Abstract
Purpose
The B-MaP-C study investigated changes to breast cancer care that were necessitated by the COVID-19 pandemic. Here we present a follow-up analysis of those patients commenced on bridging endocrine therapy (BrET), whilst they were awaiting surgery due to reprioritisation of resources.
Methods
This multicentre, multinational cohort study recruited 6045 patients from the UK, Spain and Portugal during the peak pandemic period (Feb–July 2020). Patients on BrET were followed up to investigate the duration of, and response to, BrET. This included changes in tumour size to reflect downstaging potential, and changes in cellular proliferation (Ki67), as a marker of prognosis.
Results
1094 patients were prescribed BrET, over a median period of 53 days (IQR 32–81 days). The majority of patients (95.6%) had strong ER expression (Allred score 7–8/8). Very few patients required expedited surgery, due to lack of response (1.2%) or due to lack of tolerance/compliance (0.8%). There were small reductions in median tumour size after 3 months’ treatment duration; median of 4 mm [IQR − 20, 4]. In a small subset of patients (n = 47), a drop in cellular proliferation (Ki67) occurred in 26 patients (55%), from high (Ki67 ≥ 10%) to low (< 10%), with at least one month’s duration of BrET.
Discussion
This study describes real-world usage of pre-operative endocrine therapy as necessitated by the pandemic. BrET was found to be tolerable and safe. The data support short-term (≤ 3 months) usage of pre-operative endocrine therapy. Longer-term use should be investigated in future trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In order to navigate the risk posed by the COVID-19 pandemic, changes were introduced to breast cancer care due to rationalisation of resources and prioritisation of individual patient’s cancer risks versus COVID-19 risks [1,2,3,4,5,6,7]. This occurred primarily during the first wave of infections, when the true impact of COVID-19 was unknown, vaccines were not yet available, and measures were introduced to maintain patients’ safety against the virus. This had potential to impact cancer care survival and quality of life (QoL) during the COVID-19 pandemic, but also offered a unique opportunity to prospectively observe the impact of non-standard care.
GlOBOCAN 2020 data shows that breast cancer represents 12% of all cancers diagnosed in Europe [8], and with current treatments early breast cancer prognosis is usually excellent. The management of patients diagnosed with primary breast cancer during the pandemic aimed to maintain these high standards of care whilst healthcare resources were re-prioritised to deal with the virus. This was challenging, as hospitals were considered a high-infection risk environment, and the multi-modal treatment of breast cancer involves multiple hospital visits, including surgery, radiotherapy (RT), and systemic therapy. Multidisciplinary UK, European and American guidelines, were published early in the alert phase, which helped to inform management of breast cancer during the pandemic [1, 4,5,6, 9,10,11]. The majority of recommendations did not deviate substantially from pre-COVID breast cancer management international guidance [12], but highlighted less commonly utilised alternatives, emphasizing treatment pathways that aimed to reduce the risk of and exposure to SARS-CoV-2 infection. Where theatre capacity was compromised, breast cancer treatment guidelines included use of pre-operative, or ‘bridging’, endocrine therapy (ET) [3] and priority-driven surgery scheduling based on tumour biology [6, 7, 11].
The B-MaP-C study [13, 14] investigated the changes to routine or ‘standard’ breast cancer management pathways during the first wave of the pandemic in Europe, such as alterations to use of chemotherapy (neoadjuvant and adjuvant) [11] and streamlined use of adjuvant radiotherapy (RT) [15] including the use of hypofractionated, or, 5 fraction radiotherapy (5F RT). The most frequently utilised intervention to mitigate the expected delays during the pandemic was ‘bridging’ endocrine therapy (BrET) as initial treatment for those patients with oestrogen-receptor (ER) positive cancers whilst awaiting available operating theatre capacity. The first published results of the B-MaP-C study showed that in the UK subset, of the 2216 patients who had modified MDT decisions in the pre-operative setting, 951 patients received BrET [13]. In this follow-up study, we investigated in more detail the practice and outcomes of BrET use during this period in a real-world setting across three European countries, including its effect on imaging utilisation, disease downstaging, change in surgical plan, and correlation of BrET duration with downstaging (lesion size) and tumour proliferation (Ki67).
Material and methods
The B-MaP-C study was a multicentre multinational cohort study which examined treatment recommendations during the first peak of the COVID-19 pandemic (defined as 1st February 2020 to 31st July 2020). Consecutive patients with a diagnosis of early breast cancer (invasive and DCIS) undergoing MDT-guided management were eligible for inclusion, and were identified prospectively by the local participating clinical teams in the UK, Greece, Spain and Portugal. Collection of patient demographic data, cancer-specific data and multi-disciplinary treatment recommendations in the pre-operative, operative and post-operative setting has previously been described [16, 17]. Management decisions made in the multi-disciplinary meeting (MDM) were considered as ‘standard’ if this would have been recommended pre-COVID for that particular unit, or ‘COVID-altered’ if this management recommendation was altered from that unit’s usual practice because of the COVID-19 pandemic. A ‘COVID-altered’ management decision included placing patients on BrET at the time of diagnosis; this includes patients with large hormone-receptor positive cancers having pre-operative ET, if this falls out with the unit’s standard practice. If patients had ‘standard’ treatment, no further clinicopathological data were collected other than tumour stage. Demographic, clinical (including radiological) and pathological data were captured at the time of the MDM decision to commence BrET. Patients were followed until treatment was completed or the close of the study in December 2020. Data collected included;
-
(i)
date of surgery (and hence length of BrET) and whether this was; (a) a planned date on commencement of BrET or when theatre capacity became available; (b) surgery expedited due to lack response or intolerance of BrET; or (c) continuation on ET as the primary treatment option.
-
(ii)
whether a change in surgical plan was instigated in patients with planned mastectomy as reported by the collaborator, and whether BCS was actually performed, or the patient chose to have a mastectomy
-
(iii)
whether any imaging was performed during BrET to assess response, and the reported invasive size
-
(iv)
the final pathological invasive size on the surgical excision, and whether a repeat Ki67 was performed. Ki67 change was categorised in accordance with published data as low–low (Ki67 before and after treatment < 10%); high–low (Ki67 before ≥ 10%, Ki67 after < 10%); high–high (Ki67 before and after ≥ 10%); and low–high (Ki67 before < 10%, Ki67 after ≥ 10%) [16].
We assessed response to BrET in those with invasive disease (excluding those with DCIS only) using the baseline ultrasound (USS) assessment of size, against (i) USS performed whilst on BrET and (ii) final post-surgical pathological size. The rationale behind this assumption is, that a study of the practice of BCS using image-guided localisation [17] prior to the pandemic where no delay in surgery is expected, has shown that the pathological size is marginally larger than the USS size (a median increase of 2 mm [IQR − 1, 7], N = 1521 patients).
Data were collected and managed using REDCap™ electronic data capture tools hosted at The University of Manchester [18], in accordance with Caldicott II principles. Prior to commencement of data collection, participating units were required to register the study locally with their hospital audit department and obtain local governance approvals. Ethics approval was not required according to the NHS Health Research Authority online decision tool (www.hra-decisiontools.org.uk/research/).
Data analysis
The study was reported in accordance to the STROBE guidelines for observational studies [19]. A pre-specified statistical analysis plan was approved by the study steering group. Descriptive analysis examined characteristics of patients in whom standard management was followed and in those who had BrET. Continuous variables are presented by means (standard deviation, SD) or medians (interquartile range, IQR), categorical variables are presented by frequency (percentage). Calculations for each categorical variable were performed following exclusion of missing values for this variable only. Non-parametric Mann–Whitney tests were performed to test for differences of medians between groups separately for each continuous and ordinal variable and Chi-squared tests for associations between nominal variables. Analyses were computed using Stata MP (version 16).
