Abstract
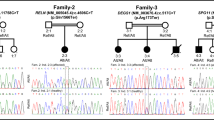
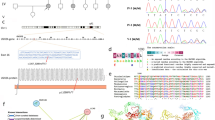
Autism is a childhood neurodevelopmental disorder with high heterogeneity. Following our genome-wide associated loci with autism, we performed sequencing analysis of the coding regions, UTR and flanking splice junctions of AMPD1 in 830 Chinese autism individuals as well as 514 unrelated normal controls. Fourteen novel variants in the coding sequence were identified, including 11 missense variants and 3 synonymous mutations. Among these missense variants, 10 variants were absent in 514 control subjects, and conservative and functional prediction was carried out. Mitochondria activity and lactate dehydrogenase assay were performed in 5 patients’ lymphoblast cell lines; p.P572S and p.S626C showed decreased mitochondrial complex I activity, and p.S626C increased lactate dehydrogenase release in medium. Conclusively, our data suggested that mutational variants in AMPD1 contribute to autism risk in Han Chinese population, uncovering the contribution of mutant protein to disease development that operates via mitochondria dysfunction and cell necrosis.
Similar content being viewed by others
References
Waterhouse L, Morris R, Allen D, Dunn M, Fein D, Feinstein C, Rapin I, Wing L (1996) Diagnosis and classification in autism. J Autism Dev Disord 26(1):59–86
Blumberg SBM, Kogan MD, Schieve LA, Jones, JR. (2013) Changes in prevalence of parent-reported autism spectrum disorder in school-aged U.S. Children: 2007 to 2011–2012. National Health Statistics Reports 65
Piven J, Palmer P, Jacobi D, Childress D, Arndt S (1997) Broader autism phenotype: evidence from a family history study of multiple-incidence autism families. Am J Psychiatry 154(2):185–190
Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M (1995) Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med 25(1):63–77
Walsh CA, Morrow EM, Rubenstein JL (2008) Autism and brain development. Cell 135(3):396–400. doi:10.1016/j.cell.2008.10.015
Kelleher RJ 3rd, Bear MF (2008) The autistic neuron: troubled translation? Cell 135(3):401–406. doi:10.1016/j.cell.2008.10.017
Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J (2007) Mapping early brain development in autism. Neuron 56(2):399–413. doi:10.1016/j.neuron.2007.10.016
Page T (2000) Metabolic approaches to the treatment of autism spectrum disorders. J Autism Dev Disord 30(5):463–469
Anitha A, Nakamura K, Thanseem I, Matsuzaki H, Miyachi T, Tsujii M, Iwata Y, Suzuki K, Sugiyama T, Mori N (2013) Downregulation of the expression of mitochondrial electron transport complex genes in autism brains. Brain Pathol 23(3):294–302. doi:10.1111/bpa.12002
Giulivi C, Zhang YF, Omanska-Klusek A, Ross-Inta C, Wong S, Hertz-Picciotto I, Tassone F, Pessah IN (2010) Mitochondrial dysfunction in autism. JAMA 304(21):2389–2396. doi:10.1001/jama.2010.1706
Xia K, Guo H, Hu Z, Xun G, Zuo L, Peng Y, Wang K, He Y, Xiong Z, Sun L, Pan Q, Long Z, Zou X, Li X, Li W, Xu X, Lu L, Liu Y, Hu Y, Tian D, Long L, Ou J, Zhang L, Pan Y, Chen J, Peng H, Liu Q, Luo X, Su W, Wu L, Liang D, Dai H, Yan X, Feng Y, Tang B, Li J, Miedzybrodzka Z, Xia J, Zhang Z, Zhang X, StClair D, Zhao J, Zhang F (2013) Common genetic variants on 1p13.2 associate with risk of autism. Mol Psychiatry. doi:10.1038/mp.2013.146
Auranen M, Nieminen T, Majuri S, Vanhala R, Peltonen L, Jarvela I (2000) Analysis of autism susceptibility gene loci on chromosomes 1p, 4p, 6q, 7q, 13q, 15q, 16p, 17q, 19q and 22q in Finnish multiplex families. Mol Psychiatry 5(3):320–322
Risch N, Spiker D, Lotspeich L, Nouri N, Hinds D, Hallmayer J, Kalaydjieva L, McCague P, Dimiceli S, Pitts T, Nguyen L, Yang J, Harper C, Thorpe D, Vermeer S, Young H, Hebert J, Lin A, Ferguson J, Chiotti C, Wiese-Slater S, Rogers T, Salmon B, Nicholas P, Petersen PB, Pingree C, McMahon W, Wong DL, Cavalli-Sforza LL, Kraemer HC, Myers RM (1999) A genomic screen of autism: evidence for a multilocus etiology. Am J Hum Genet 65(2):493–507. doi:10.1086/302497
Allen-Brady K, Miller J, Matsunami N, Stevens J, Block H, Farley M, Krasny L, Pingree C, Lainhart J, Leppert M, McMahon WM, Coon H (2009) A high-density SNP genome-wide linkage scan in a large autism extended pedigree. Mol Psychiatry 14(6):590–600. doi:10.1038/mp.2008.14
