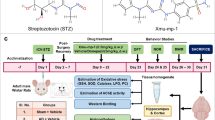
Abstract
Alzheimer’s disease (AD) causes progressive decline of memory and cognitive deficits. Because of its complicated pathogenesis, the prevention and therapy of AD remain an enormous challenge. It has been reported that catalpol possessed neuroprotective effects against AD. However, the involved mechanism still needs to be intensively studied. Therefore, the effects of catalpol on N2a/APP695swe cells and APP/PS1 mice were identified in the current study. Catalpol could improve cytotoxicity according to CCK-8 assay and ameliorate cellular morphological changes in N2a/APP695swe cells. Neuronal structural damage in the hippocampal CA1 region of APP/PS1 AD mice was improved according to HE staining and immunohistochemistry of NeuN. Meanwhile, catalpol administration ameliorated cognitive deficits confirmed by behavior performance of APP/PS1 mice. Hoechst 33,342 staining and Annexin V-FITC/PI double staining demonstrated that catalpol could reduce apoptosis in N2a/APP695swe cells. Likewise, TUNEL staining also manifested that catalpol significantly reduced apoptosis in hippocampal CA1 region of APP/PS1 mice. Catalpol administration also could improve mitochondrial functions indicated by the ameliorative mitochondrial morphology, the decreased ROS generation, and the increased MMP in N2a/APP695swe cells. Subsequently, catalpol restrained oligomerization of Aβ1-42, verified by a reduced ThT fluorescence dose- and time-dependently. Additionally, both Aβ1-40 and Aβ1-42 aggregation were decreased in N2a/APP695swe cells and APP/PS1 mice administrated with catalpol confirmed by ELISA and western blot. Western blot also showed that catalpol facilitated the phosphorylation of AKT and GSK3β, and impeded the expression of BACE1 both in vivo and in vitro. Finally, a slight alteration in lactylation, 2-hydroxyisobutyrylation, and phosphorylation were found in N2a/APP695swe cells treated with catalpol. Together, these findings manifested that catalpol served a neuroprotective effect in AD and might be a novel and expecting prophylactic or curative candidate for AD or neurodegenerative diseases therapy.










Similar content being viewed by others
References
Ahmad A, Ali T, Park HY et al (2017) Neuroprotective effect of fisetin against amyloid-beta-induced cognitive/synaptic dysfunction, neuroinflammation, and neurodegeneration in Adult Mice. Mol Neurobiol 54(3):2269–2285
Bai Y, Zhu R, Tian Y et al (2019) Catalpol in diabetes and its complications: a review of pharmacology, pharmacokinetics, and safety. Molecules 24(18):3302
Bennett DA, Yu L, Yang J et al (2015) Epigenomics of Alzheimer’s disease. Transl Res 165(1):200–220
Bi J, Jiang B, Liu JH et al (2008) Protective effects of catalpol against H2O2-induced oxidative stress in astrocytes primary cultures. Neurosci Lett 442(3):224–227
Biancalana M, Koide S (2010) Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim Biophys Acta 1804(7):1405–1412
Bloom GS, Ren K, Glabe CG (2005) Cultured cell and transgenic mouse models for tau pathology linked to beta-amyloid. Biochim Biophys Acta 1739(2–3):116–124
Bock FJ, Tait SWG (2019) Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol 21(2)
Bondi MW, Edmonds EC, Salmon DP (2017) Alzheimer’s disease: past, present, and future. J Int Neuropsychol Soc 23(9–10):818–831
Chen L, Liu S, Tao Y (2020) Regulating tumor suppressor genes: post-translational modifications. Signal Transduct Target Ther 5(1):90
Dey A, Bhattacharya R, Mukherjee A et al (2017) Natural products against Alzheimer’s disease: pharmaco-therapeutics and biotechnological interventions. Biotechnol Adv 35(2):178–216
Duyckaerts C, Potier MC, Delatour B (2008) Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol 115(1):5–38
Forloni G, Balducci C (2018) Alzheimer’s disease, oligomers, and inflammation. J Alzheimers Dis 62(3):1261–1276
Hampel H, Vassar R, De Strooper B et al (2021) The β-secretase BACE1 in Alzheimer’s disease. Biol Psychiatry 89(8):745–756
Huang W, Cheng P, Yu K et al (2017) Hyperforin attenuates aluminum-induced Aβ production and Tau phosphorylation via regulating Akt/GSK-3β signaling pathway in PC12 cells. Biomed Pharmacother 96:1–6
Hirai K, Aliev G, Nunomura A (2001) Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci 21:3017–3023
Hu H, Wang C, Jin Y et al (2019) Catalpol inhibits homocysteine-induced oxidation and inflammation via inhibiting Nox4/NF-κB and GRP78/PERK pathways in human aorta endothelial cells. Inflammation 42(1):64–80
Li Y, Zhang J, Wan J et al (2020) Melatonin regulates Aβ production/clearance balance and Aβ neurotoxicity: a potential therapeutic molecule for Alzheimer’s disease. Biomed Pharmacother 132:110887
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443(7113):787–795
