Abstract
Purpose of review
Immune checkpoint inhibitors (ICI) have modified the management of patients with cancer. Their administration is associated with an increased risk of toxicities that can affect every organ. Cardiovascular toxicities, particularly myocarditis, can occur with a low incidence (< 1%) in patients treated with ICI, but with a high fatality rate (30–50%). In this review, we discuss the mechanisms, diagnostic work-up, and management of cardiovascular toxicities associated with ICI.
Recent findings
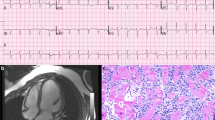
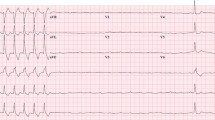
The main mechanisms of ICI-related myocarditis were first described in 2016 and are due to an infiltrate comprised T cells positive for CD3+, CD4+, and CD8+ and macrophages positive for CD68. The diagnosis of ICI-associated myocarditis remains challenging and is made on the combination of a clinical syndrome, an electrocardiogram (ECG), biomarker data, and imaging criteria. In most clinical scenarios, endomyocardial biopsy now plays a pivotal role, and the limitation of CMR in this context should be recognized. Glucocorticoids (oral or intravenous) are the first-line treatment for myocarditis confirmed by CMR and/or endomyocardial biopsy. The management of steroid-refractory myocarditis relies on the initiation of immunosuppressive therapies (ATG, infliximab, mycophenolate mofetil, and/or abatacept). However, the potential role of these drugs should be confirmed in well-designed prospective trials, as none of these strategies has been thoroughly evaluated prospectively.
Summary
ICI-related myocarditis is an emerging toxicity in patients treated with immunotherapy. The diagnosis should be made promptly since it is associated with a high fatality rate. Corticosteroids are considered as the first-line treatment.

Similar content being viewed by others
Data availability
This article does not contain any studies with human or animal subjects performed by any of the authors.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27:559–74.
•• Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–8.Study describing the fatality rate associated with ICI related myocarditis.
Michot JM, Lappara A, Le Pavec J, et al. The 2016-2019 ImmunoTOX assessment board report of collaborative management of immune-related adverse events, an observational clinical study. Eur J Cancer. 2020;130:39–50.
•• Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136:2085–7.One of the first study describing the clinical presentation of ICI related cardiovascular events including myocarditis, AF and tako-tsubo.
Cautela J, Rouby F, Salem JE, Alexandre J, Scemama U, Dolladille C, et al. Acute coronary syndrome with immune checkpoint inhibitors: a proof-of-concept case and pharmacovigilance analysis of a life-threatening adverse event. Can J Cardiol. 2020;36:476–81.
Ederhy S, Cautela J, Ancedy Y, Escudier M, Thuny F, Cohen A. Takotsubo-like syndrome in cancer patients treated with immune checkpoint inhibitors. JACC Cardiovasc Imaging. 2018;11:1187–90.
Dolladille C, Ederhy S, Allouche S, Dupas Q, Gervais R, Madelaine J, et al. Late cardiac adverse events in patients with cancer treated with immune checkpoint inhibitors. J Immunother Cancer. 2020;8.
•• Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–55.Important describtive study that analysed in depth the mechanisms of ICI related myocarditis.
Champion SN, Stone JR. Immune checkpoint inhibitor associated myocarditis occurs in both high-grade and low-grade forms. Mod Pathol. 2020;33:99–108.
•• Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–89.Review of the pharmacovigilance database showing that ICI myocarditis is not the only cardiac manifestation of ICI administration.
Pradhan R, Nautiyal A, Singh S. Diagnosis of immune checkpoint inhibitor-associated myocarditis: a systematic review. Int J Cardiol. 2019;296:113–21.
•• Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–64.Registry from MGH that gives an estimate of ICI myocarditis incidence.
Ederhy S, Voisin AL, Champiat S. Myocarditis with immune checkpoint blockade. N Engl J Med. 2017;376:290–1.
Chitturi KR, Xu J, Araujo-Gutierrez R, et al. Immune checkpoint inhibitor-related adverse cardiovascular events in patients with lung cancer. JACC: CardioOncology. 2019;1:182–92.
Zamami Y, Niimura T, Okada N, et al. Factors associated with immune checkpoint inhibitor-related myocarditis. JAMA Oncol. 2019.
Ederhy S, Dolladille C, Thuny F, Alexandre J, Cohen A. Takotsubo syndrome in patients with cancer treated with immune checkpoint inhibitors: a new adverse cardiac complication. Eur J Heart Fail. 2019;21:945–7.
Cammann VL, Sarcon A, Ding KJ, et al. Clinical features and outcomes of patients with malignancy and takotsubo syndrome: observations from the international takotsubo registry. J Am Heart Assoc. 2019;8:e010881.
