Abstract
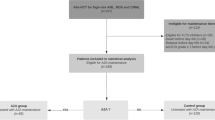
This 3+3 dose-escalation phase I multicenter study investigated the optimal dose of azacitidine (AZA) for post-hematopoietic stem cell transplantation (HSCT) maintenance, which remains unknown in Japan. Recipients of a first HSCT for high-risk myelodysplastic syndromes (MDS, n = 12) or acute myeloid leukemia (AML) with antecedent MDS (n = 3) received post-HSCT AZA maintenance in 2015–2019. The optimal AZA dose was defined as the dose at which 50–70% of patients can complete four cycles without dose-limiting toxicity (DLT). The initial dose level 1 was set as 30 mg/m2 for 5 days per 28-day cycle, and dose levels 0, 2, and 3 were set as 20, 40, and 50 mg/m2. DLT was defined as any grade 3 non-hematological or grade 4 hematological toxicity. The 15 evaluable patients were 55 (37–64) years old. The median observation of the post-HSCT survivors was 935 (493–1915) days. The median number of days post-HSCT to the start of AZA was 101 (59–176). In the first, second, and third cohorts, five of nine patients completed four cycles at dose level 1. In the final cohort, five of six additional patients completed at the same dose. In total, 10 (67%) patients tolerated AZA 30 mg/m2, which was determined as optimal. DLT occurred in five cases: grade 3 hepatotoxicity, pneumonia, enterocolitis, and grade 4 thrombocytopenia and neutropenia. The 2-year overall survival and disease-free survival rates post-HSCT were 77.0% and 73.3%. Post-HSCT AZA maintenance was well-tolerated and merits further evaluation for patients with MDS or AML with antecedent MDS. Trial registration: UMIN000018791



Similar content being viewed by others
References
Konuma T, Shimomura Y, Ozawa Y, Ueda Y, Uchida N, Onizuka M, Akiyama M, Mori T, Nakamae H, Ohno Y, Shiratori S, Onishi Y, Kanda Y, Fukuda T, Atsuta Y, Ishiyama K (2019) Induction chemotherapy followed by allogeneic HCT versus upfront allogeneic HCT for advanced myelodysplastic syndrome: a propensity score matched analysis. Hematol Oncol 37:85–95
Warlick ED, Cioc A, Defor T, Dolan M, Weisdorf D (2009) Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: importance of pretransplant disease burden. Biol Blood Marrow Transplant 15:30–38
Mielcarek M, Storer BE, Flowers ME, Storb R, Sandmaier BM, Martin PJ (2007) Outcomes among patients with recurrent high-risk hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 13:1160–1168
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz G, List A, Gore SD, Seymour JF, Bennett JM, Byrd J, Backstrom J, Zimmerman L, McKenzie D, Beach C, Silverman LR (2009) Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 10:223–232
de Lima M, Giralt S, Thall PF, de Padua SL, Jones RB, Komanduri K, Braun TM, Nguyen HQ, Champlin R, Garcia-Manero G (2010) Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer 116:5420–5431
Ali N, Tomlinson B, Metheny L, Goldstein SC, Fu P, Cao S, Caimi P, Patel RD, Varela JC, Andrade L, Balls JW, Baer L, Smith M, Smith T, Nelson M, de Lima M, Mori S (2020) Conditioning regimen intensity and low-dose azacitidine maintenance after allogeneic hematopoietic cell transplantation for acute myeloid leukemia. Leuk Lymphoma 61:2839–2849
Craddock C, Jilani N, Siddique S, Yap C, Khan J, Nagra S, Ward J, Ferguson P, Hazlewood P, Buka R, Vyas P, Goodyear O, Tholouli E, Crawley C, Russell N, Byrne J, Malladi R, Snowden J, Dennis M (2016) Tolerability and clinical activity of post-transplantation azacitidine in patients allografted for acute myeloid leukemia treated on the RICAZA trial. Biol Blood Marrow Transplant 22:385–390
Goodyear OC, Dennis M, Jilani NY, Loke J, Siddique S, Ryan G, Nunnick J, Khanum R, Raghavan M, Cook M, Snowden JA, Griffiths M, Russell N, Yin J, Crawley C, Cook G, Vyas P, Moss P, Malladi R, Craddock CF (2012) Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood 119:3361–3369
Woo J, Deeg HJ, Storer B, Yeung C, Fang M, Mielcarek M, Scott BL (2017) Factors determining responses to azacitidine in patients with myelodysplastic syndromes and acute myeloid leukemia with early post-transplantation relapse: a prospective trial. Biol Blood Marrow Transplant 23:176–179
