Abstract
Nocardia species are ubiquitous in the environment with an increasing number of species isolated from clinical sources. From 2005 to 2009, eight isolates (W9042, W9247, W9290, W9319, W9846, W9851T, W9865, and W9908) were obtained from eight patients from three states in the United States and Canada; all were from males ranging in age from 47 to 81 years old; and all were obtained from finger (n = 5) or leg (n = 3) wounds. Isolates were characterized by polyphasic analysis using molecular, phenotypic, morphologic and chemotaxonomic methods. Sequence analysis of 16S rRNA gene sequences showed the eight isolates are 100 % identical to each other and belong in the genus Nocardia. The nearest phylogenetically related neighbours were found to be the type strains for Nocardia altamirensis (99.33 % sequence similarity), Nocardia brasiliensis (99.37 %), Nocardia iowensis (98.95 %) and Nocardia tenerifensis (98.44 %). The G+C content of isolate W9851T was determined to be 68.4 mol %. The DNA–DNA relatedness between strain W9851T and the N. brasiliensis type strain was 72.8 % and 65.8 % when measured in the laboratory and in silico from genome sequences, respectively, and 95.6 % ANI. Whole-cell peptidoglycan was found to contain meso-diaminopimelic acid; MK-8-(H4)ω-cyc was identified as the major menaquinone; the major fatty acids were identified as C16:0, 10 Me C18:0, and C18:1 w9c, the predominant phospholipids were found to include diphosphatidylglycerol, phosphatidylethanolamine, phosphatidylinositol and phosphatidylinositol mannosides; whole-cell sugars detected were arabinose and galactose; and mycolic acids ranging from 38 to 60 carbon atoms were found to be present. These chemotaxonomic analyses are consistent with assignment of the isolates to the genus Nocardia. Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectra of the clinical isolates showed genus and species level profiles that were different from other Nocardia species. All isolates were resistant to ciprofloxacin, clarithromycin and imipenem but were susceptible to amikacin, amoxicillin/clavulanate, linezolid and trimethoprim/sulfamethoxazole. The results of our polyphasic analysis suggest the new isolates obtained from wound infections represent a novel species within the genus Nocardia, for which the name Nocardia vulneris sp. nov. is proposed, with strain W9851T (= DSM 45737T = CCUG 62683T = NBRC 108936T) as the type strain.


Similar content being viewed by others
References
Baba T, Nishiuchi Y, Yano I (1997) Composition of mycolic acid molecular species as criterion in nocardial classification. Int J Syst Bacteriol 47:795–801
Berd D (1973) Laboratory identification of clinically important aerobic actinomycetes. Appl Microbiol 25:665–681
Cashion P, Hodler-Franklin MA, McCully J, Franklin M (1977) A rapid method for base ratio determination of bacterial DNA. Anal Biochem 81:461–466
Clinical and Laboratory Standards Institute (2011) Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standards M24-A. Clinical Laboratory Standards Institute, Wayne
Conville PS, Witebsky FG (2007) Nocardia, Rhodococcus, Gordonia, Actinomadura, Streptomyces, and other aerobic actinomycetes. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Phaller MA (eds) Manual of clinical microbiology, 9th edn. American Society for Microbiology, Washington, pp 515–542
Conville PS, Brown JM, Steigerwalt AG, Brown-Elliott BA, Witebsky FG (2008) Nocardia wallacei sp. nov., and Nocardia blaklockiae sp. nov., human pathogens and members of the “Nocardia transvalensis complex”. J Clin Microbiol 46:1178–1184
Corti ME, Fioti MFV (2003) Nocardiosis: a review. Int J Infect Dis 4:243–250
De Ley J, Cattoir H, Reynaerts A (1970) The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem 12:133–142
Felsenstein J (1981) Evolutionary trees from DNA sequences: maximum-likelihood approach. J Mol Evol 17:368–376
Felsenstein J (2004) Inferring Phylogenies, 1st edn. Sinauer Associates, Sutherland
Goodfellow M, Maldonado LA (2012) Genus I Nocardia Trevisan 1889. In: Goodfellow M, Kämpfer P, Busse H-J, Trujillo ME, Suzuki K-I, Ludwig W, Whitman WB (eds) Bergey’s manual of systematic bacteriology, the Actinobacteria, 2nd edn. Springer, New York, pp 376–419
Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM (2007) DNA–DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol 57:81–91
Grimont PAD (1988) Use of DNA reassociation in bacterial classification. Can J Microbiol 34:541–546
Huss VAR, Festl H, Schleifer KH (1983) Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst Appl Microbiol 4:184–192
Kämpfer P, Buczolitis S, Jäckel U, Grün-Wollny I, Busee H-J (2004) Nocardia tenerifensis sp. nov. Int J Syst Evol Microbiol 54:381–383
Kim M, Oh HS, Park SC, Chun J (2014) Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol 64:346–351
