Abstract
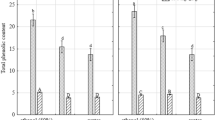
Conventional and unconventional food plants, in addition to macronutrients, are also rich in bioactive compounds, which have the antioxidant capacity and, therefore, are associated with the prevention of various diseases. There are several factors that can interfere with the concentration of these substances, such as: parts of the plant, extraction solvents, temperature, etc. The study was undertaken to extract and to characterize quantitatively the content of total phenolic compounds (TPC) in “Taioba” or Cocoyam (Xanthosoma sagittifolium) from Brazil, before and after heat treatments in different parts of the plant. TPC were performed by the Folin-Ciocalteu method and the antioxidant activity by DPPH, ABTS and FRAP methods. Principal component analysis (PCA) was used to explore the data generated. The variables (extraction solvent, parts of “Taioba” and heat treatment) influenced the TPC concentrations and antioxidant activity. The contents (mg/100 g) of TPC were higher in the nature leaf blade (75.44 ± 1.69) and the integral taioba in all treatments ranging from 44.86 ± 0.44 to 50.46 ± 0.93, in the methanolic extract when compared to the aqueous extract. PCA analysis elucidated the differences in the phenolic profile between the different parts of the “Taioba”, extraction solvent and heat treatment used. The graphs of the scores showed a clear separation of the groups according to the extraction solvents and parts of the “Taioba”, while the loading graph showed the separation of the sample groups according to the variables associated with the different heat treatments. The proposed methods were efficient for the determination of TPC in “Taioba”. Therefore, it is suggested to extend the studies to other conventional and unconventional food plants.
Graphical abstract




Similar content being viewed by others
Abbreviations
- UEP:
-
Unconventional edible plants
- TPC:
-
Total phenolic compounds
- PCA:
-
Principal component analysis
- PC:
-
Principal component
- AA:
-
Antioxidant activity
- DPPH:
-
2,2-Diphenyl-1-picryl-hydrazil
- ABTS:
-
2,2 AZINO BIS (3-ethylbenzo thiazoline 6 sulfonic acid) diammoninum salt
- FRAP:
-
Ferric antioxidant power
- TPTZ:
-
2,4,6-Tris(2-piridil)-s-triazina
- GAE:
-
Gallic acid equivalente
- TT:
-
Thermal treatments
- BI:
-
Bleaching by immersion
- BS:
-
Bleaching by steam
References
BRASIL MAPA/ACS (2010) Manual of Non-Conventional Vegetables. Ministry of Agriculture, Livestock and Supply. https://www.embrapa.br/busca-de-publicacoes/-/publicacao/857646/manual-de-hortalicas-nao-convencionais. Accessed 10 September 2021
L.S. Monteiro, B.K. Hassan, C.C.P. Estima, A.M. Souza, E.V. Junior, R. Sichieri, R.A. Pereira, Food consumption according to the days of the week – National Food Survey, 2008-2009. Rev. Saude Publica 51, 1–11 (2017)
C. Graaf, F.J. Kok, Slow food, fast food and the control of food intake. Nat Rev Endocrinol 6(5), 290–293 (2010)
V.F. Kinupp, H. Lorenzi, Non-Conventional Food Plants (PANC) in Brazil: identification guide, nutritional aspects and illustrated recipes (Plantarum, São Paulo, 2014)
L.M. Zem, C.V. Helm, K.C. Zuffellato-Ribas, H.S. Koehler, Centesimal and mineral anlysis of cupcakes base meal of leaves and stems of ora-pro-nobis (Pereskia aculeata). Rev Elet Cient UERGS 3(2), 428–446 (2017)
