Manuscript accepted on :12-10-2021
Published online on: 19-10-2021
Plagiarism Check: Yes
Reviewed by: Dr. Raina Arogya Trust

Second Review by: Dr. Salman Ahmed

Final Approval by: Dr. Francesca Gorini
Vijaya Chandra Reddy Konda* , Thulasi Gokul
, Thulasi Gokul , M Poojitha
, M Poojitha and K Umamaheswara Rao
and K Umamaheswara Rao
Department of Pharmacology, Sri Venkateswara Institute of Medical Sciences – Sri Padmavathi Medical College for Women, Tirupati, India, 517501
Corresponding Author E-mail: vijayachandrareddy@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2312
Abstract
Introduction: The pandemic due to Coronavirus disease 2019 (COVID-19) is a major health issue resulting in mortality across the globe. Multiple newer medications are being tried in treatment but with minimal success. The development of vaccines is occurring with unprecedented speed using different platforms with collaborations of academia and the pharmaceutical industries globally. These vaccines are approved for emergency use authorization by health authorities based on limited data from clinical trials. Hence, there is a need for active surveillance of vaccine recipients to monitor for safety. Methods: Adverse drug reaction monitoring centre (AMC) of our institute is actively involved in surveillance of recipients for adverse events following immunization (AEFI) who received vaccines for COVID-19 from the vaccination centre of the institute. As per the guidelines of National Coordinating Centre (NCC), Pharmacovigilance Programme of India (PvPI), designated staff of AMC follows up vaccine recipients over their registered mobile number post-vaccination for AEFI if any. This is a descriptive study of all the AEFI reported to NCC, PvPI between 16th January 2021 and 31st March 2021. Results: Of the 5793 doses of vaccination administered during the study period, 59.4% (3443) responded to follow up and 8.6% (299 of 3443) recipients reported 509 AEFI. The most common reported AEFI include fever, generalized body pains, and headache constituting 36.1%, 30.5%, and 18.5% respectively. 64.2% (327 of 509) of AEFI were reported from people younger than 45 years of age. Though females constituted 53.8% (161 of 299) of people who reported AEFI, total number of events reported from this group was 58% (295 of 509). More number of AEFI were observed after first dose of COVISHIELD compared to second dose. Conclusion: COVID-19 vaccination drive is rolled out in multiple phases for different age groups across the country. Many vaccinations are being approved for use in general public with limited data from clinical trials. Active surveillance of COVID-19 vaccines for AEFI helps us in further understanding safety issues beyond the clinical trial environment.
Keywords
Adverse Events Following Immunization; COVID-19; COVID-19 Vaccination; COVISHIELD; COVAXIN
Download this article as:| Copy the following to cite this article: Konda V. C. R, Gokul T, Poojitha M, Rao K. U. Adverse Events Following Immunization to Covid-19 Vaccines in A Tertiary Care Hospital – A Descriptive Study. Biomed Pharmacol J 2021;14(4) |
| Copy the following to cite this URL: Konda V. C. R, Gokul T, Poojitha M, Rao K. U. Adverse Events Following Immunization to Covid-19 Vaccines in A Tertiary Care Hospital – A Descriptive Study. Biomed Pharmacol J 2021;14(4). Available from: https://bit.ly/2Z4KKKB |
Introduction
Coronavirus disease 2019 (COVID-19) is a pandemic, caused by a novel coronavirus, Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). As of 12th May 2021, globally more than 159 million confirmed cases and over 3.3 million deaths were reported, with more than 23 million confirmed cases and over 2.5 million reports of deaths from India and counting 1. Many drugs are being tried for use, but with minimal benefit on the outcomes in patients with COVID-19. Development of safe and effective vaccines is critical to end the COVID-19 pandemic. World Health Organization (WHO) is working closely in collaboration with global partners in the development and manufacturing of COVID-19 vaccines, while maintaining high safety standards 2.
Various technologies and platforms that include viral-vectored, protein subunit, nucleic acid (DNA, RNA), and live attenuated and inactivated vaccines are investigated globally to develop vaccines for COVID-19. As of 11th May 2021, number of vaccines in clinical development are 99 and those in pre-clinical development are 184 [3]. COVID-19 vaccines are approved globally under emergency use authorization in order to respond quickly and effectively to the ongoing pandemic. Most of these approvals are based on interim analysis of the data of recipients followed up for a period of two to three months after administration of all the doses of vaccination. There is an immense need for continuous monitoring of use of COVID-19 vaccines in general public.
