Abstract
Purpose
Cell-based therapy is a potential treatment option for neurointestinal diseases by serving as a source of neural progenitor cells to replace missing or abnormal enteric neurons. Using an ex vivo transplantation model, we recently demonstrated that treatment with collagenase and fibronectin promotes infiltration of transplanted enteric neural crest cells (ENCCs) toward the colon lumen. The aim of this study was to determine whether this new method also promotes colonization of transplanted ENCCs in vivo.
Methods
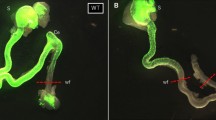
Collagenase was applied locally on the anti-mesenteric area of the recipient colon using filter paper, followed by fibronectin. Neurospheres were generated from ENCCs isolated from fetal mouse intestines and transplanted into the collagenase and fibronectin-treated colon. Engraftment of neurospheres was confirmed by immunofluorescence.
Results
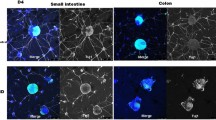
Neurospheres transplanted onto PBS- or fibronectin-treated colons were not observed to infiltrate to the muscle layer. However, when used in combination with type I collagenase and fibronectin in the recipient colon, transplanted neurospheres reached Auerbach’s plexus.
Conclusion
We demonstrated that transplanted neurospheres grow into Auerbach’s plexus in the recipient colon pretreated with collagenase and fibronectin.



Similar content being viewed by others
References
Amiel J, Sproat-Emison E, Garcia-Barcelo M, Lantieri F, Burzynski G, Borrego S, Pelet A, Arnold S, Miao X, Griseri P, Brooks AS, Antinolo G, de Pontual L, Clement-Ziza M, Munnich A, Kashuk C, West K, Wong KK, Lyonnet S, Chakravarti A, Tam PK, Ceccherini I, Hofstra RM, Fernandez R, Hirschsprung Disease C (2008) Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet 45(1):1–14
Goyal RK, Hirano I (1996) The enteric nervous system. N Engl J Med 334(17):1106–1115
Di Lorenzo C, Solzi GF, Flores AF, Schwankovsky L, Hyman PE (2000) Colonic motility after surgery for Hirschsprung’s disease. Am J Gastroenterol 95(7):1759–1764
Dasgupta R, Langer JC (2008) Evaluation and management of persistent problems after surgery for Hirschsprung disease in a child. J Pediatr Gastroenterol Nutr 46(1):13–19
Langer JC (2004) Persistent obstructive symptoms after surgery for Hirschsprung’s disease: development of a diagnostic and therapeutic algorithm. J Pediatr Surg 39(10):1458–1462
Polley TZ Jr, Coran AG, Wesley JR (1985) A ten-year experience with ninety-two cases of Hirschsprung’s disease. Including sixty-seven consecutive endorectal pull-through procedures. Ann Surg 202(3):349–355
Levitt MA, Martin CA, Olesevich M, Bauer CL, Jackson LE, Pena A (2009) Hirschsprung disease and fecal incontinence: diagnostic and management strategies. J Pediatr Surg 44(1):271–277 (discussion 277)
Stamp LA (2017) Cell therapy for GI motility disorders: comparison of cell sources and proposed steps for treating Hirschsprung disease. Am J Physiol Gastrointest Liver Physiol 312(4):G348–G354
Burns AJ, Goldstein AM, Newgreen DF, Stamp L, Schafer KH, Metzger M, Hotta R, Young HM, Andrews PW, Thapar N, Belkind-Gerson J, Bondurand N, Bornstein JC, Chan WY, Cheah K, Gershon MD, Heuckeroth RO, Hofstra RM, Just L, Kapur RP, King SK, McCann CJ, Nagy N, Ngan E, Obermayr F, Pachnis V, Pasricha PJ, Sham MH, Tam P, VandenBerghe P (2016) White paper on guidelines concerning enteric nervous system stem cell therapy for enteric neuropathies. Dev Biol 417(2):229–251
Burns AJ, Thapar N (2014) Neural stem cell therapies for enteric nervous system disorders. Nat Rev Gastroenterol Hepatol 11(5):317–328
Fattahi F, Steinbeck JA, Kriks S, Tchieu J, Zimmer B, Kishinevsky S, Zeltner N, Mica Y, El-Nachef W, Zhao H, de Stanchina E, Gershon MD, Grikscheit TC, Chen S, Studer L (2016) Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature 531(7592):105–109
Pan W, Goldstein AM, Hotta R (2021) Opportunities for novel diagnostic and cell-based therapies for Hirschsprung disease. J Pediatr Surg 57(9):61–68