Results
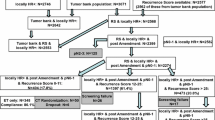
There were 6045 patients included in the B-MaP-C study (Fig. 1), including patients from UK (n = 5378), Spain (n = 269) and Portugal (n = 26). Patients’ records were excluded if the date of diagnosis was outside of the study period, if the stated date of diagnosis was after the date of surgery, there was duplicate data entry for the same patient, and where there were discrepancies in whether the management was standard or ‘COVID-altered’. There were 5673 records available for final analysis, of which 1094 patients were prescribed BrET and 2190 patients had ‘standard’ management.
Trends in management decisions
We examined the trend in ‘standard’ vs ‘BrET management decisions over time, compared to the number of positive COVID cases in the UK (data from https://coronavirus.data.gov.uk/details/cases). Figure 2 displays this data for the UK only, due to availability of raw data on COVID cases. At the start of the study, in March 2020, 40% of patients were placed on BrET, whereas by July 2020, after the end of the first COVID-19 wave in the UK, this reduced to 1%.
Use of BrET over time, compared to COVID-19 diagnosis, March–July 2022. Figure shows the changing trend in use of BrET over time (date of breast cancer diagnosis) showing UK data only. Data on COVID-19 diagnoses over period 16th March to 31st July 2022 from: https://coronavirus.data.gov.uk/details/cases
Patients on ‘Bridging’ ET
Of the 1094 patients in whom BrET was prescribed; 179 (17%) patients were pre- or perimenopausal, but the majority were post-menopausal (871; 83%). Menopausal status was not available for 44 patients. Patients receiving BrET were older, and of lower stage compared to those receiving standard treatment (T1; 51% vs 36% and N0; 84% vs 70%) (Table 1). The majority of patients having BrET (95.6%) had strong ER positivity (ER 7–8/8 *Allred score; Table 1).
Of the 179 women classified as pre/peri menopausal and receiving BrET, 132 were prescribed Tamoxifen, 9 were prescribed ovarian suppression alone and 38 prescribed an aromatase inhibitor (AI). Of those 38 patients prescribed an AI, 12 were also prescribed ovarian suppression. Of the post-menopausal women, 96% (829/867) were prescribed an AI, with the remainder prescribed Tamoxifen (supplementary Table S1).
Planning for surgery
Of the 713 patients planned for breast-conserving surgery, 632 (88.6%), had a clip- placed in the tumour (81 no clip sited, 11 were unknown). The median length of BrET in those that had surgery (990 patients, data missing in n = 104) was 53 days (IQR [32, 81]) (Fig. 3). Of these, 28.5% (282/990) had their procedure expedited due to availability of theatre capacity earlier than expected. There were 1.2% (12/990) patients where surgery was expedited due to lack of response and 0.8% (8/990) where surgery had to be expedited due to lack of tolerance/compliance with BrET. There were 8.2% (81/990) in whom ET was continued as the primary treatment approach, mostly due to patients declining surgery (31/79, missing data in n = 2, Table 2).
Imaging response to ‘Bridging’ ET
Additional post diagnostic follow-up imaging prior to surgery was undertaken in 151 (13.8%) patients on BrET. Of these 135 (89%) had ultrasound, 23 (15%) had mammogram, and 14 (9%) had MRI. Of those 135 patients who had a follow-up USS, there were 109 patients (with invasive disease only, excluding those with DCIS only) in whom data were available to assess response to BrET, using a baseline USS assessment of size against USS assessment whilst on BrET. Reductions in median USS size were small and were greater in post-menopausal than pre/perimenopausal women (− 3 mm [IQR − 7.5, 0], respectively vs − 1 mm [IQR − 3.7, 0]), and were only evident following more than 3 months BrET (median − 3 mm [IQR − 8, 0.5]) (Table 3).
Change in surgical plan
Of the 1094 patients commenced on BrET, 713 (65%) had a stated baseline surgical plan for BCS (± mastectomy as per patient choice), and 275 (25%) for mastectomy only. The baseline surgical plan was not stated in 106 patients (10%). To assess whether there was a change in surgical plan whilst on BrET, collaborators were asked to document the type of breast surgery performed post-BrET. Of 275 patients who had a baseline plan for a mastectomy, 64% (178/275) proceeded with the planned mastectomy, whilst 35.2% (97/275) after BrET had their surgical options changed from the initial plan of mastectomy to, also include breast conservation. Of these, 13.4% (13/97) patients who elected to proceed with the initially planned mastectomy although it was stated that they were considered suitable for breast conservation post-BrET. The clinicopathological data in these groups are summarised in Tables 4.
Pre-operative imaging and pathology assessment of response to ‘Bridging’ ET
There were 869 patients (with invasive disease only, excluding those with DCIS only) in whom data were available to assess response to BrET, using a final post-surgical pathological size against baseline USS assessment of size. A reduction in invasive disease size on final pathology compared to pre-operative USS was only evident after 3 months of BrET (median − 4 mm [IQR − 20, 4]) (Table 3).
Impact on tumour proliferation (Ki67) of BrET
There were 47 patients in whom Ki67 was assessed following BrET, either via repeat core biopsy prior to surgery or on the surgical excision (Fig. 4; Table 5). When patients were categorised based on their change in Ki67 as per the POETIC trial [16], most patients (n = 26, 55.3%) were in the High–Low category, where they started with a high Ki67 (≥ 10%), and dropped into the Low Ki67 group (< 10%). The remaining patients were the Low–Low (n = 14), Low–High (n = 1), and High–High (n = 6) categories accordingly. When examining the absolute changes in Ki67, there was an overall decrease in mean Ki67 from 19 to 7% with BrET. There was no correlation between this absolute reduction in Ki67, and time on BrET in days.
Discussion
The B-MaP-C study demonstrated that the management of patients presenting with primary breast cancer in the UK during the peak (Alert level 4) phase of the pandemic was, overall, in keeping with pre-covid standards. Recognising the prospect of probable limited theatre availability, and the need to deliver a priority-driven level of care, guidelines were published by the Association of Breast Surgery (ABS) and other national and international bodies [1, 4,5,6, 9,10,11]. In the absence of neo-adjuvant chemotherapy, prioritisation for surgical treatment was given to premenopausal women with triple negative or HER2 positive breast cancers. Post-menopausal women with ER-positive breast cancer were advised to commence bridging endocrine treatment, until such a time would arise that theatre capacity had improved and the COVID-19 associated surgical risks had been established. Although BrET was used, outside of routine pre-covid practice, there was a rapid reduction in patients receiving BrET, within 1 month (mid-April) of the start of the pandemic to nearer pre-pandemic practice being resumed by 4 months (Fig. 1). This transition in early April 2020 towards standard management decisions occurred before the number of positive COVID cases began to resolve (early May 2020). This was achieved due to a combination of increasing theatre capacity in a relatively ‘COVID safe’ setting, referral triaging and a reduction in volume of symptomatic new patient clinics alongside pausing of the breast screening programme.
The advice to start BrET for patients with ER-positive breast cancers, was aimed to relieve pressure on theatre capacity, to allow prioritisation of patient groups who could not have their surgical treatment delayed safely, and to postpone surgery to a time when risks of infection were better managed with appropriate theatre pathways. Reassuringly, the majority of patients placed on BrET (95.6%; Table 1) were strongly ER positive suggesting this strategy was reserved for those with tumours that were most likely to respond. However, some pre-/perimenopausal women were started on aromatase inhibitor therapy without LHRH analogue It is possible that the use of AI (± Goserelin) in this age group was due to thromboembolic considerations with Tamoxifen, particularly with the potential need to schedule surgery at short notice, but we suspect more likely reflects inaccurate menopausal status documentation and therefore uncertainty in data collection but not clinical management regarding menopausal status, leading to the categorisation as ‘peri-menopause’.
