” (easy and convenient/so–so/difficult or inconvenient).
-
4.
“How satisfied was the patient with the effect of the SQ® grass SLIT-tablet?” (very satisfied/satisfied/unsatisfied/very unsatisfied/not assessable).
AEs were recorded as reported by the patients, with date of start and end, severity (mild/moderate/severe), causality (no relation/relation unlikely/possible/probable/certain/unknown), medical treatment applied, outcome (recovered/recovered with sequelae/not yet recovered/fatal/unknown) and seriousness. An AE was defined as any untoward medical occurrence in a patient who was treated with the SQ® grass SLIT-tablet and which did not necessarily have a causal relationship with treatment. AEs that were at least possibly related to treatment were classified as adverse drug reactions (ADRs). An AE was assessed as severe when the event considerably interfered with the patient’s daily activities. A serious AE (SAE) was defined as any medical occurrence or effect that was life-threatening, required hospitalization or prolongation of hospitalization, resulted in persistent or significant disability or incapacity, resulted in death, congenital abnormalities or birth defect or any other event judged medically important.
At the final visit (V3 or V4, depending on whether the visit was after GPS2) global assessments of the patients’ status compared to the previous years and of the overall effect of the SQ® grass SLIT-tablet after the first GPS with treatment (GPS2) were made by the physician, and the overall tolerability was assessed by both patients and physicians. Finally, the continuation of treatment or discontinuation and, in case of discontinuation, the reasons were recorded.
Statistical Methods
For data analysis descriptive statistical methods and exploratory tests for the comparison of independent subgroups were used (t test, U test, χ2 test). TSQM scores of patients with evaluable TSQM data, both after 3 months (TSQM1) and after 6–9 months of treatment (TSQM2), were statistically analysed by the Wilcoxon test. Changes in TSQM scores at TSQM2 versus TSQM1 in all patients who had completed the TSQM questionnaire were analysed by the sign test of Dixon and Mood. All parameters that had been documented for the study were evaluated for the number of patients with respective entries in the CRFs. Missing data were not replaced. AEs were coded according to the current version of the Medical Dictionary for Regulatory Activities (MedDRA). Statistical Analysis System (SAS®), version 9.3 was used as statistical software (SAS Institute, Cary, NC).
Results
Patients
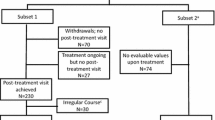
Data for 271 patients treated by 117 allergists in Germany who had their first administration of the SQ® grass SLIT-tablet in the physician’s office and treatment for a period of 6–9 months on average could be evaluated. The treatment period with the SQ® grass SLIT-tablet was between January 2008 and March 2009. Patients were observed until the end of the first GPS with treatment (GPS2) during their intended treatment period of 3 years. The patient characteristics are shown in Table 1, and the flow of patients through the study is presented in Fig. 2.
TSQM Results
The TSQM scores for all patients who completed questionnaires after 3 months of treatment with the SQ® grass SLIT-tablet at V2 (TSQM1) and after 6–9 months of treatment at V3 or V4 (TSQM2), respectively, and for patients with TSQM assessments at both time points (TSQM1 and TSQM2) analysed by Wilcoxon test are shown in Fig. 3. TSQM scores for global satisfaction and the dimensions effectiveness, side effects and convenience were higher at TSQM2 after 6–9 months of treatment with the SQ® grass SLIT tablet than those at TSQM1 after 3 months of therapy. TSQM scores were significantly increased at TSQM2 versus TSQM1 for effectiveness (p = 0.0219) and convenience (p = 0.0469), but there was no significant difference for side effects (p = 0.0778) and global satisfaction (p = 0.3756). Similar results were obtained when changes in TSQM scores were analysed in all patients who had completed the questionnaires at TSQM2 and TSQM1 by the sign-test of Dixon and Mood, except that the increase for global satisfaction was significant (p = 0.0399). Compared to the TSQM scale means from the validation study (effectiveness 68.6 ± 20.4, side effects 83.7 ± 19.5, convenience 83.2 ± 18.7, global satisfaction 71.1 ± 22.6), [27], the TSQM scores of our patients after 6–9 months of treatment with the SQ® grass SLIT-tablet were higher.
