The Prescription Characteristics, Efficacy and Safety of Spironolactone in Real-World Patients With Acute Heart Failure Syndrome: A Prospective Nationwide Cohort Study
- 1Department of Critical Care Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
- 2Department of Medicine, Catholic University Graduate School, Seoul, South Korea
- 3Division of Cardiology, Department of Internal Medicine, Seoul St. Mary's Hospital, Catholic Research Institute for Intractable Cardiovascular Disease, College of Medicine, The Catholic University of Korea, Seoul, South Korea
- 4Biostatistics Collaboration Unit, Yonsei University College of Medicine, Seoul, South Korea
- 5Department of Internal Medicine, Seoul National University Hospital, Seoul, South Korea
- 6Department of Internal Medicine, Sungkyunkwan University College of Medicine, Seoul, South Korea
- 7Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea
- 8Department of Internal Medicine, Chungbuk National University College of Medicine, Cheongju, South Korea
- 9Department of Internal Medicine, Kyungpook National University College of Medicine, Daegu, South Korea
- 10Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea
- 11Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, South Korea
- 12Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, South Korea
- 13Department of Cardiovascular Medicine, Chonnam National University Medical School, Gwangju, South Korea
- 14Department of Internal Medicine, Mediplex Sejong Hospital, Incheon, South Korea
Background: Randomized clinical trials of spironolactone showed significant mortality reduction in patients with heart failure with reduced ejection fraction. However, its role in acute heart failure syndrome (AHFS) is largely unknown.
Aim: To investigate the prescription characteristics, efficacy and safety of spironolactone in real-world patients with AHFS.
Methods: 5,136 AHFS patients who survived to hospital discharge using a nationwide prospective registry in Korea were analyzed. The primary efficacy outcome was 3-year all-cause mortality.
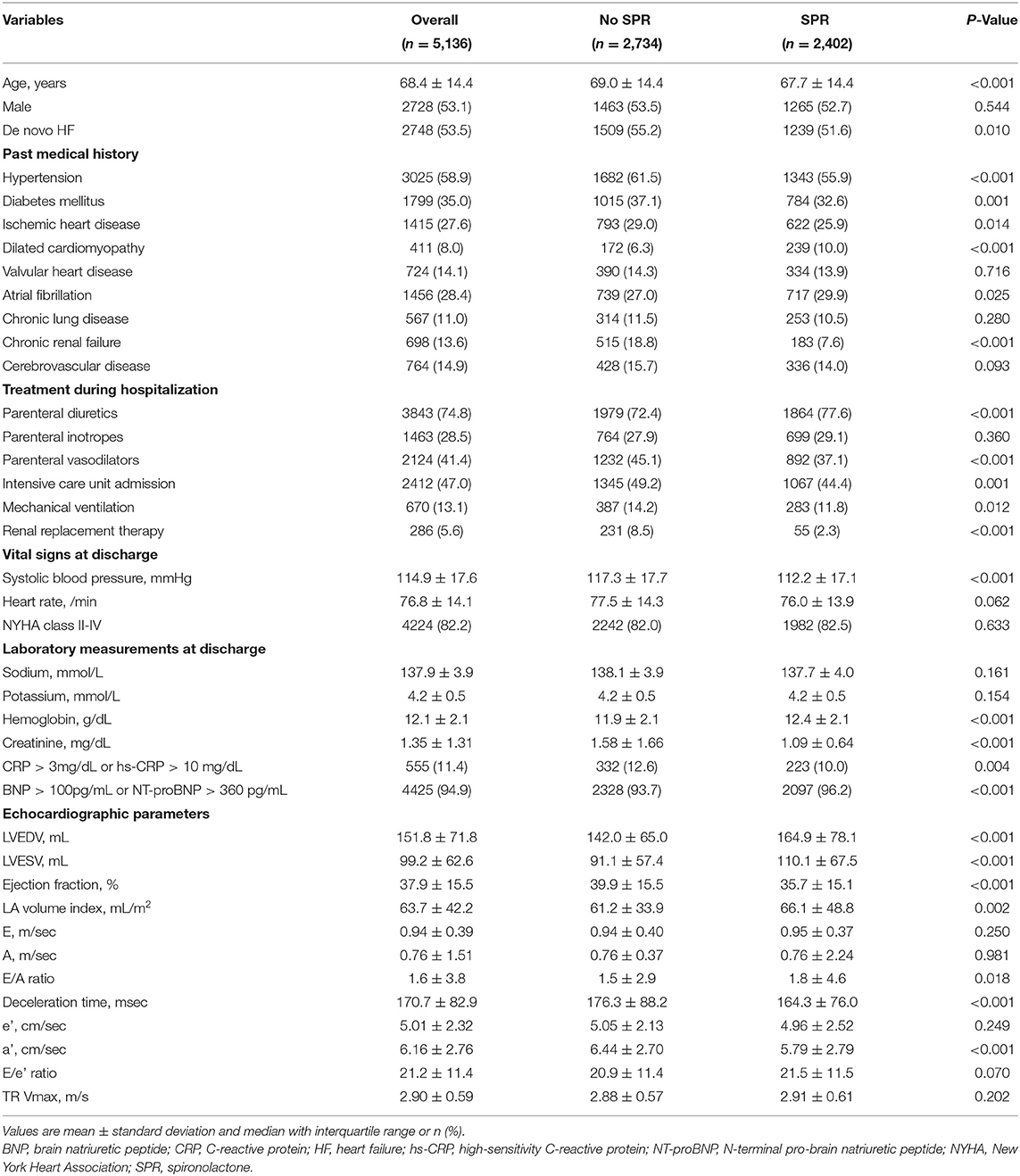
Results: Spironolactone was prescribed in 2,402 (46.8%) at discharge: <25 mg in 890 patients (37.1%), ≥25 mg, and <50 mg in 1,154 patients (48.0%), and ≥50 mg in 358 patients (14.9%). Patients treated with spironolactone had a lower proportion of chronic renal failure and renal replacement therapy during hospitalization and had lower serum creatinine level than those who did not. In overall patients, 3-year mortality was not different in both groups (35.9 vs. 34.5%, P = 0.279). The incidence of renal injury and hyperkalemia was 2.2% and 4.3%, respectively, at the first follow-up visit. The treatment effect of spironolactone on mortality was different across subpopulations according to LVEF. The use of spironolactone was associated with a significant reduction in 3-year morality in patients with LVEF ≤ 26% (33.8 vs. 44.3%, P < 0.001; adjusted HR 0.79, 95% CI 0.64–0.97, P = 0.023), but not in patients with LVEF > 26%.