Prior to the pandemic, there was infrequent utilisation of neoadjuvant ET (NET); with only 1.5% of patients diagnosed with breast cancer thing the UK breast screening programme receiving NET [20], and there is considerable variation in practice nationally and internationally. The UK NeST study demonstrated that only 14% of systemic therapy given in the neoadjuvant setting was ET despite its relative safety and tolerability compared to chemotherapy [21, 22]. The general reluctance to use NET may be driven by lack of evidence, particularly in pre-menopausal women where the evidence and subsequent guidance is less clear. The evidence around the safety and efficacy of neoadjuvant endocrine treatment compared to primary surgery is limited including regarding optimal treatment duration, although a small RCT suggested minimal differences between 4 and 6 months [23].
In this study, a change in surgical plan from planned mastectomy at the start of BrET, to BCS occurred in 35.2% of patients (97/275) after a median of 64 (IQR [37, 107]) days of BrET. Comparatively, a small, multicenter, prospective, longitudinal study [24] reported on the optimal duration of neoadjuvant letrozole that would downstage disease to accommodate BCS (139 patients). Of the 69% of patients that became eligible for BCS, the median time on NET was 7.5 months (95% CI 6.3–8.5 months). Letrozole was well-tolerated, with mostly mild-to-moderate (grade 1–2) adverse events. Similarly, a previous study of 182 patients concluded that the use of NET beyond 3 months increased the conversion to BCS from an initial requirement for mastectomy from 60% at 3 months to 72% beyond this [25]. The ACOSOG Z1031 study [26] reported downstaging to accommodate BCS in 51% of 374 patients after 16 to 18 weeks of NET. The majority of cases not undergoing BCS despite downstaging were due to patient choice (12/17). The TEAM IIA trial [27] examined the clinical and radiological response to 6 months of neoadjuvant exemestane in 102 post-menopausal, strongly ER-positive patients. A clinically measurable response was seen in 58.7% patients at 3 months and 68.3% at > 3 months, with an improved feasibility of BCS from 61.8 to 70.6% (p = 0.012). In a subsequent meta-analysis of 1452 patients from seven RCTs, the feasibility of BCS increased from 43.3 to 60.4% (p < 0·001), but BCS was performed in only 51.8%. The reluctance not to proceed with BCS despite feasibility to do so was driven by the pre-NET clinical assessment, tumour multicentricity and tumour size [28].
As the median length of BrET was relatively short in this study, there was little reduction in size on final pathology compared to baseline imaging in the overall cohort, which was only appreciable following 3 months of treatment. This difference was a median of 4 mm [IQR − 20, 4], based on the assumption of accurate sizing by USS. The results reported in this study regarding the stated changes in surgical plans should be interpreted with some caution, since, we could not exclude that considerations of unavailability or perceived unavailability of radiotherapy and/or re-excision capacity might have impacted on the baseline stated plan for mastectomy. In addition, due to ‘pooled lists’, there may have an impact onto surgical decision-making. The modest tumour size reductions, and mean baseline tumour size of 21 mm for those deemed to require mastectomy also suggest caution in interpretation and we have therefore not explicitly used the term ‘surgical downstaging’ but have reported the stated changes in treatment plan from baseline to that post-BrET prior to surgery.
Only a small proportion of patients in this study, had serial Ki67 measurement, limiting any broader conclusions from these results. The recent publication on long-term outcome and prognostic value of Ki67 after perioperative endocrine therapy in post-menopausal women reported on a higher risk of disease recurrence in patients with persistent high Ki67 score suggesting a possible benefit of additional adjuvant therapies [16]. The ability to downstage disease, with a tolerable and safe approach, along with the ability to incorporate a proven prognostic biomarker, makes pre-operative endocrine therapy an attractive option. The length of treatment remains unknown, and whether this should be 14 days of neo-adjuvant endocrine therapy to identify patients of higher risk, who would require additional therapy, or potentially 3 months for the added benefit of downstaging disease and accommodating breast conservation. To investigate the former, the POETIC-A trial [29] will aim to randomise patients with persistently high Ki67 following a short treatment window, to additional CDK4/6 inhibitor therapy.
This observational study has limitations. The length of time on BrET could be prone to reporting inaccuracy or recall bias. The date started might be the date the patient received the prescription from the clinician, and not when the patient actually commenced treatment, and drug compliance was not assessed. This variability differs to randomised controlled trials where the start of treatment is stipulated, reporting is standardised, and the exact treatment duration is documented. With regards to Tamoxifen, the practice of stopping it two weeks prior to surgery was not accounted for in this study, due to the variability in practice. There was a high number of patients (35%) in this study with a planned mastectomy who had a change in surgical plan to BCS following BrET. This is subject to bias, as the surgical decision-making prior to initiation of BrET would have been driven by the pressures of the pandemic, and may be due to apprehension around radiotherapy availability and risk of margin re-excision during the peak of the pandemic. Future work should examine trends in use of BrET post-pandemic.
Within this study, BrET was found to be tolerable, and safe, overall, as evidenced by the fact that only 1.2% of patients (12/990) required expedited surgery due to lack of response, and only 0.8% (8/990) where surgery had to be expedited due to lack of tolerance/compliance with ET. There were 8.2% (81/990) in whom ET was continued as the primary treatment approach, mostly due to patients declining surgery (31/79, Table 2). Overall drops in cellular proliferation (Ki67) occurred relatively quickly and there were definite but small reductions in median tumour size, apparent even after short-term treatment and increasing with treatment duration. The results of this study, as well as those summarised above, would support a longer period (at least 3 months) of BrET to impact surgical outcomes, however in this relatively limited study, it is not possible to infer the long-term oncological implications. EndoNET is a randomised controlled trial currently in the set-up phase which will evaluate if neo-adjuvant endocrine treatment increases breast conservation rates and/or quality of life. Post-menopausal patients with early ER+ breast cancer will be randomised to standard primary surgery versus 6 months of neo-adjuvant endocrine treatment followed by surgery [30].
Conclusions
In conclusion, short course BrET is safe, effective, well-tolerated, and has the potential to provide valuable prognostic information from serial Ki67 assessment. There was no associated adverse impact on surgical decision-making. This evidence, taken together with clinical trial data [16] supports the routine use of short-term pre-op ET to allow assessment of endocrine sensitivity, and justifies the use of this approach in situations where theatre access is constrained, as occurred during the pandemic.
Data availabilty
Anonymised patient data is the property of the collaborating unit, and has been entered onto a secure server for the purpose of this study. This data is not accessible to those who do have ownership, and is not availble to request.