Results of the Treatment Satisfaction Questionnaire for Medication (TSQM). TSQM scores are presented as the mean ± standard deviation after 3 months (n = 212 patients) and after 6–9 months of treatment (n = 174 patients) with the SQ® grass SLIT-tablet and in patients with assessments at both time points after 3 months (TSQM1) and 6–9 months of treatment (TSQM2). Changes in TSQM scores between TSQM2 and TSQM1 assessments were statistically significant (asterisk) for the dimensions effectiveness (p = 0.0219, Wilcoxon-test) and convenience (p = 0.0469); they were not significant for side effects (p = 0.0778) and global satisfaction (p = 0.3756)
Compliance and Overall Satisfaction with Treatment
Treatment satisfaction and compliance were evaluated at each follow-up visit; 91.0–92.3% of the patients showed up as scheduled for the follow-up prescription. In total, 15.2% of patients were delayed at least once in terms of keeping a scheduled appointment. Of the 231 patients evaluable (1 missing value), 196 patients (84.8%) had no delays in their visits, 30 (13.0%) had one delayed visit, three (1.3%) had two delayed visits and two (0.9%) had three delayed visits.
At each visit 75.0–79.2% of the patients reported to have taken the SQ® grass SLIT-tablet regularly, and 35.3% of patients reported irregularities at least once. Of 221 patients evaluable (11 missing values), 143 (64.7%) patients reported no deviations from taking the tablet daily as prescribed at any of the visits, 47 (21.3%) reported deviations at one visit, 25 (11.3%) reported deviations at two visits and six patients (2.7%) reported deviations at three visits. The majority of patients who did not take the SQ® grass SLIT-tablet regularly only occasionally interrupted the therapy (59 of 78 patients; 19 frequently).
The study was discontinued by 62 patients (22.9%) due to compliance reasons. Patients were considered as non-compliant if they did not return after the last documented visit, terminated treatment due to compliance reasons, did not keep an appointment to visit the physician’s office at least once and/or frequently reported not taking the SQ® grass SLIT-tablet on a regular basis. The rate of compliant patients was 175 of the 271 patients (64.6%).
At the last visit, 207 (89.6%) of the 231 evaluable patients (1 missing value) assessed the SQ® grass SLIT-tablet as “easy and convenient” to take as prescribed, 20 (8.7%) answered this question as “so–so” and four (1.7%) reported the therapy as “difficult and inconvenient”. The degree of satisfaction with the effectiveness of the SQ® grass SLIT-tablet was rated at this point in time by 86 (37.2%) patients as “very satisfied”, 107 (46.3%) as “satisfied”, 18 (7.8%) “dissatisfied” and six (2.6%) “very dissatisfied”; 14 (6.1%) patients were not assessable.
Effectiveness Assessment
Compared with the retrospective assessment for GPS1, which was prior to treatment initiation with the SQ® grass SLIT-tablet, 147 of 189 (77.8%) patients evaluable were assessed to be free of symptoms or to show improved nasal symptoms, 133/175 (76.0%) showed improvement in eye symptoms, 67/94 (71.3%) showed improvement in bronchial symptoms and 30/44 (68.2%) showed improvement for skin symptoms. Symptomatic medication was no longer used by 59 of 194 patients (31.6%; 7 missing values) at GPS2, and 76 (40.6%) patients used less medication at this time.
At the end of the observation period, 90.5% of patients were assessed as improved according to the CGI, 16.9% were assessed to be very much improved, 50.3% as much improved and 23.3% as minimally improved. Patients’ well-being was assessed to be much better or better by 87.8% of patients taking the SQ® grass SLIT-tablet, and the overall effectiveness was assessed by the physician to be very good or good in 83.1% of patients.