M.S.M. Rufino, R.E. Alves, E.S. De Brito, J. Pérez-Jiménez, F. Saura-Calixto, J. Mancini-Filho, Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem 121(4), 996–1002 (2010)
O. Oladeji, F. Adelowo, Plant phenolic compounds and health benefits. Commun. Plant Sci. 7(1–2), 20–26 (2017)
E.A. Frison, J. Cherfas, T. Hodgkin, Agricultural biodiversity is essencial for a sustainable improvement in food and nutrition security. Sustainability 3(1), 238–253 (2011)
M.A. Altieri, C.I. Nicholls, Agroecologia y resiliência al cambio climático: princípios y consideraciones metodológicas. Agroecologia 8(1), 7–20 (2013)
E.A. Jackix, E.B. Monteiro, H.F. Raposo, E.C. Vanzela, J. Amaya-Farfán, Taioba (Xanthosoma sagittifolium) leaves: nutrient composition and physiological effects on healthy rats. J Food Sci 78(12), 1929–1934 (2013)
S.J. Mayo, J. Bogner, P.C. Boyce, The genera of Araceae (Royal Botanic Garden, Kew (UK), 1997), p. 370
N.A.V.D. Pinto, V.D. Carvalho, A.D. Corrêa, A.O. Rios, Evaluation of antinutritional factors of taioba leaves (Xanthosoma sagittifolium (L.) Schott). Ciênc Agrotec 25(3), 601–604 (2001)
C.M.O. Simões, E.P. Schenkel, J.C.P. Mello, L.A. Mentz, P.R. Petrovick, Pharmacognosy: from the natural product to the medicine (Artmed, Porto Alegre, 2016), p. 502
D.M. Oliveira, D.H.M. Bastos, Phenolic acids bioavailability. Quim. Nova 34(6), 1051–1056 (2011)
B. Sultana, F. Anwar, S. Iqbal, Effect of different cooking methods on the antioxidant activity of some vegetables from Pakistan. Int. J. Food Sci. Technol. 43(3), 560–567 (2008)
C.M.J. Benevides, R.D.B. Souza, M.V. Souza, M.V. Lopes, Effect of processing on oxalate and tannin contents in gherkin (Cucumi sanguria L.), jiló (Solanum gilo), green bean (Vigna unguiculata (L.) Walp) and Andu bean (Cajanus cajan (L.) Mill sp.). Alim Nutr Araraquara 24(3), 321–327 (2013)
F. Al-juhaimi, K. Ghafoor, M.M. Özcan, M.H.A. Jahurul, E.E. Babiker, S. Jinap, F. Sahena, M.S. Sharifudin, I.S.M. Zaidul, Effect of various food processing and handling methods on preservation of natural antioxidants in fruits and vegetables. J. Food Sci. Technol. 55(10), 3872–3880 (2018)
A. Sussulini, Metabolomics: from fundamentals to clinical applications (Springer, New York, 2017), pp. 3–17
S.L.C. Ferreira, M.M.S. Junior, C.S.A. Felix, D.L.F. da Silva, A.S. Santos, J.H.S. Neto, C.T. de Souza, R.A.C. Junior, A.S. Souza, Multivariate optimization techniques in food analysis–a review. Food Chem. 273, 3–8 (2019)
M.A. Cunha, L.A.A. Paraguassú, J.G.A. Assis, A.B.P.C. Silva, R.C.V. Cardoso, Urban gardening and neglected and underutilized species in Salvador, Bahia, Brazil. J. Ethnobiol. Ethnomed. 16, 67 (2020)
IAL (2008) Physicochemical methods for food analysis. Adolfo Lutz Institute. http://www.crq4.org.br/sms/files/file/analisedealimentosial_2008.pdf. Accessed 10 September 2021
M. Naczk, F. Shahidi, Extraction and analysis of phenolics in food. J. Chromatogr. A 1054(1–2), 95–111 (2004)
M. Ikawa, T.D. Schaper, C.A. Dollard, J.J. Sasner, Utilization of Folin-Ciocalteu phenol reagent for the detection of certain nitrogen compounds. J. Agric. Food Chem. 51(7), 1811–1815 (2003)