Abbreviations
- BrET:
-
Bridging endocrine therapy
- QoL:
-
Quality of life
- RT:
-
Radiotherapy
- ET:
-
Endocrine therapy
- 5F RT:
-
5 Fraction radiotherapy
- ER:
-
Oestrogen-receptor
- DCIS:
-
Ductal carcinoma in situ
- IQR:
-
Interquartile range
- NET:
-
Neoadjuvant endocrine therapy
References
(NICE) NIfHaCE. COVID-19 rapid guideline: delivery of systemic anticancer treatments. NICE guideline [NG161] 27 April 2020. https://www.nice.org.uk/guidance/ng161. Accessed 15 June 2020
(NICE) NIfHaCE (2020) COVID-19 rapid guideline: delivery of radio- therapy. https://www.nice.org.uk/guidance/ng162
Dowsett M, Ellis MJ, Dixon JM, Gluz O, Robertson J, Kates R et al (2020) Evidence-based guidelines for managing patients with primary ER+ HER2- breast cancer deferred from surgery due to the COVID-19 pandemic. NPJ Breast Cancer 6:21
England N (2020) Clinical guide for the management of non- coronavirus patients requiring acute treatment: cancer. https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/specialty-guide-acute-treatment-cancer-23-march-2020.pdf
Imaging ESoB (2020) EUSOBI recommendations for breast imaging and cancer diagnosis during and after the COVID-19 pandemic. https://www.eusobi.org/news/recommendations-breast-covid19/
Oncology ESoM (2020) ESMO management and treatment adapted recommendations in the COVID-19 era: breast cancer. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/breast-cancer-in-the-covid-19-era
Dietz JR, Moran MS, Isakoff SJ, Kurtzman SH, Willey SC, Burstein HJ et al (2020) Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic the COVID-19 pandemic breast cancer consortium. Breast Cancer Res Treat 181(3):487–497
Cancer IAfRo (2020) Global Cancer Observatory. https://gco.iarc.fr/
van de Haar J, Hoes LR, Coles CE, Seamon K, Fröhling S, Jäger D et al (2020) Caring for patients with cancer in the COVID-19 era. Nat Med 26(5):665–671
Coles CE, Aristei C, Bliss J, Boersma L, Brunt AM, Chatterjee S et al (2020) International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol (R Coll Radiol) 32(5):279–281
Association of Breast Surgery (2020) Statement from the association of breast surgery, 15th march 2020. Confidential advice for health professionals. https://associationofbreastsurgery.org.uk/media/252009/abs-statement-150320-v2.pdf. Accessed 26 May 2020
National Institute for Health and Care Excellence (NICE) (2018) Early and locally advanced breast cancer: diagnosis and management (NICE Guideline NG101). https://www.nice.org.uk/guidance/ng12. Accessed 3 June 2020
Dave RV, Kim B, Courtney A, O’Connell R, Rattay T, Taxiarchi VP et al (2021) Publisher Correction: Breast cancer management pathways during the COVID-19 pandemic: outcomes from the UK “Alert Level 4” phase of the B-MaP-C study. Br J Cancer 124:1785
Courtney A, O’Connell R, Rattay T, Kim B, Cutress RI, Kirwan CC et al (2020) The B-MaP-C study: breast cancer management pathways during the COVID-19 pandemic. Study protocol. Int J Surg Protoc 24:1–5
Coles C (2020) Guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. https://www.rcr.ac.uk/sites/default/files/breast-cancer-treatment-covid19.pdf. Accessed 26 May 2020
Smith I, Robertson J, Kilburn L, Wilcox M, Evans A, Holcombe C et al (2020) Long-term outcome and prognostic value of Ki67 after perioperative endocrine therapy in postmenopausal women with hormone-sensitive early breast cancer (POETIC): an open-label, multicentre, parallel-group, randomised, phase 3 trial. Lancet Oncol 21(11):1443–1454
Dave RV, Barrett E, Morgan J, Chandarana M, Elgammal S, Barnes N et al (2022) Wire- and magnetic-seed-guided localization of impalpable breast lesions: iBRA-NET localisation study. Br J Surg 109(3):274–282
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP et al (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370(9596):1453–1457
PHE. NHS Breast Screening Programme & Association of Breast Surgery. An audit of screen detected breast cancers for the year of screening April 2017 to March 2018. Public Health England. https://associationofbreastsurgery.org.uk/media/65088/nhsbsp-abs-audit-2017-to-2018.pdf. Accessed 28 Nov 2019
Irwin GW, Bannon F, Coles CE, Copson E, Cutress RI, Dave RV et al (2019) The NeST (neoadjuvant systemic therapy in breast cancer) study – Protocol for a prospective multi-centre cohort study to assess the current utilization and short-term outcomes of neoadjuvant systemic therapies in breast cancer. Int J Surg Protoc 18:5–11
McIntosh SA, Irwin GW, Bannon F, Coles C, Copson E, Cutress R, Dave R, Grayson M, Holcombe C, Irshad S, O'Brien CS, O'Connell R, Palmieri C, Shaaban AM, Sharma N, Singh J, Whitehead I, Potter S (2020) National utilisation of neoadjuvant systemic therapy and impact on surgical treatment: a prospective multi-centre cohort study. https://doi.org/10.1158/1538-7445SABCS19-P2-16-04
Hojo T, Kinoshita T, Imoto S, Shimizu C, Isaka H, Ito H et al (2013) Use of the neo-adjuvant exemestane in post-menopausal estrogen receptor-positive breast cancer: a randomized phase II trial (PTEX46) to investigate the optimal duration of preoperative endocrine therapy. Breast 22(3):263–267
Carpenter R, Doughty JC, Cordiner C, Moss N, Gandhi A, Wilson C et al (2014) Optimum duration of neoadjuvant letrozole to permit breast conserving surgery. Breast Cancer Res Treat 144(3):569–576
Dixon JM, Renshaw L, Macaskill EJ, Young O, Murray J, Cameron D et al (2009) Increase in response rate by prolonged treatment with neoadjuvant letrozole. Breast Cancer Res Treat 113(1):145–151
Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A et al (2011) Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype–ACOSOG Z1031. J Clin Oncol 29(17):2342–2349
Fontein DB, Charehbili A, Nortier JW, Meershoek-Klein Kranenbarg E, Kroep JR, Putter H et al (2014) Efficacy of six month neoadjuvant endocrine therapy in postmenopausal, hormone receptor-positive breast cancer patients–a phase II trial. Eur J Cancer 50(13):2190–2200
Karakatsanis A, Tasoulis MK, Wärnberg F, Nilsson G, MacNeill F (2018) Meta-analysis of neoadjuvant therapy and its impact in facilitating breast conservation in operable breast cancer. Br J Surg 105(5):469–481
Research TIoC. POETIC-A: Pre-Operative Endocrine Therapy for Individualised Care with Abemaciclib https://www.icr.ac.uk/our-research/centres-and-collaborations/centres-at-the-icr/clinical-trials-and-statistics-unit/clinical-trials/poetic-a
Registry I (2022) Randomised trial looking at the effectiveness of hormone therapy (neoadjuvant endocrine treatment) in post-menopausal women with early breast cancer. https://doi.org/10.1186/ISRCTN11896599
Acknowledgements