Tolerability Assessment
Adverse events were observed in 150 of 271 patients who were treated in total (55.4%) during the entire observation period. Twelve patients (4.4%) received a premedication at first administration of the SQ® grass SLIT-tablet, predominantly antihistamines. ADRs after first administration of the SQ® grass SLIT-tablet were reported in 134 (49.4%) patients; these were assessed in 131 (48.3%) patients to be tolerable and in three (1.1%) patients to be intolerable. In 102 patients with tolerable reactions, the AEs were not further specified in the CRF (85 patients had tolerable reactions to first administration and no AEs during follow-up, 49 had reactions to first administration and AEs during follow-up and 16 patients had no reactions at first administration and no AEs during follow-up). The most frequent events after the first administration recorded in the CRF (32 patients, 47 events) were MedDRA-preferred terms paraesthesia oral (3.3% of patients), oral pruritus (1.5%) and oedema mouth (1.5%). In three patients with “intolerable” reactions, treatment was initially continued after first administration but discontinued after 27 days of treatment in one patient and discontinued due to ADRs in the later course of treatment in two patients. In 64 (23.6%, 114 events) patients of the 65 patients (24.0%, 121 events) with AEs during follow-up, the AEs were assessed to be related to treatment with the SQ® grass SLIT-tablet, and thus were ADRs. The most frequent MedDRA-preferred terms were paraesthesia oral (5.5% of patients), oral pruritus (2.2%) and throat irritation (2.2%); all other events accounted for <2.0%. No SAEs were observed. Global tolerability of the SQ® grass SLIT-tablet was assessed by 86.4% of patients and by 86.3% of physicians to be very good (48.5% of patients/50.0% of physicians) or good (37.9% of patients/36.3% of physicians).
Discussion
In this non-interventional, open-label, observational study that involved 271 patients treated by 117 allergists in Germany, we recorded treatment satisfaction in patients receiving the SQ® grass SLIT-tablet using the scores of the validated TSQM instrument as the patient reported outcome (PRO) [26, 27]. Mean TSQM scores were significantly increased after 6–9 months of treatment (TSQM2) versus the first assessment after 3 months of treatment (TSQM1) for the TSQM dimensions effectiveness and convenience, and they were not significantly different for the TSQM dimensions of side effects and global satisfaction. Global satisfaction, effectiveness, side effects and convenience were observed to be higher after 6–9 months of treatment with the SQ® grass SLIT-tablet than the mean TSQM scores from the TSQM validation study [27]. Physician assessment suggested that more than 75% of the patients took the tablet regularly, as prescribed, while treatment was discontinued in 22.9% of patients due to compliance issues. Treatment with the SQ® grass SLIT-tablet continued after the last post-seasonal follow-up visit of the study in 62.4% of the total number of patients included in the study. Patient well-being when taking the SQ® grass SLIT-tablet was assessed to be much better or better by 87.8% of patients, and the effectiveness assessed by the physician was very good or good in 83.1% of patients.
The majority of patients observed in our study had moderate to severe rhinoconjunctivitis symptoms before treatment with AIT, and about 24% had concomitantly asthma. About 60% of the patients were quoted to be dissatisfied with the effect of symptomatic treatment in the previous GPS. Data on assessments of dissatisfaction with symptomatic medication in patients with allergic rhinoconjunctivitis have been reported previously [31, 32]. In a non-interventional, observational study involving patients treated routinely with the SQ® grass SLIT-tablet and symptomatic medication as needed, and patients treated only with symptomatic medication, disease-specific and general health-related quality of life were observed to improve markedly during the GPS compared with the previous GPS before the start of treatment in those patients receiving the SQ® grass SLIT-tablet, while patients treated only by symptomatic medication without AIT showed no improvement [25].
Tolerability of treatment with the SQ® grass SLIT-tablet in adults and children [21, 23], feasibility of an intra-seasonal start of treatment [22] and tolerability in patients treated with concomitant AIT [24] have been investigated in other studies in the real life setting. In our study, treatment satisfaction with the SQ® grass SLIT-tablet using the validated TSQM instrument was investigated in the real-life setting.
Increases in the TSQM scores after 6–9 months versus 3 months of treatment were predictably not very large because the tablet is taken daily by the patient from day 1 of treatment. The largest and significant increase in the TSQM score was observed for effectiveness with increasing duration of treatment. This was to be expected because patients experienced symptoms of grass pollen exposure for the first time after the start of treatment with the SQ® grass SLIT-tablet at the TSQM assessment after 6–9 months of treatment (TSQM2). The significant increase in the score for satisfaction with the convenience of the treatment may be explained by an increasing adaption to the daily use of the tablet.