Schroeder T, Czibere A, Platzbecker U, Bug G, Uharek L, Luft T, Giagounidis A, Zohren F, Bruns I, Wolschke C, Rieger K, Fenk R, Germing U, Haas R, Kröger N, Kobbe G (2013) Azacitidine and donor lymphocyte infusions as first salvage therapy for relapse of AML or MDS after allogeneic stem cell transplantation. Leukemia 27:1229–1235
Schroeder T, Rachlis E, Bug G, Stelljes M, Klein S, Steckel NK, Wolf D, Ringhoffer M, Czibere A, Nachtkamp K, Dienst A, Kondakci M, Stadler M, Platzbecker U, Uharek L, Luft T, Fenk R, Germing U, Bornhäuser M, Kröger N, Beelen DW, Haas R, Kobbe G (2015) Treatment of acute myeloid leukemia or myelodysplastic syndrome relapse after allogeneic stem cell transplantation with azacitidine and donor lymphocyte infusions – a retrospective multicenter analysis from the German Cooperative Transplant Study Group. Biol Blood Marrow Transplant 21:653–660
Oran B, de Lima M, Garcia-Manero G, Thall PF, Lin R, Popat U, Alousi AM, Hosing C, Giralt S, Rondon G, Woodworth G, Champlin RE (2020) A phase 3 randomized study of 5-azacitidine maintenance vs observation after transplant in high-risk AML and MDS patients. Blood Adv 4:5580–5588
Bewersdorf JP, Allen C, Mirza AS, Grimshaw AA, Giri S, Podoltsev NA, Gowda L, Cho C, Tallman MS, Zeidan AM, Stahl M (2021) Hypomethylating agents and FLT3 inhibitors as maintenance treatment for acute myeloid leukemia and myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation – a systematic review and meta-analysis. Transplant Cell Ther 27:997.e1-997.e11
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J (1997) International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 89:2079–2088
Storer BE (1989) Design and analysis of phase I clinical trials. Biometrics 45:925–937
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED (1995) 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 15:825–828
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, Cowen EW, Dinndorf P, Farrell A, Hartzman R, Henslee-Downey J, Jacobsohn D, McDonald G, Mittleman B, Rizzo JD, Robinson M, Schubert M, Schultz K, Shulman H, Turner M, Vogelsang G, Flowers ME (2005) National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. diagnosis and staging working group report. Biol Blood Marrow Transplant 11:945–956
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Breems DA, Van Putten WL, De Greef GE, Van Zelderen-Bhola SL, Gerssen-Schoorl KB, Mellink CH, Nieuwint A, Jotterand M, Hagemeijer A, Beverloo HB, Löwenberg B (2008) Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol 26:4791–4797
Choi J, Ritchey J, Prior JL, Holt M, Shannon WD, Deych E, Piwnica-Worms DR, DiPersio JF (2010) In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood 116:129–139
Sánchez-Abarca LI, Gutierrez-Cosio S, Santamaría C, Caballero-Velazquez T, Blanco B, Herrero-Sánchez C, García JL, Carrancio S, Hernández-Campo P, González FJ, Flores T, Ciudad L, Ballestar E, Del Cañizo C, San Miguel JF, Pérez-Simon JA (2010) Immunomodulatory effect of 5-azacytidine (5-azaC): potential role in the transplantation setting. Blood 115:107–121
Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH et al (2015) International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 126:291–299
Seymour JF, Döhner H, Butrym A, Wierzbowska A, Selleslag D, Jang JH, Kumar R, Cavenagh J, Schuh AC, Candoni A, Récher C, Sandhu I, Del Castillo TB, Al-Ali HK, Falantes J, Stone RM, Minden MD, Weaver J, Songer S, Beach CL, Dombret H (2017) Azacitidine improves clinical outcomes in older patients with acute myeloid leukaemia with myelodysplasia-related changes compared with conventional care regimens. BMC Cancer 17:852
Liu W, Zhou Z, Chen L, Wang X (2021) Comparison of azacitidine and decitabine in myelodysplastic syndromes and acute myeloid leukemia: a network meta-analysis. Clin Lymphoma Myeloma Leuk 21:e530–e544
Lindsley RC, Mar BG, Mazzola E, Grauman PV, Shareef S, Allen SL, Pigneux A, Wetzler M, Stuart RK, Erba HP, Damon LE, Powell BL, Lindeman N, Steensma DP, Wadleigh M, DeAngelo DJ, Neuberg D, Stone RM, Ebert BL (2015) Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 125:1367–1376
Walter MJ, Shen D, Shao J, Ding L, White BS, Kandoth C et al (2013) Clonal diversity of recurrently mutated genes in myelodysplastic syndromes. Leukemia 27:1275–1282