Klatte S, Kroppenstedt RM, Rainey FA (1994) Rhodococcus opacus sp. nov., an unusual nutritionally versatile Rhodococcus species. Syst Appl Microbiol 17:355–360
Kluge AG, Farris FS (1969) Quantitative phyletics and the evolution of anurans. Syst Zool 18:1–32
Kroppenstedt RM (1982) Separation of bacterial menaquinones by HPLC using reverse phase (RP18) and a silver loaded ion exchanger as stationary phases. J Liq Chromatogr 5:2359–2367
Kroppenstedt RM (1985) Fatty acid and menaquinone analysis of actinomycetes and related organisms. In: Goodfellow M, Minnikin DE (eds) Chemical methods in bacterial systematics. Academic Press, London, pp 173–199
Lasker BA, Moser BD, Brown JM (2011) Gordonia. In: Liu D (ed) Molecular detection of human bacterial pathogens. CRC Taylor & Francis Group, Boca Raton, pp 95–110
Lechevalier MP, Lechevalier HA (1970) Chemical composition as a criterion in the classification of aerobic actinomycetes. Int J Syst Bacteriol 20:435–443
Meier-Kolthoff JP, Auch AF, Klenk H-P, Göker M (2013a) Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 14:60–73
Meier-Kolthoff JP, Göke M, Spröer C, Klenk H-P (2013b) When should a DDH experiment be mandatory in microbial taxonomy? Arch Microbiol 195:413–418
Mesbah M, Premachandran U, Whitman WB (1989) Precise measurement of the G + C content of deoxyribonucleic acid by high-performance liquid chromatography. Int J Syst Bacteriol 39:159–167
Minnikin DE, O’Donnell AG, Goodfellow G, Alderson G, Athalye M, Schaal A, Parlett JH (1984) An integrated procedure for the extraction of bacterial isoprenoid quinones and polar lipids. J Microbiol Methods 2:233–241
Rhuland LE, Work E, Denman RF, Hoare DS (1955) The behavior of the isomers of α, ε-diaminopimelic acid on paper chromatograms. J Am Chem Soc 77:4844–4846
Saitou N, Nei M (1987) The neighbor-joining method: a new method for constructing phylogenetic trees. Mol Biol Evol 4:406–425
Sasser M (1990) Identification of bacteria by gas chromatography of cellular fatty acids. USFCC Newsl 20:16
Sneath PHA (1989) Analysis and interpretation of sequence data for bacterial systematics: the view of a numerical taxonomist. System Appl Microbiol 12:15–31
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Töth EM, Schumann P, Borsodi AK, Kéki Z, Kovács AL, Márialigeti K (2008) Wohlfahrtiimonas chitiniclastica gen. nov., a new gammaproteobacterium isolated from Wohlfahrtia magnifica (Diptera: scarcophagidae). Intl J Syst Evol Microbiol 58:976–981
Trevisan V (1889) I Generi e le Specie Delle Batteriacee. Zanaboni and Gabuzzi, Milano
Vandamme P, Pot B, Gillis M, de Vos P, Kersters K, Swings J (1996) Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbial Rev 60:407–438
Vera-Cabrera L, Ortiz-Lopez R, Elizondo-Gonzalez R, Ocampo-Candianu J (2013) Complete genome sequence analysis of Nocardia brasiliensis HUJEG-1 reveals a saprobic lifestyle and the genes needed for human pathogenesis. PLoS One 8:1–8
Wallace RJ Jr, Steele LC, Sumter G, Smith JM (1988) Antimicrobial susceptibility patterns of Nocardia asteroides. Antimicrob Agents Chemother 32:1776–1779
Wayne LG, Brenner DJ, Colwell RR, Grimont PAD, Kandle O, Krichevsky MI, Moore LH, Moore WEC, Murray RGE, Stackebrandt E, Starr MP, Trüper HG (1987) Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int J Syst Bacteriol 37:463–464
Weyant RS, Moss CW, Weaver RE, Hollis DG, Jordan JG, Cook EC, Daneshvar MI (1996) Identification of unusual pathogenic Gram-negative and facultatively anaerobic bacteria, 2nd edn. Williams and Wilkins, Baltimore
Yamaguchi H, Sekimoto E, Shirakami A, Shibata H, Ozaki S, Shigekiyo T, Noda T, Shikji T, Kanda K, Hirose T, Matsuzawa T, Gonoi T (2013) Testicular nocardiosis accompanied by cutaneous lesions in an immunocompetent man. Intern Med 52:129–133
Yassin AF, Rainey FA, Brzezinka H, Burghardt J, Lee HJ, Schaal KP (1995) Tsukamurella inchonensis sp. nov. Int J Syst Bacteriol 45:522–527
Acknowledgments
We thank Jean Euzéby for nomenclatural advice and Gabi Pötter (DSMZ) for help in chemotaxonomic analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10482_2014_226_MOESM1_ESM.pptx
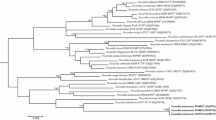
Supplementary material 1 Fig. S1 Neighbour-joining phylogenetic tree of aligned 16S rRNA gene sequences showing the position of the eight clinical isolates to validly named type strains within the genus Nocardia. Bootstrap percentages are shown based on 1,000 replications; only values ≥50 are shown. The tree was rooted using the sequence of Mycobacteriun tuberculosis ATCC 27294T as the outgroup. Bar, 0.005 substitutions per nucleotide position (PPTX 78 kb)
Rights and permissions
About this article
Cite this article
Lasker, B.A., Bell, M., Klenk, HP. et al. Nocardia vulneris sp. nov., isolated from wounds of human patients in North America. Antonie van Leeuwenhoek 106, 543–553 (2014). https://doi.org/10.1007/s10482-014-0226-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-014-0226-0