The first phase of COVID-19 vaccination drive began in India from 16th January 2021 initially targeting the frontline workers, followed by second phase for people more than 60 years and for those older than 45 years with any of the 20 comorbidities identified by the Ministry of Health and Family Welfare and later extended to people even without any comorbidities 4. Currently, on 01st May 2021, government of India rolled out the third phase of COVID-19 vaccination drive for those above the age of 18. However, this process is delayed in many states due to shortage of vaccines 5.
ChAd0x1 nCoV- 19 Corona Virus Vaccine, COVISHIELD, is a recombinant, replication-deficient chimpanzee adenovirus vector encoding the SARS-CoV-2 Spike (S), produced in genetically modified human embryonic kidney (HEK) 293 cells 6. India’s indigenous COVID-19 vaccine, COVAXIN, is developed by Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR) – National Institute of Virology (NIV), is a whole virion inactivated corona virus vaccine produced using a vero cell-based platform, and is approved by Central Drug Standard Control Organization (CDSCO) on 2nd January 2021 for emergency use authorization 7. The third vaccine that got approval in India under emergency use authorization is the Russia’s Sputnik V COVID-19 vaccine on 12th April 2021 8.
Since the start of vaccination drive, Adverse Drug Reaction Monitoring Centre (AMC) of our institute, as per the guidelines of National Coordination Centre (NCC), Pharmacovigilance Programme of India (PvPI), is actively following up recipients for Adverse Events Following Immunization (AEFI) in COVID-19 vaccine recipients. Very little is known about the safety of these vaccines intended to be used in general public beyond the clinical trial environment. As far as our knowledge goes, there are no studies published to date on AEFI to COVID-19 vaccines in India. The present study aimed at describing various AEFI reports for COVID-19 vaccines reported from our institute.
Material and methods
This is a descriptive study of all the AEFI reported by personnel of AMC of this institute as part of active surveillance of COVID-19 vaccine recipients who were vaccinated at the vaccination centre of the institute between 16th January 2021 to 31st March 2021. As per the guidelines provided to AMC by NCC, PVPI, vaccine recipients were actively followed up by contacting them over their registered mobile number for AEFI if any atleast 24 hours after the vaccination. If there was no response from the recipient when contacted for the first time, they were also contacted for the second time on the subsequent day. All the information obtained from vaccine recipients with adverse events were noted in suspected adverse drug reaction notification form for nonserious events9 and in AEFI form for serious events 10. Reported event was considered serious if it has resulted in death, congenital-anomaly, life threatening, disability, hospitalization or other medically important condition. All these reports were submitted to NCC, PvPI in a timely manner by entering into VigiFlow, a web-based individual case safety report management system, provided by NCC, PvPI to all AMCs 11.
This study was started after obtaining approval from the Institutional Ethics Committee. All the AEFI reported by AMC personnel by active follow up of vaccine recipients in the specified forms during the study period were included in the study. Reported forms lacking any of the details like recipient age, gender, type of vaccine, dose of vaccine (first or second), adverse events, seriousness of the events, and reporter details were not included in this study. Information on comorbidities like history of diabetes, hypertension, cardiac disease, stroke, epilepsy, bronchial asthma, smoking, alcohol and medications used if any for treatment of the reported adverse events were also collected from recipients when contacted over mobile phone. Causality assessment was done as per the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) causality assessment scale. Data were expressed as numbers and percentage using Microsoft Excel 2016.
Results
This study primarily focussed on describing the various adverse events following COVID-19 vaccination at our institute. During the study period, there was administration of 5793 vaccinations in the COVID immunization centre of the institute. Among them, 3814, 1439 & 540 vaccines recipients received 1st dose of COVISHIELD, 2nd dose of COVISHIELD & 1st dose of COVAXIN respectively. Of the total vaccine recipients, we were able to actively follow-up 59.4% (3443 out of 5793). There were 509 AEFI reported from 299 vaccine recipients that accounts to 8.7% (299 out of 3443) of people who reported AEFI on active follow up. During the study period none of the vaccine recipients have received 2nd dose of COVAXIN. Details of type of vaccinations administered and the number of people responded to active follow up during the above specified study period are given in figure 1.