Abbas Imran: Royal Cornwall Hospital Truro. Abdalla SAAD ABDALLA AL-ZAWI: Basildon & Thurrock University Hospital. Abeera Abbas: The Royal Marsden NHS Foundation Trust. Ada Chrysafi: Nobles Hospital Isle of Man. Adam Critchley: Newcastle upon Tyne Hospitals NHS Foundation Trust. Adam Walsh: Musgrove Park Hospital. Ahmed Hamad: University Hospitals of Derby and Burton NHS Foundation Trust. Ajay Sahu: North Bristol NHS Trust. Alex Knight: Luton and Dunstable University Hospital. Alexandra Humphreys: Gloucestershire NHS Foundation Trust. Ali Salih: Basildon & Thurrock University Hospital. Alice Chambers: North Bristol NHS Trust. Alice Moody: North Bristol NHS Trust. Alsarah DIAB: Northern Ireland Medical Dental Training Agency. Amanda Taylor: Milton Keynes University Hospital. Amanda Thorne: Musgrove Park Hospital. Amani Asour: Medway Maritime NHS Foundation Trust. Amit Agrawal: Addenbrookes Hospital. Amit Goyal: University Hospitals of Derby and Burton NHS Foundation Trust. Amtul Carmichael: University Hospitals of Derby and Burton NHS Foundation Trust. Amtul Sami: Lincoln County Hospital. Andreas Larentzakis: Attikon University Hospital, Medical School, University of Athens, Greece. Andrew Pieri: Newcastle upon Tyne Hospitals NHS Foundation Trust. Angeline Tanhueco: Norfolk and Norwich University Hospital. Anita Hargreaves: Countess of Chester NHS Foundation Trust. Anita Sharma: Royal Devon & Exeter Hospital. Anjana Satpathy: Manchester University NHS Foundation Trust. Anna Heeney: Beaumont Hospital, Dublin 9, Ireland. Anna R Hurley: The Royal Marsden NHS Foundation Trust. Anne Tansley: Royal Liverpool University Hospital. Antonio Piñero-Madrona: Hospital Clínico Universitario "Virgen de la Arrixaca"-IMIB. Murcia. Spain. Anu Sandhya: East Surrey Hospital. Anu Shrotri: Aintree University Hospital. Anup Sharma: St George's Hospital. Anushka Chaudhry: Great Western Hosp tal NHS Foundation Trust. Anzors Gvaramadze: United Lincolnshire County Hospitals. Aonghus Ansari: University Hospitals of Leicester. Arjun Kattakayam: Aintree University Hospital. Arnold DK Hill: Beaumont Hospital, Dublin 9, Ireland. Asha Adwani: Oxford University Hospital NHS Foundation Trust. Ashok Chouhan: Brighton and Sussex university hospitals NHS Trust. Ashutosh Kothari: Guy's and St Thomas' NHS foundation trust. Ashvina Segaran: Oxford University Hospital NHS Foundation Trust. Atanu Ray: St. Helens and Knowsley Trust. Bahar Mirshekar-Syahkal: West Suffolk NHS Hospital. Bahaty Riogi: St. Helens and Knowsley Trust. Bashar Zeidan: University Hospital Southampton. Beatrix Elsberger: NHS Grampian. Bernadette Pereira: North Middlesex University Hospital. Brian Hogan: St. James's University Hospital, Leeds. Brian Isgar: Royal Wolverhampton NHS Trust. Carl Podesta: Royal Wolverhampton NHS Trust. Carol-Ann Courtney: University Hospitals of Derby and Burton NHS Foundation Trust. Caroline Mortimer: Ipswich Hospital NHS Trust. Caroline Pogson: Surrey and Sussex NHS Trust. Catherine Krzyzanowska: Taunton and Somerset NHS Foundation Trust. Cátia Felício: Centro Hospitalar Lisboa Norte. Channegowda Navin: St. James's University Hospital, Leeds. Charles Zammit: Brighton and Sussex university hospitals NHS Trust. Charlotte Ives: Royal Devon & Exeter Hospital. Charlotte Kallaway: Royal United Hospital Bath. Cheryl Lobo: University College London Hospital. Chloe Williams: Cardiff and Vale University Health Board. Christiana Laban: Royal United Hospital, Bath. Christopher W.J Cartlidge: NHS Fife. Christopher Wilson: Gartnavel General Hospital. Ciara McGoldrick: Queen's University Belfast. Ciaran Hollywood: Chesterfield Royal Hospital. Claire Louise Rutherford: Gartnavel General Hospital. Claudia Harding-Mackean: Countess of Chester NHS Foundation Trust. Claudiu Simonca: Primrose Breast Care Centre, University Hospitals Plymouth NHS. Colm Power: Beaumont Hospital, Dublin 9, Ireland. D.H.B.Ubayawansa: East and North Hertfordshire. Dalia Elfadl: Chelsea and Westminster NHS foundation trust. David Dodwell: Oxford University Hospital NHS Foundation Trust. David Mark Sibbering: University Hospitals of Derby and Burton NHS Foundation Trust. David Rew: University Hospital Southampton. Deepika Akolekar: Maidstone & Tunbridge Wells NHS Trust. Demetrios Hadjiminas: Imperial College Healthcare NHS Trust. Dennis Remoundos: Oxford University Hospital NHS Foundation Trust. Dheer Singh Rana: Chelsea and Westminster NHS foundation trust. Diana Gonçalves: Centro Hospitalar Universitário de São João—Breast Center. Dibendu Betal: Western Sussex Hospitals NHS Foundation Trust. Dibyesh Banerjee: St George's Hospital. Dinesh Thekkinkattil: Lincoln County Hospital. Disha Mehta: Maidstone & Tunbridge Wells NHS Trust. Donna Egbeare: Cardiff and Vale University Health Board. Dorin Dumitru: Hull University Teaching Hospitals NHS Trust. Douglas Ferguson: Royal Devon & Exeter Hospital. Duraisamy Ravichandran: Luton and Dunstable University Hospital. E Rahman: The Royal Wolverhampton NHS Trust. Edel Quinn: Manchester University NHS Foundation Trust. Edward R C St John: Imperial College Healthcare NHS Trust. Eiman Khalifa: Hull University Teaching Hospitals NHS Trust. El-Rasheed Abdalla: Nobles Hospital, Isle of Man. Elaine Borg: St James University Hospital. Elaine Hyett: Royal Berkshire NHS Foundation Trust. Eleanore J Massey: Gloucestershire NHS Foundation Trust. Eleni Ntakomyti: University College London Hospital. Eleri Davies: Cardiff and Vale University Health Board. Eliana Kalakouti: Chelsea and Westminster NHS Foundation Trust. Elizabeth Clayton: Royal Surrey NHS Foundation Trust. Elizabeth Smyth: NHS Grampian. Ellora Barua: Maidstone & Tunbridge Wells NHS Trust. Emanuele Garreffa: University Hospitals of Derby and Burton NHS Foundation Trust. Emma de Sousa: Royal Liverpool University Hospital. Emma MacInnes: St. James's University Hospital, Leeds. Ennio Agabiti: West Hertfordshire Hospitals NHS trust. Erum Najeeb: Plymouth University Hopsitals NHS trust. Evangelos Mallidis: Ipswich Hospital NHS Trust. Fabio Rapisarda: Western Sussex Hospitals NHS Foundation Trust. Farrokh Pakzad: Royal Surrey NHS Foundation Trust. Fathi Salem: Royal Wolverhampton NHS Trust. Fayyaz Mazari: University Hospital Southampton. Firas Eddin Bachir Alkistawi: Basildon & Thurrock University Hospital. Frances Kenny: University Hospitals of Leicester. Frank Trollman: University Hospitals of Leicester. Gael MacLean: Oxford University Hospital NHS Foundation Trust. Gareth W Irwin: Queen's University Belfast. George Boundouki: Royal Devon & Exeter Hospital. Georgette Oni: Nottingham Breast Institute. Georgios Exarchos: Imperial College Healthcare NHS Trust. Georgios Karagiannidis: Ipswich Hospital NHS Trust. Gerald Gui: The Royal Marsden NHS Foundation Trust. Geraldine Mitchell: Royal Liverpool University Hospital. Gerard Byrne: Manchester University NHS Foundation Trust. Gillian Clayton: Broomfield Breast Unit, Mid and South Essex NHS Foundation Trust. Giulio Cuffolo: Oxford University Hospital NHS Foundation Trust. Giuseppina Mondani: Royal Cornwall Hospital Truro. Gordon Urquhart: NHS Grampian. Hannah Knowles: Maidstone & Tunbridge Wells NHS Trust. Haresh Devalia: Maidstone & Tunbridge Wells NHS Trust. Hazem Khout: Nottingham Breast Institute. Helen Dent: Surrey and Sussex Healthcare. Helen M Sweetland: Cardiff and Vale University Health Board. Helen Mathers: Craigavon Area Hospital. Henrique Mora: Centro Hospitalar Universitário de São João—Breast Center. Henry Cain: Newcastle upon Tyne Hospitals NHS Foundation Trust. Henry Douglas Robb: Imperial College