M.S.M. Rufino, R.E. Alves, E.S. Brito, S.M. Morais, C.G. Sampaio, J. Pérez-Jiménez, F.D. Saura-Calixto (2007) Metodologia Científica: Determinação da atividade antioxidante total em frutas pela captura do radical livre ABTS•+ 4 p.: il – (Documentos /Embrapa Agroindústria Tropical, ISSN 1679-6535; 128). https://ainfo.cnptia.embrapa.br/digital/bitstream/CNPAT/10225/1/Cot_128.pdf
M.S.M. Rufino, R.E. Alves, E.S. Brito, S.M. Morais, C.G. Sampaio, J. Pérez-Jiménez, F.D. Saura-Calixto (2007) Metodologia Científica: determinação da atividade antioxidante total em frutas pela captura do radical livre DPPH 4 p.: il – (Documentos /Embrapa Agroindústria Tropical, ISSN 1679-6535; 127). https://ainfo.cnptia.embrapa.br/digital/bitstream/CNPAT/10224/1/Cot_127.pdf
M.S.M. Rufino, R.E. Alves, E.S. Brito, S.M. Morais, C.G. Sampaio, J. Pérez-Jiménez, F.D. Saura-Calixto (2006) Metodologia Científica: determinação da atividade antioxidante total em frutas pelo método de redução do ferro (FRAP) 4 p.: il – (Documentos /Embrapa Agroindústria Tropical, ISSN 1679-6535; 127). https://ainfo.cnptia.embrapa.br/digital/bitstream/CNPAT-2010/11964/1/cot-125.pdf
K. Mishra, H. Ojha, N.K. Chaudhury, Estimation of antiradical properties of antioxidants using DPPHassay: A critical review and results. Food Chem. 130, 1036–1043 (2012)
C. Sánchez-Moreno, J.A. Larrauri, F. Saura-Calixto, A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 76, 270–276 (1998)
B.B. Menezes, L.M. Frescura, R. Duarte, M.A. Villetti, M.B. Rosa, A critical examination of the DPPH method: Mistakes and inconsistencies in stoichiometry and IC50 determination by UV–Vis spectroscopy. Anal. Chim. Acta 1157, 338398 (2021)
N. Kumar, N. Goel, Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. (Amst) 24, e00370 (2019)
D.M. Kasote, S.S. Katyare, M.V. Hegde, H. Bae, Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 11(8), 982–991 (2016)
A.V.B. Moreira, J. Mancini Filho, Antioxidant activity of mustard, cinnamon and fennel spices in aqueous and lipid systems. Nutrire Rev. Soc. Bras. Aliment Nutr. 25, 31–46 (2003)
E. Wada, T. Feyissa, K. Tesfaye, Proximate, mineral and antinutrient contents of Cocoyam (Xanthosoma sagittifolium (L.) Schott) from Ethiopia. Int. J. Food Sci. 2019, 8965476 (2019)
M.S. Hossain, M. Asaduzzaman, M.S. Uddin, M.A.A. Noor, M.A. Rahman, M.S. Munira, Investigation of the in vitro antioxidant and cytotoxic activities of Xanthosoma sagittifolium leaf. Indo. Am. J. Pharm. Res. 5(10), 3300 (2015)
W.G. Sganzerla, R. Schmit, M.D. Melo, M.S. Azevedo, P.I. Ferreira, A.P.D. Veeck, J.P. Ferrareze, Rumex obtusifolius is a wild food plant with great nutritional value, high content of bioactive compounds and antioxidant activity. Emir. J. Food Agric. 31(4), 315–320 (2019)
H.A.B.D. Oliveira, P.C. Anunciação, B.P.D. Silva, Â.M.N.D. Souza, S.S. Pinheiro, C.M. Della Lucia, L.M. Cardoso, L.C.V. Castro, H.M. Pinheiro-Sant’Ana, Nutritional value of non-conventional vegetables prepared by family farmers in rural communities. Cienc Rural 49(8), e20180918 (2019)
Y. Maghsoudlou, M.A. Ghajari, S. Tavasoli, Effects of heat treatment on the phenolic compounds and antioxidant capacity of quince fruit and its tisane’s sensory properties. J. Food Sci. Technol. 56(5), 2365–2372 (2019)
F. Zhang, F. Liu, A.M. Abbasi, X. Chang, X. Guo, Effect of steaming processing on phenolic profiles and cellular antioxidant activities of Castanea mollissima. Molecules 24(4), 703 (2019)