Wei AH, Döhner H, Pocock C, Montesinos P, Afanasyev B, Dombret H et al (2020) Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med 383:2526–2537
de Lima M, Oran B, Champlin RE, Papadopoulos EB, Giralt SA, Scott BL, William BM, Hetzer J, Laille E, Hubbell B, Skikne BS, Craddock C (2018) CC-486 maintenance after stem cell transplantation in patients with acute myeloid leukemia or myelodysplastic syndromes. Biol Blood Marrow Transplant 24:2017–2024
Bernard E, Nannya Y, Hasserjian RP, Devlin SM, Tuechler H, Medina-Martinez JS et al (2020) Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat Med 26:1549–1556
Lindsley RC, Saber W, Mar BG, Redd R, Wang T, Haagenson MD, Grauman PV, Hu ZH, Spellman SR, Lee SJ, Verneris MR, Hsu K, Fleischhauer K, Cutler C, Antin JH, Neuberg D, Ebert BL (2017) Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med 376:536–547
Cluzeau T, Sebert M, Rahmé R, Cuzzubbo S, Lehmann-Che J, Madelaine I et al (2021) Eprenetapopt plus azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia: a phase II study by the Groupe Francophone des Myélodysplasies (GFM). J Clin Oncol 39:1575–1583
Sallman DA, DeZern AE, Garcia-Manero G, Steensma DP, Roboz GJ, Sekeres MA et al (2021) Eprenetapopt (APR-246) and azacitidine in TP53-mutant myelodysplastic syndromes. J Clin Oncol 39:1584–1594
Acknowledgements
We thank all members of the KSGCT study and the staff of the data center of the KSGCT. We also thank Dr. Gaku Oshikawa for the preparation of the initial study protocol.
Author information
Authors and Affiliations
Contributions
YN, T. Tachibana, YK, TI, and AY designed the study. YN and SM analyzed data. YN, T. Tachibana, YT, KT, YA, T. Toya, AI, MT, ES, RA, MO, TK, ND, and KO performed patient care. YN wrote the manuscript. All of the authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
The protocol of this study was approved by the Institutional Review Board of each participating center. All procedures involving human participants were conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Conflict of interest
YN reports speakers’ bureau fees from Pfizer outside the submitted work. T. Tachibana reports speakers’ bureau fees from Otsuka Pharmaceutical, Novartis Pharma, Pfizer, BMS, Daiichi Sankyo, and Amgen outside the submitted work. ES reports speakers’ bureau fees and/or grants from BMS, Pfizer, Novartis Pharma, and Celgene and grants from Kyowa Kirin, Chugai Pharmaceutical, and Ono Pharmaceutical outside the submitted work. SM reports speakers’ bureau fees and/or grants from Boehringer Ingelheim, AstraZeneca, BMS, Chugai Pharmaceutical, Eli Lilly and Company, Ono Pharmaceutical, Pfizer, and Taiho Pharmaceutical outside the submitted work. SO reports speakers’ bureau fees and/or grants from Astellas Pharma, Eisai, Otsuka Pharmaceutical, Ono Pharmaceutical, Kyowa Kirin, Sanofi, Daiichi Sankyo, Takeda Pharmaceutical, Chugai Pharmaceutical, Novartis Pharma, Mochida Pharmaceutical, JCR Pharma, Pfizer Nihon Shinyaku, and BMS and grants from Asahi Kasei Pharma, Shionogi, Dainihon Sumitomo, and Teijin Pharma outside the submitted work. YK reports speakers’ bureau fees and/or grants from Astellas Pharmaceuticals, Dainihon Sumitomo, Chugai Pharmaceutical, Eisai, MSD, Pfizer, Novartis Pharma, Otsuka Pharmaceutical, and Janssen Pharmaceutical and grants from Celgene, Kyowa Kirin, Ono Pharmaceutical, Takeda Pharmaceutical, and Shionogi outside the submitted work. The other authors declare no competing financial interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• This first prospective study evaluating post-transplant azacitidine (AZA) maintenance in Japanese allo-HSCT recipients revealed that four cycles of AZA 30 mg/m2 for 5 days are feasible.
• For the recipients with high-risk MDS or AML with a prior MDS history, post-transplant AZA maintenance produced a 73% disease-free survival rate at 2 years post-HSCT, Providing the basis for further clinical trials.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Najima, Y., Tachibana, T., Takeda, Y. et al. Dose-finding trial of azacitidine as post-transplant maintenance for high-risk MDS: a KSGCT prospective study. Ann Hematol 101, 2719–2729 (2022). https://doi.org/10.1007/s00277-022-04981-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-022-04981-x