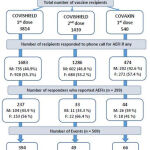 |
Figure 1: Details of type of vaccination administered in our institute during the study period. |
Of the 299 people reported with adverse events, 94 (31.5%) recipients were between the ages of 18 to 30 years who reported 178 (34.9%) adverse events following immunization. Among other age groups, 82 (27.4%) recipients between 31 to 45 years of age reported 149 (29.3%) events. Further details on age wise distribution of AEFI are shown in table 1.
Table 1: Age wise distribution of number of AEFIs
|
Age group |
Number of recipients reported AEFIs
n (%) |
AEFIs
n (%) |
| 18-30 | 94 (31.5) | 178 (34.9) |
| 31-45 | 82 (27.4) | 149 (29.3) |
| 46-60 | 54 (18) | 88 (17.3) |
| 61-75 | 67 (22.4) | 91 (17.9) |
| >75 | 2 (0.7) | 3 (0.6) |
| Total | 299 | 509 |
Of the 3443 who responded for follow up, 1559 (45.3%) were males and the remaining 1884 (54.7%) were females. Among 299 people who reported AEFI, 138 (46.2%) were male who reported 214 (42%) AEFI and 161 (53.8%) were female who reported 295 (58%) AEFI during the study period as shown in the figure 2.
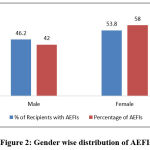 |
Figure 2: Gender wise distribution of AEFIs. |
Among 509 AEFIs reported during the study period, 184 (36.1%), 155 (30.5%) and 94 (18.5%) events were due to fever, generalized body pains and headache respectively. Of all the events, 394 (77.4%), 49 (9.6%) and 66 (13%) were reported after 1st dose of COVISHIELD, 2nd dose of COVISHIELD and 1st dose of COVAXIN respectively. Details of all the AEFIs for various doses of vaccinations are shown in table 2.
Table 2: AEFI after 1st & 2nd dose of COVISHIELD and 1st dose of COVAXIN
|
S.No |
AEFI |
1st Dose COVISHIELD | 2nd Dose COVISHIELD | 1st Dose COVAXIN | Total AEFI | ||||
| n | % | n | % | n | % | n | % | ||
| 1 | Fever | 142 | 27.9 | 15 | 2.9 | 27 | 5.3 | 184 | 36.1 |
| 2 | Generalized body pains | 123 | 24.2 | 21 | 4.1 | 11 | 2.2 | 155 | 30.5 |
| 3 | Headache | 68 | 13.4 | 11 | 2.2 | 15 | 2.9 | 94 | 18.5 |
| 4 | Chills | 17 | 3.3 | 2 | 0.4 | 0 | 0.0 | 19 | 3.7 |
| 5 | Dizziness | 14 | 2.8 | 0 | 0.0 | 5 | 1.0 | 19 | 3.7 |
| 6 | Cough | 8 | 1.6 | 0 | 0.0 | 0 | 0.0 | 8 | 1.6 |
| 7 | Chest pain | 4 | 0.8 | 0 | 0.0 | 0 | 0.0 | 4 | 0.8 |
| 8 | Itching | 3 | 0.6 | 0 | 0.0 | 0 | 0.0 | 3 | 0.6 |
| 9 | Burning sensation of eye | 3 | 0.6 | 0 | 0.0 | 0 | 0.0 | 3 | 0.6 |
| 10 | Nausea | 1 | 0.2 | 0 | 0.0 | 2 | 0.4 | 3 | 0.6 |
| 11 | Sore throat | 1 | 0.2 | 0 | 0.0 | 2 | 0.4 | 3 | 0.6 |
| 12 | Drowsiness | 1 | 0.2 | 0 | 0.0 | 2 | 0.4 | 3 | 0.6 |
| 13 | Diarrhoea | 1 | 0.2 | 0 | 0.0 | 1 | 0.2 | 2 | 0.4 |
| 14 | Running nose | 1 | 0.2 | 0 | 0.0 | 1 | 0.2 | 2 | 0.4 |
| 15 | Heart burn | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| 16 | Joint pains | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| 17 | Dehydration | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| 18 | Rashes | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| 19 | Sleeplessness | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| 20 | Breathing difficulty | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| 21 | BP changes | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| Total | 394 | 77.4 | 49 | 9.6 | 66 | 13.0 | 509 | 100.0 | |
Of the 299 people who reported AEFI, 102 (34.1%) have history of comorbid conditions like diabetes, hypertension, cardiovascular diseases, stroke, epilepsy, bronchial asthma, history of surgery, smoking and alcohol intake. All the reported events occurred within 24 hours of vaccination. All these events were considered as nonserious. Causality assessment done as per WHO-UMC scale showed that all the reported events were probably related to vaccination. All the events were reported with reasonable time duration, unlikely attributable any other diseases and all events were resolved within 2-3 days after the onset of event.
