Abstract
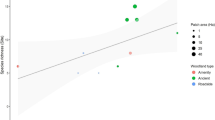
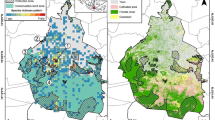
The matrix-tolerance hypothesis suggests that the most abundant species in the inter-habitat matrix would be less vulnerable to their habitat fragmentation. This model was tested with leaf-litter frogs in the Atlantic Forest where the fragmentation process is older and more severe than in the Amazon, where the model was first developed. Frog abundance data from the agricultural matrix, forest fragments and continuous forest localities were used. We found an expected negative correlation between the abundance of frogs in the matrix and their vulnerability to fragmentation, however, results varied with fragment size and species traits. Smaller fragments exhibited stronger matrix-vulnerability correlation than intermediate fragments, while no significant relation was observed for large fragments. Moreover, some species that avoid the matrix were not sensitive to a decrease in the patch size, and the opposite was also true, indicating significant differences with that expected from the model. Most of the species that use the matrix were forest species with aquatic larvae development, but those species do not necessarily respond to fragmentation or fragment size, and thus affect more intensively the strengthen of the expected relationship. Therefore, the main relationship expected by the matrix-tolerance hypothesis was observed in the Atlantic Forest; however we noted that the prediction of this hypothesis can be substantially affected by the size of the fragments, and by species traits. We propose that matrix-tolerance model should be broadened to become a more effective model, including other patch characteristics, particularly fragment size, and individual species traits (e.g., reproductive mode and habitat preference).


Similar content being viewed by others
References
Antongiovanni M, Metzger JP (2005) Influence of the matrix habitats to the occurrence of insectivorous bird species in Amazonian Forest fragments. Biol Conserv 122:441–451
Argôlo AJS (2004) As serpentes dos Cacauais do Sudeste da Bahia. Editora da UESC, Ilhéus
Becker CG, Fonseca CR, Haddad CFB et al (2007) Habitat split and the global decline of amphibians. Science 318:1775–1777
Blaustein AR, Wake D, Sousa WP (1994) Amphibians declines: judging stability, persistence, and susceptibility of populations to local and global extinctions. Conserv Biol 8:60–71
Cook WM, Anderson RM, Schweiger EW (2004) Is the matrix really inhospitable? Vole runway distribution in an experimentally fragmented landscape. OIKOS 104:5–14
Corn PS (1994) Standard techniques for inventory and monitoring—straight-line drift fences and pitfall traps. In: Heyer WR, Donnelly MA, McDiarmid RW et al (eds) Measuring and monitoring biological diversity, standard methods for amphibians. Smithsonian Institution Press, Washington, London, pp 118–124
Daily GC, Ehrlich PR, Sanchez-Azofeifa GA (2001) Countryside biogeography: utilization of human dominated habitats by the avifauna of southern Costa Rica. Ecol Appl 11:1–13
Dixo M (2001) Efeito da fragmentação da floresta sobre a comunidade de sapos e lagartos de serrapilheira no sul da Bahia. MSc dissertation, Universidade de São Paulo, Brazil
Dixo M (2005) Diversidade de sapos e lagartos de serrapilheira numa paisagem fragmentada do Planalto Atlântico de São Paulo. PhD dissertation, Universidade de São Paulo, Brazil
Eterovick PC, Carnaval ACOQ, Borges-Nojosa DM et al (2005) Amphibian declines in Brazil: an overview. Biotropica 32:166–179
Ewers RM, Didham RK (2006) Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev 81:117–142
Faria D, Paciencia MLB, Dixo M et al (2007) Ferns, frogs, lizards, birds and bats in forest fragments and shaded cacao plantations of two contrasting landscapes in the Atlantic forest, Brazil. Biodivers Conserv 16:2335–2357
Gardner TA, Barlow J, Peres CA (2007) Paradox, presumption and pitfalls in conservation biology: the importance of habitat change for amphibians and reptiles. Biol Conserv 138:166–179
Gascon C (1993) Breeding-habitat use by five Amazonian frogs at forest edge. Biodivers Conserv 2:438–444
Gascon C, Lovejoy TE, Bierregaard RO et al (1999) Matrix habitat and species persistence in tropical forest remnants. Biol Conserv 91:223–229
Haddad CFB, Prado CPA (2005) Reproductive modes in frogs and their unexpected diversity in the Atlantic Forest of Brazil. Bioscience 55:207–217
Henle K, Davies KF, Kleyer CM et al (2004) Predictors of species sensitivity to fragmentation. Biodivers Conserv 13:207–251
Hobbs RJ, Saunders DA, Arnold GW (1993) Integrated landscape ecology: a Western-Australian perspective. Biol Conserv 64:231–238
Laurance WF (1991) Ecological correlates of extinction proneness in Australian tropical rain forest mammals. Conserv Biol 5:79–89