Conclusions: Although spironolactone was frequently used at lower doses in real-world practice, use of spironolactone significantly reduced 3-year mortality in patients with severely reduced LVEF with acceptable safety profile. However, our findings remain prone to various biases and further prospective randomized controlled studies are needed to confirm these findings.
Introduction
Aldosterone has gained interest as a therapeutic target due to its independent and significant role in the pathophysiology of heart failure (HF). Beyond maintaining sodium and water homeostasis, aldosterone is involved in myocardial hypertrophy, fibrosis, and endothelial dysfunction (1). After the results of the Randomized Aldactone Evaluation Study (RALES) trial, which demonstrated an association between spironolactone and considerable mortality risk reduction in patients with severe HF, mineralocorticoid antagonists became a component of treatment for HF with reduced ejection fraction (HFrEF) (2–4). There was an attempt to reconsider for spironolactone to expand its therapeutic range to HF with preserved ejection fraction (HFpEF), and recently the U.S Food and Drug Administration's advisory committee reviewed a labeled indication for spironolactone in the treatment of adults with HFpEF (5).
However, data on the efficacy and safety of spironolactone in patients with acute heart failure syndrome (AHFS) including HFpEF are still limited. Even in the Aldosterone Antagonist Therapy for Adults with Heart Failure and Preserved Systolic Function (TOPCAT) trial, which investigated the use of spironolactone in HFpEF, outcome improvement was identified only in patients enrolled in the Americas, not all participants (6, 7). In addition, spironolactone showed conflicting results in a broad unselected population with HF outside of clinical trials (8). Considering the potential risk of adverse effects of spironolactone, such as renal impairment and hyperkalemia (9, 10), it is necessary to collect data on the efficacy and safety of spironolactone in real clinical AHFS practice to establish guidance for the use of spironolactone.
Therefore, we aimed to present the current spironolactone prescription pattern, efficacy, and safety, and to evaluate whether the efficacy of spironolactone could be varied depending on the left ventricular ejection fraction (LVEF) in Korean patients with AHFS.