Among people who reported AEFI, 113 (37.8%) have used medications like paracetamol as and when necessary. There is no information on the dose and duration of paracetamol, and on other medications used.
Discussion
This is a descriptive study of reported AEFI submitted by AMC of the institute to NCC, PvPI, India. In the present study there were 509 AEFI reported from 299 vaccine recipients.
During the study period of 16th January 2021 to 31st March 2021, the total number of vaccination doses administered all over India were around 15 million and the reported AEFI constituted 0.015% 12. In the present study, 8.7% (299 of 3443 who responded to active follow up) reported AEFI. This large difference in reporting of AEFI from our institute compared to national statistics could be due to limitation in active follow up of all vaccine recipients at the national level. Even at the institute level, we were unable to actively follow up 40.6% (2350 out of 5793) of vaccine recipients. Various reasons for lack of follow-up are mobile number switched off, not reachable, not responding or the number provided does not exist or a wrong number. In this context, even the chances of human errors while collecting data related to mobile numbers at the registration centre cannot be ruled out.
Considering the age of the vaccine recipients, in the present study a relatively large number of AEFI were reported in people less than 45 years of age. There were 58.9% (176 of 299) of vaccine recipients, who reported 64.2 % (327 of 509) of AEFI in the age group of 18 to 45 years. Similar findings were observed in the interim analysis of clinical trials data for COVISHIELD that showed adverse reactions were generally milder and reported less frequently in older adults ≥ 65 years 6. Polack F.P. et al. observed similar findings in the study on safety and efficacy of BNT162b2 mRNA COVID-19 vaccine, which showed a relatively larger people in the age group of 16 to 55 years reporting more local reactions (like pain at injection site, redness & swelling) and systemic events (like fatigue, fever, headache, muscle pain, chills & joint pains) compared to people aged more than 55 years [13]. However, based on the findings in the present study it cannot be concluded that AEFI will be observed more in young than the aged people. One possible factor to be considered is, at the start of nation-wide vaccination program for COVID-19, the initial phase was targeted at frontline workers across the country and then followed by for people more than 45 years of age. At our institute, most of the frontline workers were vaccinated during the study period and at the same time, their relative responses to follow up might also be more resulting in projection of more number of events being reported in younger age groups. In contrast, data from COWIN during the study period showed that more vaccine recipients were from the age group of 46 to 60 years, followed by the age groups of more than 60 years, 31 to 45 years and lastly 18 to 30 years group. Well-designed prospective studies in this aspect are needed to confirm this finding of the present study.
Response for follow up post vaccination was more in females compared to males (54.7% vs 45.3%). In proportion to the number of females (53.8% vs 46.2%) who reported AEFI, the number of events reported in this group was higher compared to males (58% vs 42%). This indicates that number of AEFI are reported more in females. In the study by Kadali et al. to study side effects of BNT162b2 mRNA COVID-19 vaccine in healthcare workers after vaccination, it was observed that responses to online questionnaire was higher in females (86.55%) compared to males 14. Interim analysis of pooled data from four clinical trials conducted in the United Kingdom, Brazil and South Africa that aimed at studying the safety and efficacy of ChAdOx1 nCoV-19 vaccine (COVISHIELD) also showed more females participants (55.8% vs 44.2%) 15.