Healthcare NHS Trust. Hiba Fatayer: Manchester University NHS Foundation Trust. Hisham Hamed: Guy's and St Thomas' NHS foundation trust. Hudhaifah Shaker: North Manchester General Hospital. Hussein Tuffaha: Ipswich Hospital NHS Trust. Hyunjin Shin: Croydon University Hospital. Iain Brown: Royal Cornwall Hospital Truro. Ian Daltrey: Highland Breast Centre, Raigmore Hospital. Ian Whitehead: Royal Liverpool University Hospital. Ian Young: NHS Fife. Iman Azmy: Chesterfield Royal Hospital. Imran Abbas: Royal Cornwall Hospital Truro. Inga Peerlink: Ipswich Hospital NHS Trust. Irene Athanasiou: Whittington Health NHS Trust. Irene Osorio-Silla: Hospital Fundación Jiménez Díaz. Madrid. Isabella Dash: North Bristol NHS Trust. James Bentley: Royal Devon & Exeter Hospital. James Cook: North Bristol NHS Trust. James Harvey: Manchester University NHS Foundation Trust. Jamie McIntosh: Royal United Hospital, Bath. Jamie Vatish: Warwick Hospital. Jane Aitken: West Suffolk NHS Hospital. Jane Ooi: Countess of Chester NHS Foundation Trust. Jane Ralph: Newcastle upon Tyne Hospitals NHS Foundation Trust. Jane Turner: Croydon University Hospital. Jarin Louis Noronha: Aintree University Hospital. Jaroslaw Krupa: University Hospitals of Leicester. Jasdeep Gahir: North Middlesex University Hospital. Jasper Gill: Musgrove Park Hospital. Jennifer E Rusby: The Royal Marsden NHS Foundation Trust. Jennifer Isherwood: Royal Berkshire NHS Foundation Trust. Jennifer Long: Royal Glamorgan Hospital, Cwm Taf Morgannwg Health Board. Jennifer Peck: Great Western Hospital NHS Foundation Trust. Jenny Banks: Royal Devon & Exeter Hospital. Jeremy Batt: North Bristol NHS Trust. Jibril Jibril: United Lincolnshire County Hospitals. Jo Mondani: Breast Oncoplastic Surgery Royal Cornwall Hospital Truro. Joanna Franks: University College London Hospital. Joanna Seward: Countess of Chester NHS Foundation Trust. John Benson: Addenbrookes Hospital. John Murphy: Manchester University NHS Foundation Trust. Jonathan D Horsnell: Royal Surrey NHS Foundation Trust. Jonathan Lund: Wirral University Teaching Hospital. Jonida Selmani: Attikon University Hospital, Medical School, University of Athens, Greece. Jose I. Sanchez-Mendez: Hospital Universitario La Paz. Joseph Maalo: West Hertfordshire Hospitals NHS trust. Julia Henderson: Royal Liverpool University Hospital. Julia Massey: Chesterfield Royal Hospital. Julie Doughty: Gartnavel General Hospital. Julie Dunn: Royal Devon & Exeter Hospital. Kalliope Valassiadou : University Hospitals of Leicester. Kamal Pushdary: St Helens & Knowsley Teaching Hospitals NHS Trust. Karen Bosch: North Middlesex University Hospital. Karen James: Wirral University Teaching Hospital. Karina Cox: Maidstone & Tunbridge Wells NHS Trust. Karyn Shenton: Kingston Hospital NHS Foundation Trust. Kat McEvoy: University Hospital Coventry and Warwickshire NHS Trust. Katalin Zechmeister: Norfolk and Norwich University Hospital. Katarina Lakatosova: Kingston Hospital NHS Foundation Trust. Kate E Williams: North Manchester Care Organisation. Katharine Kirkpatrick: Luton and Dunstable University Hospital. Katherine Fairhurst: Royal United Hospital Bath. Katherine Krupa: The Royal Marsden NHS Foundation Trust. Kathryn E Harborough: The Royal Marsden NHS Foundation Trust. Katy Hogben: Imperial College Healthcare NHS Trust. Kelly Lambert: University Hospitals of Leicester. Kelvin Chong: West Hertfordshire Hospitals NHS trust. Khalid Amin: Airedale NHS Foundation Trust. Kristjan Asgeirsson : Nottingham Breast Institute. Kwok-Leung Cheung: University Hospitals of Derby and Burton NHS Foundation Trust. Laszlo Romics: Gartnavel General Hospital. Lee Martin: Aintree University Hospital. Lee-Min Lai: West Hertfordshire Hospitals NHS trust. leena Chagla: St. Helens and Knowsley Trust. Lisa Whisker: Nottingham Breast Institute. Loraine Kalra: Newcastle upon Tyne Hospitals NHS Foundation Trust. Lorna Cook: Western Sussex Hospitals NHS Foundation Trust. Louise Alder: University Hospital Southampton. Louise Merker: Musgrove Park Hospital. Lubna Noor: Croydon University Hospital. Lucie Jones: South Warwickshire NHS Foundation Trust. Lucy R Khan: NHS Forth Valley Royal Hospital. Lydia Prusty: Lincoln County Hospital. Lyndsey Highton: Manchester University NHS Foundation Trust. M Bilal Elahi: Hull University Teaching Hospitals NHS Trust. Maged Hussien: Norfolk and Norwich University Hospital. Mairead Savage: Altnagelvin Area Hospital (Western Health and Social Care Trust). Mairi Fuller: NHS Grampian. Manoj Gowda: University Hospitals of North Midlands. Maria Bramley: North Manchester Care Organisation. Maria Callaghan: Wirral University Teaching Hospital. Maria Vernet-Tomas: Parc de Salut Mar, Barcelona, Spain. Maria Verroiotou: Primrose Breast Care Centre, University Hospitals Plymouth NHS. Marta Jimenez Gomez : Parc de Salut Mar, Barcelona, Spain. Massimiliano Cariati: University College London Hospital. Masuma Sarker: Hull University Teaching Hospitals NHS Trust. Matthew Hague: Royal Surrey NHS Foundation Trust. Matthew Rowland: Royal Liverpool University Hospital. Michael Faheem: Barts Health NHS Trust. Michael J Allen: Beaumont Hospital, Dublin 9, Ireland. Michelle Mullan: North Bristol NHS Trust. Mike Shere: North Bristol NHS Trust. Mina Girgis: West Suffolk NHS Hospital. Mina M G Youssef: Norfolk and Norwich University Hospital. Mini V Sardar: University Hospitals of Leicester. Mohamed Elamass: Basildon & Thurrock University Hospital. Mohamed Elkorety: West Hertfordshire hospitals NHS Trust. Mohamed Lafi: Aintree University Hospital. Mohamed Razick Sait: University Hospital Coventry and Warwickshire NHS Trust. Mohammad Amir Sharif: Manchester University NHS Foundation Trust. Mohammed Absar: Pennine Acute Hospital NHS Trust. Mohsin Dani: Maidstone & Tunbridge Wells NHS Trust. Mona Sulieman: Royal Cornwall Hospital Truro. Monika Kaushik : University Hospitals of Leicester. Muhammad Salman: Nobles Hospital Isle of Man. Musa Barkeji: Chelsea and Westminster NHS foundation trust. Mysore Chandrashekar: Royal Liverpool University Hospital. Nabila Nasir: North Manchester Care Organisation. Nader Touqan: Northern Care Alliance. Nadine Betambeau: St George's Hospital. Nathan Coombs: Great Western Hospital NHS Foundation Trust. Neil Johns: East and North Hertfordshire. Neill Patani: University College London Hospital. Ngee-Ming Goh: University Hospitals Plymouth NHS Trust. Nicholas Gallegos: North Bristol NHS Trust. Nicholas Holford: Royal Berkshire Hospital. Nick Abbott: Highland Breast Centre, Raigmore Hospital. Nicola Barnes: Manchester University NHS Foundation Trust. Nicola Laurence: Royal United Hospital, Bath. Nicola Roche: The Royal Marsden NHS Foundation Trust. Nikitas Dimopoulos: Manchester University NHS Foundation Trust. Nikolaos V Michalopoulos: Attikon University Hospital, Medical School, University of Athens, Greece. Norah Scally: Craigavon Area Hospital. Noyko Stanilov: University College London Hospital. Nur Amalina Che Bakri: Imperial College Healthcare NHS Trust. Oladapo Fafemi: North Middlesex University Hospital. Olubunmi Odofin: Western Sussex Hospitals NHS Foundation Trust. Panagiotis Kokoropoulos: Attikon University Hospital, Medical School, University of Athens, Greece. Pankaj Roy: Oxford University Hospital NHS Foundation Trust. Parto Forouhi: Addenbrookes Hospital. Paul Thiruchelvam: Imperial College Healthcare NHS Trust. Pawel Trapszo: Darent Valley Hospital, Dartford. Penelope McManus: University Hospitals of Morecambe Bay. Peter A Barry : The Royal Marsden NHS Foundation Trust. Peter Liptay-Wagner: East Suffolk and North Essex Foundation Trust. Peter Mallon: Queen's University Belfast. Petros Charalampoudis: University College London Hospital. Philip Drew: Royal Cornwall Hospital Truro. Philip Turton: St. James's University Hospital, Leeds. Pilar Matey: Royal Wolverhampton NHS Trust. Polly King: Royal Cornwall Hospital Truro. Polly Partlett: Royal Surrey NHS Foundation Trust. Primeera Wignarajah: Addenbrookes Hospital. Rachel Ainsworth: North Bristol NHS Trust. Rachel Elizabeth English: Royal Cornwall Hospital Truro. Rachel Foster: Countess of Chester NHS Foundation Trust. Rachel Soulsby: Milton Keynes University Hospital. Rachel Tillett: Royal Devon & Exeter Hospital. Rachel Xue Ning Lee: Nottingham Breast Institute. Radhika Chadha: The Royal Bekshire NHS Trust. Ragheed Al-Mufti: Imperial College Healthcare NHS Trust. Raj Achuthan: St. James's University Hospital, Leeds. Raja Eid: Royal liverpool university hospital. Rajaram Burrah: Wirral University Teaching Hospital. Rajiv Vashisht: Chelsea and Westminster NHS foundation trust. Rajive Nair: Lincoln County Hospital. Ralia Bunza: Chelsea and Westminster NHS foundation trust. Raman Vinayagam: Wirral University Teaching Hospital. Rami Tabbakh: North Manchester Care Organisation. Raouef Ahmed Bichoo: Hull University TeachingHospital NHS Trust. Rathi Rathinaezhil: Brighton and Sussex university hospitals NHS Trust. Rebekah Law: The Royal Marsden NHS Foundation Trust. Reem Salman: Craigavon Area Hospital. Reginald Salvador: Norfolk and Norwich University Hospital. Ricardo Pardo: Hospital Fundación Jiménez Díaz. Madrid. Riccardo Bonomi: Western Sussex Hospitals NHS Foundation Trust. Richard Johnson: Manchester University NHS Foundation Trust. Richard Sutton: Royal United Hospital, Bath. Rishikesh Parmeshwar: University Hospitals of Morecambe Bay. Ritchie Chalmers: Maidstone & Tunbridge Wells NHS Trust. Ritika Rampal: Airedale NHS Foundation Trust. Rob Hardy: Aintree University Hospital. Robert Macmillan: Nottingham Breast Institute. Robert Thomas: Newcastle upon Tyne Hospitals NHS Foundation Trust. Rogelio Andrés-Luna: Centro Hospitalar Lisboa Norte. Rosamond Jacklin: East Suffolk and North Essex Foundation Trust. Rosie Simson: Gloucestershire NHS Foundation Trust. Russell Mullen: Highland Breast Centre, Raigmore Hospital. Ruth James: Luton and Dunstable University Hospital. Ruvinder Athwal: South Warwickshire NHS Foundation Trust. Sa'ed Ramzi: Primrose Breast Care Centre, University Hospitals Plymouth NHS. Sabrina Bezzaa: St George's Hospital. Sadaf Jafferbhoy: University Hospitals of North Midlands. Sam Jeffreys: Royal Glamorgan Hospital, Cwm Taf Morgannwg Health Board. Samantha A Sloan: Queen's University Belfast. Samantha K Williams: Great Western Hospital NHS Foundation Trust. Samir Laali: University Hospital Southampton. Samy Shaheed: Brighton and Sussex university hospitals NHS Trust. Sanjay Joshi: Croydon University Hospital. Sankaran Chandrasekharan: East Suffolk and North Essex Foundation Trust. Sankaran Narayanan: University Hospitals of North Midlands. Santosh Somasundaram: University Hospitals of Morecambe Bay. Sarah Barker: Queen Elizabeth University Hospital, Glasgow. Sarah Horne: Croydon University Hospital. Sascha Dua: Broomfield Breast Unit, Mid and South Essex NHS Foundation Trust. Sasi Govindarajulu: North Bristol NHS Trust. Saung Hnin Phyu: Chelsea and Westminster NHS foundation trust. Sekhar Marla: University Hospitals of North Midlands. Senthurun Mylvaganam: Royal Wolverhampton NHS Trust. Shabbir Poonawala: Wirral University Teaching Hospital. Shamaela Waheed: Surrey and Sussex NHS Trust. Sharat Chopra: Cardiff and Vale University Health Board. Sharon Wallace: Norfolk and Norwich University Hospital. Sheila Shokuhi: University Hospitals of Leicester. Sheila Stallard: Gartnavel General Hospital. Shelley Potter: North Bristol NHS Trust. Sherif Monib: West Hertfordshire Hospitals NHS Trust. Shireen Mckenzie: St. James's University Hospital, Leeds. Simon Harries: South Warwickshire NHS Foundation Trust. Simon Hawkins: Great Western Hospital NHS Foundation Trust. Simon Marsh: East Suffolk and North Essex Foundation Trust. Simon Pain: Norfolk and Norwich University Hospital. Simon Pilgrm : University Hospitals of Leicester. Simon Smith: Broomfield Breast Unit, Mid and South Essex NHS Foundation Trust. Simon Thomson: West Hertfordshire Hospitals NHS trust. Siobhan Rooney : Addenbrookes Hospital. Sisse Olsen: Royal Devon & Exeter Hospital. Soni Soumian: University Hospitals of North Midlands. Sonia Bathla: St. Helens and Knowsley Trust. Stacy Wardle: Western Sussex Hospitals NHS Foundation Trust. Stephanie C Jenkins: Primrose Breast Care Centre, University Hospitals Plymouth NHS. Stephen McCulley: Nottingham Breast Institute. Stuart Robertson: University Hospital Coventry and Warwickshire NHS Trust. Sumit Goyal: Cardiff and Vale University Health Board. Sumohan Chatterjee: Manchester University NHS Foundation Trust. Sunita Saha: East Suffolk and North Essex Foundation Trust (Colchester hospital). Susan Williams-Jones: University Hospitals of Derby and Burton NHS Foundation Trust. Syeda Nadia Shah Gilani: Nottingham Breast Institute. Tamara Kiernan: St. Helens and Knowsley Trust . Tania S de Silva: Surrey and Sussex NHS Trust. Tapan Sircar: Royal Wolverhampton NHS Trust. Tasha Gandamihardja: Broomfield Breast Unit, Mid and South Essex NHS Foundation Trust. Theodoros A Sidiropoulos: Attikon University Hospital, Medical School, University of Athens, Greece. Thomas Stroud: University Hospital Southampton. Tin Aung Sein: Warwick Hospital. Toral Gathani: Oxford University Hospital NHS Foundation Trust. Tracey Irvine: Royal Surrey NHS Foundation Trust. Tuabin Rasheed: Nottingham Breast Institute. Urvashi Jain: Guy's and St Thomas' NHS foundation trust. Usama Suleiman: Surrey and Sussex NHS Trust. Uzma Andaleeb : Medway Maritime NHS Foundation Trust. Vallipuran Gopalan: Primrose Breast Care Centre, University Hospitals Plymouth NHS. Vasileios Sakellariou: University Hospital Southampton. Venla Kantola : St. James's University Hospital, Leeds. Vinod Mathen: Manchester University NHS Foundation Trust. Wail Al Sarakbi: Croydon University Hospital. Walid Sasi: University Hospitals of Leicester. Wendy Sotheran: Western Sussex Hospitals NHS Foundation Trust. William H Allum: The Royal Marsden NHS Foundation Trust. Yasmin Wahedna: University Hospitals of Derby and Burton NHS Foundation Trust. Yazan Masannat: NHS Grampian. Youhana Mikhael: West Hertfordshire hospitals NHS Trust. Yousuf Sabah: Cardiff and Vale University Health Board. Zaid Al-Ishaq: Royal