Van den Berghe G, Bontemps F, Vincent MF, Van den Bergh F (1992) The purine nucleotide cycle and its molecular defects. Prog Neurobiol 39(5):547–561
Dale O, Salo M (1996) The Helsinki declaration, research guidelines and regulations: present and future editorial aspects. Acta Anaesthesiol Scand 40(7):771–772
Anderson MA, Gusella JF (1984) Use of cyclosporin A in establishing Epstein-Barr virus-transformed human lymphoblastoid cell lines. In Vitro 20(11):856–858
Bernier FP, Boneh A, Dennett X, Chow CW, Cleary MA, Thorburn DR (2002) Diagnostic criteria for respiratory chain disorders in adults and children. Neurology 59(9):1406–1411
Chan FK, Moriwaki K, De Rosa MJ (2013) Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol Biol 979:65–70. doi:10.1007/978-1-62703-290-2_7
Gross M, Morisaki H, Morisaki T, Holmes EW (1994) Identification of functional domains in AMPD1 by mutational analysis. Biochem Biophys Res Commun 205(2):1010–1017. doi:10.1006/bbrc.1994.2767
Kroemer G, Dallaporta B, Resche-Rigon M (1998) The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol 60:619–642. doi:10.1146/annurev.physiol.60.1.619
Sabina RL, Swain JL, Patten BM, Ashizawa T, O’Brien WE, Holmes EW (1980) Disruption of the purine nucleotide cycle. A potential explanation for muscle dysfunction in myoadenylate deaminase deficiency. J Clin Invest 66(6):1419–1423. doi:10.1172/JCI109995
Spiegel EK, Colman RF, Patterson D (2006) Adenylosuccinate lyase deficiency. Mol Genet Metab 89(1–2):19–31. doi:10.1016/j.ymgme.2006.04.018
Persico AM, Militerni R, Bravaccio C, Schneider C, Melmed R, Trillo S, Montecchi F, Palermo MT, Pascucci T, Puglisi-Allegra S, Reichelt KL, Conciatori M, Baldi A, Keller F (2000) Adenosine deaminase alleles and autistic disorder: case–control and family-based association studies. Am J Med Genet 96(6):784–790. doi:10.1002/1096-8628(20001204)96:6<784:AID-AJMG18>3.0.CO;2-7
Akizu N, Cantagrel V, Schroth J, Cai N, Vaux K, McCloskey D, Naviaux RK, Van Vleet J, Fenstermaker AG, Silhavy JL, Scheliga JS, Toyama K, Morisaki H, Sonmez FM, Celep F, Oraby A, Zaki MS, Al-Baradie R, Faqeih EA, Saleh MA, Spencer E, Rosti RO, Scott E, Nickerson E, Gabriel S, Morisaki T, Holmes EW, Gleeson JG (2013) AMPD2 regulates GTP synthesis and is mutated in a potentially treatable neurodegenerative brainstem disorder. Cell 154(3):505–517. doi:10.1016/j.cell.2013.07.005
Yang J, Weimer RM, Kallop D, Olsen O, Wu Z, Renier N, Uryu K, Tessier-Lavigne M (2013) Regulation of axon degeneration after injury and in development by the endogenous calpain inhibitor calpastatin. Neuron. doi:10.1016/j.neuron.2013.08.034
Glynn P (2000) Neural development and neurodegeneration: two faces of neuropathy target esterase. Prog Neurobiol 61(1):61–74
Wong M (2013) Mammalian target of rapamycin (mTOR) pathways in neurological diseases. Biomed J 36(2):40–50. doi:10.4103/2319-4170.110365
White JF (2003) Intestinal pathophysiology in autism. Exp Biol Med (Maywood) 228(6):639–649
Depino AM (2013) Peripheral and central inflammation in autism spectrum disorders. Mol Cell Neurosci 53:69–76. doi:10.1016/j.mcn.2012.10.003
Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa R, Mostofsky SH (2006) Motor signs distinguish children with high functioning autism and Asperger’s syndrome from controls. J Autism Dev Disord 36(5):613–621. doi:10.1007/s10803-006-0109-y
Al Abdulmohsen T, Kruger TH (2011) The contribution of muscular and auditory pathologies to the symptomatology of autism. Med Hypotheses 77(6):1038–1047. doi:10.1016/j.mehy.2011.08.044
Acknowledgments
We would like to thank our patients and their family for participating in the study, and thank Prof. Jia da Li and Fengyu Zhang for providing comments that improve manuscript. We also thank laboratory colleagues for the help and advices during the experiment. This work was supported by National Basic Research Program of China (2012CB517902), National Natural Science Foundation of China (81330027, 81161120544) and National Key Technology R&D Program of China (2012BAI03B02).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, L., Ou, J., Xu, X. et al. AMPD1 functional variants associated with autism in Han Chinese population. Eur Arch Psychiatry Clin Neurosci 265, 511–517 (2015). https://doi.org/10.1007/s00406-014-0524-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-014-0524-6