Methods
Study Design and Population
Data for this study were from the Korean Acute Heart Failure (KorAHF) registry. Details on the study design and rationale of the KorAHF registry were previously reported (11, 12). The KorAHF registry is a nationwide prospective multicenter cohort study that evaluates the clinical characteristics, management, and outcomes of patients hospitalized for AHFS in Korea. Patients were enrolled at 10 tertiary university-affiliated hospitals from March 2011 to February 2014. Patients with signs or symptoms of HF and either (1) lung congestion defined as congestion on a chest X-ray or as rales on physical examination or (2) objective findings of LV systolic dysfunction or structural heart disease were eligible for the registry. A total of 5,625 consecutive patients were enrolled in the registry. Because our study aimed to identify whether spironolactone have a homogeneous treatment effect among patient subpopulations with different ejection fraction, we included the whole range of LVEF. Among them, patients without documented data of LVEF and patients who died during index hospitalization were excluded in this study (Supplementary Figure S1). The institutional review board or ethics committee at each participating hospital approved the study protocol and waived the need for written informed consent. This study complied with the Declaration of Helsinki principles.
Data Collection and Clinical Outcomes
Data were collected by attending physicians in each participating center using a web-based case-report form in the Clinical Research and Trial Management System (iCReaT) supported by the Korean National Institute of Health with the assistance of a clinical research coordinator. Information about patient demographic characteristics including comorbidities, etiology of HF, vital signs, laboratory and echocardiographic measurements, treatments, and clinical outcomes were obtained prospectively at the time of admission, discharge, and during the follow-up period. Data were periodically reviewed by an independent data monitoring team.
For C-reactive protein (CRP)/ high sensitivity CRP (hs-CRP) and brain natriuretic peptide (BNP)/ N-terminal pro-BNP (NT-proBNP), only one of the two variables was measured for each hospital. Therefore, these variables were classified as follows, referring to previous publications, in order to reduce missing values: BNP < 100 pg/mL or NT-proBNP < 360 pg/mL and BNP ≥ 100 pg/mL or NT-proBNP ≥ 360 pg/mL, and CRP level ≤ 10 mg/L or hsCRP ≤ 3.0 mg/L and CRP level > 10 mg/L or hsCRP > 3.0 mg/L (6, 13, 14).
The primary outcome in this study was all-cause mortality 3 years after hospital discharge. The mortality data for patients who were lost to follow-up was collected from the National Insurance data or National Death Records. Mortality and cause of death were verified by a Clinical Event Committee, which was composed of independent experts in HF who have not participated in patient enrolment.
Definitions
The use of spironolactone was assessed at hospital discharge. The prescription for initiation, dose adjustment or discontinuation of medications including spironolactone was left to the discretion of the physician in charge, but the decision-making generally followed the guidelines (3, 4). The safety of spironolactone treatment was evaluated at the first post-discharge outpatient follow-up visit. Renal injury was defined as a doubling of serum creatinine based on the Risk, Injury, Failure, Loss, and End-stage Kidney (RIFLE) classification creatinine doubling, and hyperkalemia was defined as potassium greater than 5.5 mmol/L (15).
Echocardiography was performed by a board-certified cardiologist or echocardiography technician. Quantitative assessment of LVEF using the modified Simpson's method was recommended, but LVEF that measured by M-mode or visual estimation was also used for HF categorization when the accuracy of the biplane method was limited due to a poor acoustic window (16). We defined the HF classification based on LVEF using the criteria of American College of Cardiology/American Heart Association (ACC/AHA) and the European Society of Cardiology (ESC) guidelines as follows: HFrEF, HF with LVEF ≤40%; HF with mid-range ejection fraction (HFmrEF), HF with LVEF 41–49%, HFpEF, HF with LVEF ≥50% (4, 17).