Liu Z, Zhu P, Zhang L et al (2018) Autophagy inhibition attenuates the induction of anti-inflammatory effect of catalpol in liver fibrosis. Biomed Pharmacother 103:1262–1271
Lopez OL, Kuller LH (2019) Epidemiology of aging and associated cognitive disorders: prevalence and incidence of Alzheimer’s disease and other dementias. Handb Clin Neurol 167:139–148
Marcelli S, Corbo M, Iannuzzi F et al (2018) The involvement of post-translational modifications in Alzheimer’s disease. Curr Alzheimer Res 15(4):313–335
Meng T, Fu S, He D et al (2021) Evodiamine inhibits lipopolysaccharide (LPS)-induced inflammation in BV-2 cells via regulating AKT/Nrf2-HO-1/NF-κB signaling axis. Cell Mol Neurobiol 41(1):115–127
Nalivaeva NN, Turner AJ (2019) Targeting amyloid clearance in Alzheimer’s disease as a therapeutic strategy. Br J Pharmacol 176(18):3447–3463
Neddens J, Temmel M, Flunkert S et al (2018) Phosphorylation of diferent tau sites during progression of Alzheimer’s disease. Acta Neuropathol Commun 6:52
O’Brien RJ, Wong PC (2011) Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci 34:185–204
Obulesu M, Lakshmi MJ (2014) Apoptosis in Alzheimer’s disease: an understanding of the physiology, pathology and therapeutic avenues. Neurochem Res 39(12):2301–2312
Perez Ortiz JM, Swerdlow RH (2019) Mitochondrial dysfunction in Alzheimer’s disease: role in pathogenesis and novel therapeutic opportunities. Br J Pharmacol 176(18):3489–3507
Rehman MU, Wali AF, Ahmad A et al (2019) Neuroprotective strategies for neurological disorders by natural products: an update. Curr Neuropharmacol 17(3):247–267
Ren H, Wang D, Zhang L et al (2019) Catalpol induces autophagy and attenuates liver steatosis in ob/ob and high-fat diet-induced obese mice. Aging (albany NY) 11(21):9461–9477
Schliebs R, Arendt T (2011) The cholinergic system in aging and neuronal degeneration. Behav Brain Res 221(2):555–563
Venne AS, Kollipara L, Zahedi RP (2014) The next level of complexity: crosstalk of posttranslational modifications. Proteomics 14(4–5):513–524
Wang D, Wang C, Liu L et al (2018) Protective effects of evodiamine in experimental paradigm of Alzheimer’s disease. Cogn Neurodyn 12:303–313
Wang LY, Yu X, Li XX et al (2019) Catalpol exerts a neuroprotective effect in the MPTP mouse model of Parkinson’s disease. Front Aging Neurosci 11:316
Wang W, Zhao F, Ma X et al (2020) Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: recent advances. Mol Neurodegener 15(1):30
Xia Z, Zhang R, Wu P et al (2012) Memory defect induced by β-amyloid plus glutamate receptor agonist is alleviated by catalpol and donepezil through different mechanisms. Brain Res 1441:27–37
Xia Z, Wang F, Zhou S et al (2017) Catalpol protects synaptic proteins from beta-amyloid induced neuron injury and improves cognitive functions in aged rats. Oncotarget 8(41):69303–69315
You L, Peng H, Liu J et al (2021) Catalpol protects ARPE-19 cells against oxidative stress via activation of the Keap1/Nrf2/ARE pathway. Cells 10(10):2635
Yu M, Chen X, Liu J et al (2019) Gallic acid disruption of Aβ1-42 aggregation rescues cognitive decline of APP/PS1 double transgenic mouse. Neurobiol Dis. https://doi.org/10.1016/j.nbd.2018.11.009
Zhang H, Zhao C, Cao G et al (2017) Berberine modulates amyloid-beta peptide generation by activating AMP-activated protein kinase. Neuropharmacology. 2017; 125:408–417
Zhang Y, Huang NQ, Yan F et al (2018) Diabetes mellitus and Alzheimer's disease: GSK-3β as a potential link. Behav Brain Res. 339:57-65.
Zhao L, Wang Y, Liu Q (2019) Catalpol inhibits cell proliferation, invasion and migration through regulating miR-22-3p/MTA3 signalling in hepatocellular carcinoma. Exp Mol Pathol 109:51–60
Zhu H, Wang Y, Yang X et al (2019) Catalpol improves axonal outgrowth and reinnervation of injured sciatic nerve by activating Akt/mTOR pathway and regulating BDNF and PTEN expression. Am J Transl Res 11(3):1311–1326
Funding
This work was supported by National Natural Science Foundation of Guangdong Province (21202107190000430 and 2022A1515010638), Project of Educational Commission of Guangdong Province (4SG22049G, 4SG22068G, and 4SG22006G), Project of Administration of Traditional Chinese Medicine of Guangdong Province (20222100), Shenzhen Science and Technology Program (JCYJ20210324123211030), GDMU Students’ Innovative Entrepreneurial Training Program (ZZDM002 and ZYDM004), and Special Funds for the Cultivation of Guangdong College Students’ Scientific and Technological Innovation Climbing Program Special Funds (PDJH2022B0222).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Research Involving Human and Animal participants
This work does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Du, J., Liu, J., Huang, X. et al. Catalpol Ameliorates Neurotoxicity in N2a/APP695swe Cells and APP/PS1 Transgenic Mice. Neurotox Res 40, 961–972 (2022). https://doi.org/10.1007/s12640-022-00524-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-022-00524-4