Laurance WF, Bierregaard RO Jr (1997) Tropical forest remnants: ecology, management, and conservation of fragmented communities. University Chicago Press, Chicago
Metzger JP (2009) Conservation issues in the Brazilian Atlantic Forest. Biol Conserv 142:1138–1140
Metzger JP, Martensen AC, Dixo M et al (2009) Time-lag in biological responses to landscape changes in a highly dynamic Atlantic forest region. Biol Conserv 142:1166–1177
Mittermeier RA, Meyers N, Gil PR et al (1999) Hotspots: earth’s biologically richest and most endangered terrestrial ecoregions. CEMEX, México city, Mexico
Myers N, Mittermeier RA, Mittermeier GG et al (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Pardini R (2004) Effects of forest fragmentation on small mammals in an Atlantic Forest landscape. Biodivers Conserv 13:2567–2586
Pough FHR, Andrews M, Cadle JE et al (1998) Herpetology. Prentice Hall, Upper Saddle River
Ribeiro MC, Metzger JP, Martensen AC et al (2009) The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biol Conserv 142:1141–1153
Ricketts TH (2001) The matrix matters: effective isolation in fragmented landscapes. Am Nat 158:87–99
Ruckelshaus M, Hartway C, Kareiva P (1997) Assessing the data requirements of spatially explicit dispersal models. Conserv Biol 11:1298–1306
Seabra M (1971) Vargem Grande: organização e transformações de um setor do cinturão-verde paulistano. Instituto de Geografia, Universidade de São Paulo, São Paulo
Silvano DL, Segalla MV (2005) Conservation of Brazilian Amphibians. Conserv Biol 19:653–658
Silvano DL, Colli G, Dixo M (2003) Anfíbios e Répteis. In: Rambaldi D, Oliveira DAS et al (eds) Fragmentação de ecossistemas: causas, efeitos sobre a biodiversidade e recomendações de políticas públicas. MMA/SBF, Brasília, pp 183–200
SOS Mata Atlântica and Instituto Nacional de Pesquisas Espaciais (2008) Atlas dos remanescentes florestais da Mata Atlântica, período de 2000 a 2005. www.sosmatatlantica.org.br
Statsoft Inc (2001) STATISTICA (data analysis software system), version 6. http://www.statsoft.com/
Stevens VM, Polus E, Wesselingh RA et al (2004) Quantifying functional connectivity: experimental evidence for patch-specific resistance in the Natterjack frog (Bufo calamita). Landscape Ecol 19:829–842
Stuart SN, Chanson JS, Cox NA et al (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306:1783–1786
Teixeira AMG, Soares-Filho BS, Freitas SR et al (2009) Modeling landscape dynamics in an Atlantic Rainforest region: Implications for conservation. Forest Ecol Manag 257:1219–1230
Tocher MD, Gascon C, Zimmerman BL (1997) Fragmentation effects on a Central Amazonian frog community: a ten-year study. In: Laurance WF, Bierregaard RO (eds) Tropical forest remnants: ecology, management, and conservation of fragmented communities. University of Chicago Press, Chicago, pp 124–137
Umetsu F, Metzger JP, Pardini R (2008) Importance of estimating matrix quality for modeling species distribution in complex tropical landscapes: a test with Atlantic forest small mammals. Ecography 31:359–370
Urbina-Cardona JN, Olivares-Perez M, Reynoso VH (2006) Herpetofauna diversity and microenvironment correlates across a pasture-edge-interior ecotone in tropical Rainforest fragments in the Los Tuxtlas Biosphere Reserve of Veracruz, Mexico. Biol Conserv 132:61–75
Vallan D (2000) Influence of forest fragmentation on amphibian diversity in the nature reserve of Ambohitantely, highland Madagascar. Biol Conserv 96:31–43
Wake D (1998) Action on amphibians. Trends Ecol Evol 13:363–379
Wiens JA (1995) Landscapes mosaics and ecological theory. In: Hansson L, Fahrig L, Merriam G (eds) Mosaic landscape and ecological process. Chaptam and Hall, London, pp 1–16
Acknowledgments
We appreciate the cooperation during all the steps of this study of members of the project “Biodiversity conservation in fragmented landscapes at the Atlantic Plateau of São Paulo”. We thank Vanessa K. Verdade for helping identifying the frogs, José Mario Ghellere for his help in the field work, Guarino R. Colli, Marcio Martins, Ricardo J. Sawaya, Ana Carolina O. Q. Carnaval, and Jaime Bertoluci, for helpful comments in a previous version of the manuscript. We are thankful to SABESP, which support our work in the Morro Grande Reserve and also all private owners that authorized the use of their fragments for our research. Financial support was provided by FAPESP—Fundação de Amparo à Pesquisa do Estado de São Paulo (project n. 99/05123-4; PhD fellowship for MD n. 01/07916-3) and Fundação O Boticário de Proteção à Natureza (0564_20022).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dixo, M., Metzger, J.P. The matrix-tolerance hypothesis: an empirical test with frogs in the Atlantic Forest. Biodivers Conserv 19, 3059–3071 (2010). https://doi.org/10.1007/s10531-010-9878-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-010-9878-x