In the study by Polack et al., it was observed that there was slight male predominance in the trial compared to female (50.6 vs 49.4%) [13]. There is no mention of data related to gender differences if any in occurrence of AEFI in any of the studies mentioned above. Future research aimed at studying these aspects of gender differences in distribution of AEFI are needed.
Comorbid conditions like diabetes, hypertension, cardiovascular diseases, stroke, epilepsy, asthma, history of surgery, smoking and alcohol intake were present in 34.1 % of people who reported AEFI in the present study. Though there was enrolment of participants with various comorbid conditions listed above into various COVID-19 vaccine trials, none of them reported any safety data specifically in those participants. Future studies in this direction helps in further understanding the differences in AEFI if any among people with comorbid conditions.
All the events reported in the present study are nonserious and occurred within 24 hours of administration of vaccine. In the present study, almost all the people were reporting pain at injection site. It was felt by AMC of the institute that it is common to have pain at intramuscular injection site by any medication for a day or two, so it was decided not to report any events related to pain at injection site for the first 2 days post vaccination. In view of this there were no events reported by this institute related to pain at injection site to NCC, PvPI during the study period. Fever was the predominant AEFI followed by generalized body pains and headache that contributed to 36.1%, 30.5% & 18.5% respectively of the total number reported in the present study. All the reported events resolved within 2-3 days of their appearance. As per the interim analysis data, the most frequently reported adverse reactions to COVISHIELD were injection site tenderness (>60%); injection site pain, headache, fatigue (>50%); myalgia, malaise (>40%); pyrexia, chills (>30%); and arthralgia, nausea (>20%) 6. As per the data of phase 2 trial published, pain at the injection site, followed by headache, fatigue, and fever were the common adverse events reported to COVAXIN 16. In the study by Polack et al. major adverse events reported were pain at injection site (74.5%), fatigue (47.8%), headache (39.5%), muscle pains (25.3%), chills (19.5%), joint pains (15.3%) and fever (4%) [13]. Similar type of adverse events were reported in about 50% of vaccine recipients after MODERNA vaccine 17. The most common adverse events following Sputnik V were flu like illness in 15.2% and local reaction in 5.4% of the participants in the vaccine group 18.
Of the total AEFI reported for COVISHIELD (n=443) in the present study, 88.9% were reported after the first dose and the remaining 11.1% were reported after the second dose. Similar observations of more number of AEFI after first dose were noted from interim analysis of clinical trials for COVISHIELD [6, 15]. The study did not depict any data on AEFI for 2nd dose of COVAXIN as the duration for administration of 2nd dose was well beyond this study period. However, as per the results of phase 2 published by Ella et al., there was no association between the dose of vaccine and the number of adverse events reported [16]. In contrast, based on the data from clinical trials involving mRNA based vaccines, it was observed that more number AEFI were reported following second dose compared to that reported after first dose 17, 18.
All the vaccines for COVID-19 that are presently administered got emergency use authorization with the objective to curb the ongoing pandemic. Though their efficacy and safety are established based on the interim analysis of trial data published based on the follow up of few months after the second dose for various vaccines, outcomes on long-term follow up are yet to be known. As for any new vaccine/drug released into market, much is unknown on how these behave when used in the general public beyond the clinical trial environment. Additionally identification of new mutant strains of COVID-19 that are more infective than those used to develop vaccines is of concern on the efficacy and safety of vaccines used now. Hence, active surveillance studies on AEFI caused by vaccines help us in further understanding the safety in larger populations beyond clinical trials.
The present study has several limitations. Being a descriptive study of the reported AEFI based on active follow-up of the vaccine recipients by calling over their registered mobile number, there is limitation in the availability of some data like weight, height, body mass index, data on severity of the reported event, various medications used in the treatment of those events. Contacting all the vaccine recipients over their registered mobile number was challenging due to reasons explained above. Local reaction like pain at injection site were not considered to be reported by AMC of this institute. Most of the events reported were observed in the early post-vaccination period only that is within 3 – 5 days. Any AEFI occurred beyond the follow up of AMC personnel might have been missed from being reported. Though the events were reported post-vaccination, some of the events may be due to pre-existing chronic medical problems and its role in the reported event could not be completely ruled out as the reported events were based purely on recipient’s response. The exact time lag between the administration of vaccination and onset of adverse events could not be assessed. Most of these limitations of the present study can be overcome by prospective studies conducted in multiple centres with active follow up for few months after complete vaccination.