Druckenbrod NR, Epstein ML (2009) Age-dependent changes in the gut environment restrict the invasion of the hindgut by enteric neural progenitors. Development 136(18):3195–3203
Hotta R, Anderson RB, Kobayashi K, Newgreen DF, Young HM (2010) Effects of tissue age, presence of neurones and endothelin-3 on the ability of enteric neurone precursors to colonize recipient gut: implications for cell-based therapies. Neurogastroenterol Motility 22(3):331-e386
Nishida S, Yoshizaki H, Yasui Y, Kuwahara T, Kiyokawa E, Kohno M (2018) Collagen VI suppresses fibronectin-induced enteric neural crest cell migration by downregulation of focal adhesion proteins. Biochem Biophys Res Commun 495(1):1461–1467
Wagner JP, Sullins VF, Dunn JC (2014) Transplanted skin-derived precursor stem cells generate enteric ganglion-like structures in vivo. J Pediatr Surg 49(8):1319–1324
Zhang L, Zhao B, Liu W, Ma R, Wu R, Gao Y (2017) Cotransplantation of neuroepithelial stem cells with interstitial cells of Cajal improves neuronal differentiation in a rat aganglionic model. J Pediatr Surg 52(7):1188–1195
Fujiwara N, Miyahara K, Nakazawa-Tanaka N, Akazawa C, Yamataka A (2022) In vitro investigation of the differentiation of enteric neural crest-derived cells following transplantation of aganglionic gut in a mouse model. Pediatr Surg Int 38(5):755–759
Yasui Y, Yoshizaki H, Kuwahara T, Nishida S, Kohno M, Okajima H (2022) Transplanted neural crest cells migrate toward Auerbach’s plexus layer instead of the colon surface in recipient colon pretreated with collagenase and fibronectin. Biochem Biophys Res Commun 601:116–122
Nishiyama C, Uesaka T, Manabe T, Yonekura Y, Nagasawa T, Newgreen DF, Young HM, Enomoto H (2012) Trans-mesenteric neural crest cells are the principal source of the colonic enteric nervous system. Nat Neurosci 15(9):1211–1218
Nagashimada M, Ohta H, Li C, Nakao K, Uesaka T, Brunet JF, Amiel J, Trochet D, Wakayama T, Enomoto H (2012) Autonomic neurocristopathy-associated mutations in PHOX2B dysregulate Sox10 expression. J Clin Investig 122(9):3145–3158
Morrison SJ, White PM, Zock C, Anderson DJ (1999) Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell 96(5):737–749
Schlieve CR, Fowler KL, Thornton M, Huang S, Hajjali I, Hou X, Grubbs B, Spence JR, Grikscheit TC (2017) Neural crest cell implantation restores enteric nervous system function and alters the gastrointestinal transcriptome in human tissue-engineered small intestine. Stem Cell Reports 9(3):883–896
Cheng LS, Hotta R, Graham HK, Belkind-Gerson J, Nagy N, Goldstein AM (2017) Postnatal human enteric neuronal progenitors can migrate, differentiate, and proliferate in embryonic and postnatal aganglionic gut environments. Pediatr Res 81(5):838–846
Nakazawa N, Miyahara K, Okawada M, Yamataka A, Suzuki R, Akazawa C, Tomikawa-Ichikawa N, Arikawa-Hirasawa E (2013) Laminin-1 promotes enteric nervous system development in mouse embryo. Pediatr Surg Int 29(11):1205–1208
Acknowledgements
We thank Dr. Hideki Enomoto (Kobe University) for provision of Ednrb-hKikGR mice and Dr. Mamoru Tanida (Kanazawa Medical University) for helpful suggestions. We also thank Mitchell Arico from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. This work was supported in part by Innovation in Cell Therapy in the Hokuriku District of Japan through the Private University Branding Project of MEXT (RP2017-11), Collaborative Research from Kanazawa Medical University (C2016-3 and C2017-1), and JSPS KAKENHI (19K22669 and 22K06050).
Author information
Authors and Affiliations
Contributions
T.K., Y.Y. and H.Y. designed the studies and wrote the manuscript. T.K., Y.Y. and M.M. designed experiments, conducted in vivo studies, and analyzed data. H.Y., M.K. and H.O. supervised the project and edited the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuwahara, T., Yasui, Y., Yoshizaki, H. et al. Recipient colon preoperative treatment with type I collagenase and fibronectin promotes the growth of transplanted enteric neural crest cells into Auerbach’s plexus. Pediatr Surg Int 38, 1793–1798 (2022). https://doi.org/10.1007/s00383-022-05224-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-022-05224-w