The TSQM score for side effects did not significantly increase, which is consistent with the finding in RCTs with the SQ® grass SLIT-tablet that local reactions at the oral mucosa observed in the majority of patients at the beginning of treatment tend to decline during the first 3 months of treatment [33].
Global satisfaction did not change significantly in patients who completed the TSQM questionnaire at both time points (TSQM1 and TSQM2). Analysis of the changes in TSQM scores at TSQM2 versus TSQM1 in all patients who had completed the TSQM questionnaire at the two time points by the sign test (Dixon and Mood) revealed a significant difference, suggesting that treatment was discontinued by more patients with a lower level of global satisfaction than patients with high level of global satisfaction. In the TSQM validation study, global satisfaction with treatment has been found to influence the decision of a patient to continue or discontinue a treatment [27].
Analysis of the results of the TSQM validation study revealed that TSQM ratings are influenced by the route of administration of the drug. Oral applications had higher global and convenience ratings and lower effectiveness ratings than injectables [27]. In a prospective, observational study with 30 patients allergic to house dust mites in Spain that were treated with a SCIT product (Dermatophagoides pteronyssinus allergoids), TSQM mean scores of 65.2 for effectiveness, 76.2 for convenience, 96.4 for side effects and 68.5 for global satisfaction were obtained after 1 year of treatment [28].
Lower satisfaction levels were observed concomitantly with a higher severity of illness and poorer appraisal of general health during the TSQM validation study [27]. Compared with ratings in the TSQM validation study for other chronic diseases in patients with a mildly to moderately serious illness and a very good/good appraisal of general health, as well as with treatment duration of between 31.0 and 125 weeks [27], we observed in our study higher mean TSQM scores with the sublingually applied SQ® grass SLIT-tablet after 6–9 months of treatment, with the largest differences for global satisfaction, side effects and convenience, and only a small difference for effectiveness.
The results for the TSQM scores in our study correspond well to the assessments of compliance, convenience of taking the tablet and effect of treatment recorded in the CRF. More than 90% of the patients attended the scheduled visits to the physician’s office for follow-up prescriptions of SQ® grass SLIT-tablets and rated the use of the SQ® grass SLIT-tablet to be easy and convenient, with 65% of patients reporting that they used the SQ® grass SLIT-tablets on a regular basis. Irregularities in taking the SQ® grass SLIT-tablet daily as prescribed were mainly caused by occasional interruptions in taking the tablet. At the last visit, 83.5% of all patients gave a global rating of very satisfied or satisfied with treatment by the SQ® grass SLIT-tablet. Data for tolerability in our study are consistent with data obtained in other non-interventional studies with the SQ® grass SLIT-tablet and confirm the tolerability profile obtained from RCTs [10, 12].
The limitations of our study are those consistent with a prospective, open-label, uncontrolled and observational study. Patients were recruited at sites across Germany. Physicians were asked to include patients in a consecutive order dependent on the consent of the patient in order to reduce a potential selection bias. Sources of potential bias with respect to the effectiveness assessments in our study is the amount of pollen grains each individual patient was exposed to and the natural variability of the grass pollen load in Germany in the two GPS compared. Such variability could potentially induce an increase in the perceived effect of treatment by patients in the case of a lower pollen exposure in GPS2 after 6–9 months of treatment compared with the baseline GPS1.
Conclusion
In summary, our non-interventional, open-label, observational study using the TSQM instrument as the patient reported outcome to measure satisfaction with treatment by the SQ® grass SLIT-tablet indicates that global satisfaction with the treatment, as measured by the TSQM instrument, was similar to or higher than average values for treatments of other chronic diseases during routine usage. Satisfaction ratings for effectiveness and convenience were statistically significantly increased between ratings after 3 months and 6–9 months of treatment with the SQ® grass SLIT-tablet, and they were not significantly different for side effects and global satisfaction.