L. Arfaoui, Dietary plant polyphenols: Effects of food processing on their content and bioavailability. Molecules 26(10), 2959 (2021)
M.K. Roy, M. Takenaka, S. Isobe, T. Tsushida, Antioxidant potential, anti-proliferative activities, and phenolic content in water-soluble fractions of some commonly consumed vegetables: Effects of thermal treatment. Food Chem. 103(1), 106–114 (2007)
K.A. Arbos, R.J.S. Freitas, S.C. Stertz, M.F. Dornas, Antioxidant activity and phenolic content in organic and conventional vegetables. Food Sci. Technol. 30(2), 501–506 (2010)
T. Herrera, Y. Aguilera, M. Rebollo-Hernanz, E. Bravo, V. Benítez, N. Martinez-Sáez, S.M. Arribas, M.D. Castilho, M.A. Martín-Cabreias, Teas and herbal infusions as sources of melatonin and other bioactive non-nutrient components. LWT - Food Sci. Technol. 89, 65–73 (2018)
H.F.S. Moura, F.S. Dias, L.B. Souza e Souza, B.E.A. Magalhães, C.A. Tannus, W.C. Carvalho, G.C. Brandão, W.N.L. dos Santos, M.G.A. Korn, D.C.M.B. Santos, M.V. Lopes, D.A. Santana, A.F. Santos Júnior, Evaluation of multielement/proximate composition and bioactive phenolics contents of unconventional edible plants from Brazil using multivariate analysis techniques. Food Chem. 363, 129995 (2021)
J.P.E. Spencer, M.M.A. El Mohsen, A.M. Minihane, J.C. Mathers, Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br. J. Nutr. 99(1), 12–22 (2008)
M. Kussmann, M. Affolter, K. Nagy, B. Holst, L.B. Fay, Mass spectrometry in nutrition: Understanding dietary health effects at the molecular level. Mass Spec. Rev. 26(6), 727–750 (2007)
D. Stagos, Antioxidant activity of polyphenolic plant extracts. Antioxidants (Basel) 9(1), 19 (2020)
R.R. Sá, R.A. Matos, V.C. Silva, J.D. Caldas, M.C.D. Sauthier, W.N.L. dos Santos, H.I.F. Magalhaes, A.F. Santos Júnior, Determination of bioactive phenolics in herbal medicines containing Cynara scolymus, Maytenus ilicifolia Mart ex Reiss and Ptychopetalum uncinatum by HPLC-DAD. Microchem. J. 135, 10–15 (2017)
C. Simó, C. Ibáñez, A. Valdés, A. Cifuentes, V. García-Cañas, Metabolomics of genetically modified crops. Int. J. Mol. Sci. 15(10), 18941–18966 (2014)
G. Cao, Y. Zhang, J.A. Feng, H. Cai, C.R. Zhang, M.J. Ding, X.D. Cong, B.C. Cai, A rapid and sensitive assay for determining the main components in processed Fructus corni by UPLC-Q-TOF-MS. Chromatographia 73(1–2), 135–141 (2011)
M.A. Farag, L.A. Wessjohann, Metabolome classification of commercial Hypericum perforatum (St. Johns Wort) preparations via UPLC–qTOF–MS and chemometrics. Planta Med. 78(5), 488–496 (2012)
M.A. Farag, M.G.S. Eldin, H. Kassem, M. Abou el Fetouh, Metabolome classification of Brassica napus L. organs via UPLC-QTOF-PDA-MS and their anti-oxidant potential. Phytochem. Anal. 24(3), 277–287 (2013)
Acknowledgements
The authors are grateful for State University of Bahia (UNEB), “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES)”, “Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)”, and “Fundação de Amparo à Pesquisa do Estado da Bahia”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Jesus Benevides, C.M., da Silva, H.B.M., Lopes, M.V. et al. Multivariate analysis for the quantitative characterization of bioactive compounds in “Taioba” (Xanthosoma sagittifolium) from Brazil. Food Measure 16, 1901–1910 (2022). https://doi.org/10.1007/s11694-021-01265-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-01265-2