Conclusion
In this study, the commonly reported AEFI are fever, generalized body pains and headache. All the reported AEFI were nonserious. The number of AEFI after first dose of COVISHIELD were more compared to that of second dose. There is limited data available on AEFI to approved COVID-19 vaccines in India beyond the clinical trials. Hence, further long term follow up studies are required to understand the safety of these vaccines in multiple age groups and with comorbidities.
Acknowledgement:
The authors would acknowledge Professor Dr. K. R. Subash for his continuous encouragement and valuable inputs. The authors are grateful to Indian Pharmacopeia Commission, NCC, PvPI, for their support to AMC of our institute. We would like to thank the Pharm D interns for their support in following up of vaccine recipients
Conflict of Interest
Authors do not have any conflict of interest.
Funding Source
This study received no external funding.
References
- World Health Organization. WHO Coronavirus (COVID-19) dashboard. Available from https://covid19.who.int/. [Accessed on 12th May 2021].
- World Health Organization. Coronavirus disease (COVID-190: Vaccine safety. Available from https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-(covid-19)-vaccines-safety. [Accessed on 12th May 2021].
- World Health Organization. Draft landscape and tracker of COVID19 candidate vaccines. Available from https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. [Accessed on 12th May 2021].
- Science the Wire. India kicks off Phase 2 of vaccination drive, COWIN opens for public registration. https://science.thewire.in/health/india-covid-19-vaccination-drive-co-win-covaxin-covishield/. [Accessed on 12th May 2021].
- India Today. Phase 3 of Covid vaccination drive delayed in many states amid vaccine shortage. Available from https://www.indiatoday.in/coronavirus-outbreak/vaccine-updates/story/phase-3-covid-vaccination-drive-delayed-states-shortage-1797092-2021-05-02. [Accessed on 12th May 2021].
- Serum Institute of India. Coronavirus disease (COVID-19): Vaccines. Available from https://www.seruminstitute.com/product_covishield.php. [Accessed on 12th May 2021].
- Bharat Biotech. Bharat Biotech’s COVAXIN emergency use authorization approval by DCGI-CDSCO, MoH & FW, a significant landmark in India’s scientific discovery, and scientists capability. Available from https://www.bharatbiotech.com/images/press/bharat-biotech-covaxin-emergency-use-authorization-approval-by-dcgi-cdsco-moh-and-fw.pdf. [Accessed on 12 May 2021].
- The Hindu. Expert panel approves Russia’s Sputnik V COVID-19 vaccine in India. Available from https://www.thehindu.com/sci-tech/health/expert-panel-recommends-granting-approval-to-covid-19-vaccine-sputnik-v-for-emergency-use-in-india/article34301838.ece. [Accessed on 12th May 2021].
- Pharmacovigilance Programme of India. Suspected ADRs reporting form (for HCPs). Available from https://ipc.gov.in/images/ADR-Reporting-Form1.3.pdf. [Accessed on 15th January 2021].
- Pharmacovigilance Programme of India. Serious AEFI case notification form. Available from https://www.ipc.gov.in/PvPI/adr/Serious%20AEFI%20Case%20notification%20form.pdf. [Accessed on 15th January 2021].
- Uppsala Monitoring Centre. VigiFlow. Available from https://www.who-umc.org/global-pharmacovigilance/vigiflow/about-vigiflow/. [Accessed on 13th May 2021].
- Ministry of Health and Family WEelfare, Government of India. National Co-Win Statistics. Available from https://dashboard.cowin.gov.in/. [Accessed on 12th May 2021].
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615.
CrossRef - Kadali RAK, Janagama R, Peruru S, Malayala SV. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis. 2021;106:376-381.
CrossRef - Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99-111.
CrossRef - Ella R, Reddy S, Jogdand H, Sarangi V, Ganneru B, Prasad S et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021;S1473-3099(21)00070-0.
CrossRef - Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403-416.
CrossRef - Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021 02 20; 397(10275):671-681
CrossRef