Wolverhampton NHS Trust. Zarghuna Taraki: University College London Hospital. Zenon Rayter: North Bristol NHS Trust. Abigail Tomlins: Gloucestershire NHS Foundation Trust. Alda Correia: Centro Hospitalar Universitário de São João—Breast Center. Amir Sharif: Manchester University NHS Foundation Trust. André Magalhães: Centro Hospitalar Universitário de São João—Breast Center. Anjana Sathpathy: Manchester University NHS Foundation Trust. Antonio Piñero Madrona: Breast Unit. Hospital Clínico Universitario "Virgen de la Arrixaca". Murcia. Spain. Asma Al-Allak: Royal Glamorgan Hospital, Cwm Taf Morgannwg Health Board, Wales. Aurea Manso de Lema: Hospital Universitario La Paz. Bashar Zedian: University Hospital Southampton. Balendra Kumar: West Suffolk Hospital. Brendan Smith: The Royal Bekshire NHS Trust. C Navin: St. James's University Hospital, Leeds. Caroline Richardson: Kingston Hospital NHS Foundation Trust. Chandra Sekharan: East Suffolk and North Essex Foundation Trust (Colchester hospital). Chloe Constantinou: Kingston Hospital NHS Foundation Trust. Chris Wayte: Royal United Hospital Bath. Christina Summerhayes: University Hospital Southampton. Clare Fowler: Gloucestershire NHS Foundation Trust. Claire Murphy: Airedale NHS Foundation Trust. Colin Rogers: University Hospitals of Derby and Burton NHS Foundation Trust. Covadonga Marti Alvarez: Hospital Universitario La Paz. Douglas Macmillan: Nottingham Breast Institute. Eamonn Coveney: West Suffolk Hospital. Eleanor Gutteridge: Nottingham Breast Institute. Eleftheria Kleidi: Cambridge University Hospitals. Elisa York Pineda: Hospital Universitario La Paz. Ellen Copson: University Hospital Southampton. Fernando Osório: Centro Hospitalar Universitário de São João—Breast Center. Fiona Court: Gloucestershire NHS Foundation Trust. Francis Kenny: University Hospitals of Leicester. Gary Osborn: Royal Glamorgan Hospital, Cwm Taf Morgannwg Health Board, Wales. Georgina Yiasoumis: Manchester University NHS Foundation Trust. Gloria Petralia: Whittington Health NHS Trust. Harleen Deol: East and North Hertfordshire. Richard Hunt: Gloucestershire NHS Foundation Trust. John Robertson: University Hospitals of Derby and Burton NHS Foundation Trust. José Luis Fougo: Centro Hospitalar Universitário de São João—Breast Center. Kieran Horgan: St. James's University Hospital, Leeds. Lara Miralles Olivar: Hospital Universitario La Paz. Laura Johnson: Barts Health NHS Trust . Mahwash Baber: Whittington Health NHS Trust. Marcel Segura Badia : Parc de Salut Mar, Barcelona, Spain. MD Zaker Ullah: Barts Health NHS Trust. D Hassanally : Medway Maritime NHS Foundation Trust. Nicola Dunne: The Royal Bekshire NHS Trust. Susie Connolly: The Royal Bekshire NHS Trust. Mohsin El-Gammal: Medway Maritime NHS Foundation Trust. Brendan Skelly: Altnagelvin Area Hospital (Western Health and Social Care Trust). Ibrahim Ahmed : Medway Maritime NHS Foundation Trust. PW Crane: East and North Hertfordshire. Lucy Satherley: Cardiff and Vale University Health Board. Tracey Simoes: University Hospital Southampton. Natarajan Vaithilingam: Barts Health NHS Trust. Nikolaos Arkadopoulos: Attikon University Hospital, Medical School, University of Athens, Greece. Nikolaos Danias: Attikon University Hospital, Medical School, University of Athens, Greece. Nuria Argudo: Parc de Salut Mar, Barcelona, Spain. P Macmanus: University Hospitals of Morecambe Bay. Pantelis Vassiliu: Attikon University Hospital, Medical School, University of Athens, Greece. Pau Nicolau Batalla : Parc de Salut Mar, Barcelona, Spain. Pilar Zamora Auñon: Hospital Universitario La Paz. Ramsey Cutress: University Hospital Southampton. Rachel Tillett: Royal Devon & Exeter Hospital. Sarah B Vestey: Gloucestershire NHS Foundation Trust. Sarah Tang: St George's Hospital. Sergio Salido: Hospital Fundación Jiménez Díaz. Madrid. Shweta Aggarwal: Barts Health NHS Trust. Simon Pilgrim: University Hospitals of Leicester. Susy Costa: Centro Hospitalar Universitário de São João—Breast Center. Zoe Winters: Kingston Hospital NHS Foundation Trust
Author information
Authors and Affiliations
Consortia
Contributions
RVD conceived the study. RVD, BK, RIC, AG and CCK designed the pilot study. RVD, BK, AC, ROC and TR designed and trialed the pilot data collection forms. RVD, BK, AC, ROC, TR, VPT, JJK, EMC, PF, NS, CWJC, KH, SAM, DRL, RV, SP, CH, EC, CEC, RIC, AG, CCK contributed to final study design and finalised data collection forms. AC, RVD, ROC and TR designed the study website, and coordinated collaborator recruitment and provided collaborator support. RVD, BK, KH, PF, NS, CWJC, RV, SP, SAM, DRL, CH, EC, CEC, RIC, AG and CCK provided clinical leadership and promoted unit participation and data collection. VPT, JJK and EMC provided methodological support. RVD, VPT, JJK and EMC drafted the statistical analysis plan and analysed the data. All authors contributed to data interpretation. RVD led the study and wrote the first draft of the paper, with support from BK, JJK, KH, SAM, RIC, AG and CCK. All authors reviewed and critically revised the manuscript and approved it before submission.
Corresponding author
Ethics declarations
Conflict of interest
Baek Kim, Alona Courtney, Rachel O’Connell, Vicky P Taxiarchi, Raghavan Vidya, Jamie J Kirkham, Patricia Fairbrother, Nisha Sharma, Christopher W.J. Cartlidge, Kieran Horgan, Shelley Potter, Ashu Gandhi, Stuart A McIntosh, Elizabeth Camacho, Daniel R Leff and Chris Holcombe have nothing to declare. Rajiv V Dave (RVD) has received honoraria from Roche and support from Endomag. Dr Tim Rattay (TR) is currently an NIHR Clinical Lecturer. Charlotte E Coles (CEC) is supported by the National Institute Health Research Cambridge Biomedical Research Centre. Ramsey I Cutress (RIC) has equipment provided by Seca to analyse body composition to University Hospital Southampton as part of a NIHR model industry collaborative agreement (MiCA). This equipment is used in an Academic Investigator led charity funded study of which Ramsey Cutress is CI. Ellen Copson (EC) declares honoraria from: Roche, Pfizer, Astra-Zeneca, Lilly, Nanostring and expert panel work for World Cancer Research Fund. Cliona C Kirwan (CCK) is Royal College of Surgeons/University of Manchester Professor of Surgical trials funded by a Royal College of Surgeons of England / Masonic Charitable Foundation professorship.
Ethical approval and consent to participate
Ethics approval was not required according to the NHS Health Research Authority online decision tool (www.hra-decisiontools.org.uk/research/).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This is a collaborative authorship, and Pubmed-citable authors are noted below, in alphabetical order in acknowledgement section.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dave, R.V., Elsberger, B., Taxiarchi, V.P. et al. Bridging pre-surgical endocrine therapy for breast cancer during the COVID-19 pandemic: outcomes from the B-MaP-C study. Breast Cancer Res Treat 199, 265–279 (2023). https://doi.org/10.1007/s10549-023-06893-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06893-4