Saade A, Mansuet-Lupo A, Arrondeau J, Thibault C, Mirabel M, Goldwasser F, et al. Pericardial effusion under nivolumab: case-reports and review of the literature. J Immunother Cancer. 2019;7:266.
Lee Chuy K, Oikonomou EK, Postow MA, Callahan MK, Chapman PB, Shoushtari AN, et al. Myocarditis surveillance in patients with advanced melanoma on combination immune checkpoint inhibitor therapy: the Memorial Sloan Kettering Cancer Center Experience. Oncologist. 2019;24:e196–7.
Sarocchi M, Grossi F, Arboscello E, Bellodi A, Genova C, Dal Bello MG, et al. Serial troponin for early detection of nivolumab cardiotoxicity in advanced non-small cell lung cancer patients. Oncologist. 2018;23:936–42.
• Awadalla M, Mahmood SS, Groarke JD, Hassan MZO, Nohria A, Rokicki A, et al. Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor-related myocarditis. J Am Coll Cardiol. 2020;75:467–78.Global longitudinal strain at the time of ICI myocarditis allow to identify a high risk population prone to develop MACCE.
Zhang L, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, Thuny F, et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J. 2020;41:1733–43.
•• Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A, et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. 2019;140:80–91.A pivotal study that provides a framework and guidelines to improve management and diagnosis of ICI myocarditis in the setting of cancer patients.
Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31:171–90.
Lyon AR, Dent S, Stanway S, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020. https://doi.org/10.1002/ejhf.1920.
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714–68.
•• Salem JE, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ, et al. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med. 2019;380:2377–9.First study supporting the use of Abatacept in patient presenting with an ICI corticosteroid refractory myocarditis.
Zhang L, Zlotoff DA, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation. 2020;141:2031–4.
Acknowledgments
Editorial support was provided by Sophie Rushton-Smith, PhD (MedLink Healthcare Communications) and was funded by the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A.A.C. received a research grant from RESICARD (research nurses) and consultant and lecture fees from Amgen, AstraZeneca, Bayer Pharma, Alliance BMS-Pfizer, Novartis, and Sanofi-Aventis. Stéphane Champiat received Honoraria: Amgen, AstraZeneca, BMS, Janssen, MSD, Novartis, and Roche. As part of the Drug Development Department (DITEP) =.Principal/sub-Investigator of Clinical Trials for Abbvie, Adaptimmune, Aduro Biotech, Agios Pharmaceuticals, Amgen, Argen-X Bvba, Arno Therapeutics, Astex Pharmaceuticals, Astra Zeneca Ab, Aveo, Basilea Pharmaceutica International Ltd., Bayer Healthcare Ag, Bbb Technologies Bv, Beigene, Blueprint Medicines, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Myers Squibb, Ca, Celgene Corporation, Chugai Pharmaceutical Co, Clovis Oncology, Cullinan-Apollo, Daiichi Sankyo, Debiopharm, Eisai, Eisai Limited, Eli Lilly, Exelixis, Forma Therapeutics, Gamamabs, Genentech, GlaxoSmithKline, H3 Biomedicine, Hoffmann La Roche Ag, Imcheck Therapeutics, Innate Pharma, Institut De Recherche Pierre Fabre, Iris Servier, Janssen Cilag, Janssen Research Foundation, Kyowa Kirin Pharm. Dev, Lilly France, Loxo Oncology, Lytix Biopharma As, Medimmune, Menarini Ricerche, Merck Sharp & Dohme Chibret, Merrimack Pharmaceuticals, Merus, Millennium Pharmaceuticals, Molecular Partners Ag, Nanobiotix, Nektar Therapeutics, Novartis Pharma, Octimet Oncology Nv, Oncoethix, Oncopeptides, Orion Pharma, Ose Pharma, Pfizer, Pharma Mar, Pierre Fabre, Medicament, Roche, Sanofi Aventis, Sotio A.S, Syros Pharmaceuticals, Taiho Pharma, Tesaro, Xencor Research Grants from AstraZeneca, BMS, Boehringer Ingelheim, Janssen Cilag, Merck, Novartis, Pfizer, Roche, Sanofi Non-financial support (drug supplied) from AstraZeneca, Bayer, BMS, Boringher Ingelheim, Medimmune, Merck, NH TherAGuiX, Pfizer, and Roche.
Other authors have no conflicts of interest.
Ethics approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Consent to participate
This article does not contain any studies with human or animal subjects performed by any of the authors.
Consent for publication
This article does not contain any studies with human or animal subjects performed by any of the authors.
Code availability
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardio-oncology
Rights and permissions
About this article
Cite this article
Ederhy, S., Benhamou-Tarallo, I., Chauvet-Droit, M. et al. Cardiotoxicity Related to Immune Checkpoint Inhibitors. Curr Treat Options Cardio Med 23, 4 (2021). https://doi.org/10.1007/s11936-020-00878-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s11936-020-00878-y