References
Bousquet J, Khaltaev N, Cruz AA, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63[Suppl 86]:8–160.
Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass pollen immunotherapy. N Engl J Med. 1999;341:468–75.
Naclerio RM, Proud D, Moylan B, et al. A double-blind study of the discontinuation of ragweed immunotherapy. J Allergy Clin Immunol. 1997;100:293–300.
Ott H, Sieber J, Brehler R, et al. Efficacy of grass pollen sublingual immunotherapy for three consecutive seasons and after cessation of treatment: the ECRIT study. Allergy. 2009;64:179–86.
Radulovic S, Calderon MA, Wilson D, Durham S. Systematic review of sublingual immunotherapy (SLIT). Allergy. 2011;66:740–52.
Malling HJ, Lund L, Ipsen H, Poulsen LK. Safety and immunological changes during specific sublingual immunotherapy with SQ standardized grass allergen tablets. J Investig Allergol Clin Immunol. 2006;16:162–8.
Bender BG, Oppenheimer J. The special challenge of nonadherence with sublingual immunotherapy. J Allergy Clin Immunol Pract. 2014;2:152–5.
Kleine-Tebbe J, Ribel M, Herold DA. Safety of a SQ-standardized grass allergen tablet for sublingual immunotherapy: a randomized controlled trial. Allergy. 2006;61:181–4.
Dahl R, Stender A, Rak S. Specific immunotherapy with SQ standardised grass allergen tablet in asthmatics with rhinoconjunctivitis. Allergy. 2006;61:185–90.
Durham SR, Yang HW, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivits. J Allergy Clin Immunol. 2006;117:802–9.
Calderon M, Essendrop M. Specific immunotherapy with high dose SQ standardised grass allergen tablets was safe and well tolerated. J Investig Allergol Clin Immunol. 2006;16:338–44.
Dahl R, Kapp A, Colombo G, et al. Efficacy and safety of sublingual immunotherapy with grass allergen tablet for seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;118:434–40.
Ibañez MD, Kaiser F, Knecht R, et al. Safety of specific sublingual immunotherapy with SQ standardized grass allergen tablets in children. Pediatr Allergy Immunol. 2007;18:516–22.
Dahl R, Kapp A, Colombo G, et al. Sublingual grass allergen tablet provides sustained clinical benefit with progressive immunological changes over 2 years. J Allergy Clin Immunol. 2008;121:512–8.
Bufe A, Eberle P, Franke-Beckmann E, et al. Safety and efficacy in children of an SQ-standardized grass allergen tablet for sublingual immunotherapy. J Allergy Clin Immunol. 2009;123:167–73.
Durham SR, Emminger W, Kapp A, et al. Long-term clinical efficacy in grass pollen-induced rhinoconjunctivitis after treatment with SQ-standardized grass allergy immunotherapy tablet. J Allergy Clin Immunol. 2010;125:131–8.
Durham SR, Emminger W, Kapp A, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129:717–25.
Blaiss M, Maloney J, Nolte H, et al. Safety of timothy grass allergy immunotherapy tablets in North American children and adolescents. J Allergy Clin Immunol. 2011;127:64–71.
Nelson HS, Nolte H, Creticos P, et al. Efficacy and safety of timothy grass allergy immunotherapy tablet in North American adults. J Allergy Clin Immunol. 2011;127:72–80.
Maloney J, Bernstein DI, Nelson H, et al. Efficacy and safety of grass sublingual immunotherapy tablet, MK.7243: a large randomized controlled trial. Ann Allergy Asthma Immunol. 2014;112:146–53.
Gronke C, Wolf H, Schnitker J, Wüstenberg E. Treatment with the SQ-standardised grass allergy immunotherapy tablet is well tolerated in children, adolescents and adults in real life application-a non-interventional observational study. J Allergy Ther. 2013;4:146. doi:10.4172/2155-6121.1000146.
Schwab JA, Wolf H, Schnitker J, Wüstenberg E. Safety and tolerability of an intra-seasonal initiation of the SQ-standardised grass allergy immunotherapy tablet: a non-interventional observational study investigating the feasibility during routine administration. Clin Drug Investig. 2013;33:719–26.
Vitzthum HG, Wolf H, Schnitker J, Wüstenberg E. Tolerability of the SQ-standardised grass sublingual immunotherapy tablet in adult patients during routine administration-a non-interventional observational study. J Allergy Ther. 2016;5:198. doi:10.4172/2155-6121.1000198.
Reiber R, Keller M, Keller W, Wolf H, Schnitker J, Wüstenberg E. Tolerability of the SQ-standardised grass sublingual immunotherapy tablet in patients treated with concomitant allergy immunotherapy: a non-interventional observational study. Clin Transl Allergy. 2016;6:9. doi:10.1186/s13601-016-0097-8.
Horn A, Zeuner H, Wolf H, Schnitker J, Wüstenberg E. Health-related quality of life during routine treatment with the SQ-standardised grass allergy immunotherapy tablet: a non-interventional observational study. Clin Drug Investig. 2016;36:453–62.
Atkinson MJ, Kumar R, Cappelleri JC, Hass SL. Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM version II) among outpatient consumers. Value in Health. 2005;8[Suppl 1]:S9–24.
Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, Rowland CR. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12–25.
Roger A, Depreux N, Jurgens Y, Serra AT, Heath MD, Garcia G, Skinner MA. A novel microcrystalline tyrosine adsorbed, mite-allergoid subcutaneous immunotherapy: 1-year follow-up report. Immunotherapy. 2016;8:1169–74.
GRAZAX®. 75,000 SQ-T oral lyophilisate. Summary of Product Characteristics. Wedel, Germany: ALK; 2006.
Clinical Guy W, Clinical Global Impression. ECDEU Assessment Manual for Psychopharmacology, revised. Rockville, MD: National Institute of Mental Health; 1976.
Nathan RA. The burden of allergic rhinitis. Allergy Asthma Proc. 2007;28:3–9.
Valovirta E, Myrseth SE, Palkonen S. The voice of the patients. Allergic rhinitis is not a trivial disease. Curr Opinion Allergy Clin Immunol. 2008;8:1–9.
Rak S, Yang WH, Pedersen MR, Durham SR. Once-daily sublingual allergen-specific immunotherapy improves quality of life in patients with grass pollen-induced allergic rhinoconjunctivitis, a double-blind, randomised study. Qual Life Res. 2007;16:191–201.
Acknowledgements
The study and article processing charges were funded by ALK. The authors were involved in trial design, data collection or analysis and interpretation. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. We would like to thank all physicians who participated in the study.
Compliance with Ethics Guidelines
All procedures in this study were in accordance with the 1964 Helsinki declaration (and its amendments), since only data on treatment as part of the routine medical practice were recorded the study was not subject to approval by an independent ethics committee. Written informed consent was not required because only data as part of the routine medical practice were recorded.
Disclosures
T.-F. Tjan received remuneration from ALK for the documentation of patient data from his practice and for reviewing the manuscript. J. Schnitker was funded by ALK as Clinical Research Organisation (CRO) in the study. H. Wolf is an employee of ALK and holds stock options for ALK. E. Wüstenberg is an employee of ALK and holds stock options of ALK. The investigators received remuneration from ALK for the documentation of treatment of their patients. IAS GmbH received as a CRO remuneration from ALK for its services in biometrical planning, data management and statistical analysis of the study.
Data Availability
The datasets during and/or analysed during the current study are available from the corresponding author upon reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/4B87F0604E1EC3B6.
Data in this article were published in abstract form as: Liefold N, Wolf H, Schnitker J, Wüstenberg E. Treatment satisfaction during routine treatment with the SQ-standardised grass allergy immunotherapy tablet. Allergy 2010;65[Suppl 92]:569. (Poster presentation at the 29th EAACI-Congress, 05–09 June 2010, London, UK).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tjan, TF., Wolf, H., Schnitker, J. et al. Treatment Satisfaction During Routine Treatment with the SQ®-Standardised Grass Allergy Immunotherapy Tablet: A Non-interventional Observational Study. Pulm Ther 3, 149–161 (2017). https://doi.org/10.1007/s41030-017-0030-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-017-0030-x