Statistical Analysis
To compare clinical characteristics and outcomes between the two groups, we analyzed categorical variables as numbers and percentages using the χ2 test or Fisher's exact test. Continuous variables are reported as mean ± standard deviation and were compared using the t-test. We performed subpopulation treatment effect pattern plot (STEPP) and the Contal and O'Quigley to evaluate whether the effect of spironolactone varies according to LVEF and to identify the cut-off value. To evaluate the effect of the spironolactone use and to identify risk factors for the 3-year all-cause mortality, we performed Cox proportional hazard regression analysis. Variables deemed clinically relevant from previous studies were considered candidate variables in multivariable Cox regression models. The hazard ratio (HR) of each variable is reported with the 95% confidence interval (CI). Survival curves were constructed by the Kaplan-Meier method, and the significance level was assessed using the log rank test to assess the effect of spironolactone with respect to the primary outcome according to classification of HF. To reduce the effects of potential confounders and selection bias, we performed a sensitivity analysis using propensity score matching. Propensity scores were estimated using a logistic regression model of the treatment on the covariate included in the Cox regression models. The patients were matched 1:1 by propensity scores. For all analyses, a two-tailed test with a P-value less than 0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R software package (R Foundation for Statistical Computing, Vienna, Austria).
Results
Prescription Pattern of Spironolactone
Of the 5,136 eligible patients, 2,402 (46.8%) patients were treated with spironolactone. The proportion of patients prescribed spironolactone decreased as the degree of renal function worsened (Supplementary Figure S2). The prescribed doses of spironolactone were < 25 mg in 890 (37.1%), ≥ 25 mg and < 50 mg in 1,154 (48.0%), and ≥ 50 mg in 358 (14.9%) in overall patients. About 75% of survivors in the spironolactone group were followed up until 3 years after hospital discharge, and 51.8% of them maintained spironolactone treatment during the 3-year follow-up period (Supplementary Figure S3).
There were significant differences in clinical and in-hospital treatment characteristics between patients treated with spironolactone and without spironolactone (Table 1). The proportion of de novo HF, hypertension, diabetes, and ischemic heart disease were higher in the no spironolactone group, and the proportion of dilated cardiomyopathy, and atrial fibrillation were higher in the spironolactone group. In the spironolactone group, chronic renal failure and need of renal replacement therapy during hospitalization were less common and serum creatinine was significantly lower than in the no spironolactone group. In addition, the spironolactone group had lower LVEF than the no spironolactone group.
Mortality and Adverse Events
Overall, 1,810 (35.2%) patients died during the 3-year follow-up after discharge and there was no significant difference in 3-year (35.9% vs. 34.5%, P = 0.279) all-cause mortality between the two groups (Figure 1). As renal function worsened, the 3-year mortality tended to increase and the difference in 3-year mortality between the two groups was not significant except for patients with glomerular filtration rate <15 mL/min/1.73m2 (57/132 vs. 2/14, P = 0.036) (Supplementary Table S1).
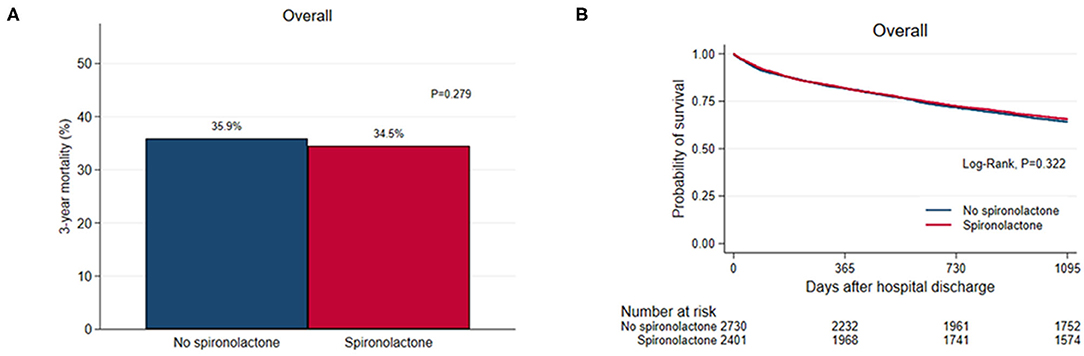
Figure 1. (A) Bar graph and (B) Kaplan–Meier curves of the 3-year all-cause mortality after hospital discharge according to spironolactone treatment in overall patients.
Among the spironolactone group, 1,324 (55.1%) patients had post-discharge outpatient follow-up visits with blood sampling within an average of 14.9 days. There were significant differences in systolic blood pressure (113.1 mmHg vs. 109.9 mmHg, P < 0.001), serum creatinine (1.08 mg/dL vs. 1.18 mg/dL, P < 0.001), and serum potassium (4.2 mmol/L vs. 4.6 mmol/L, P < 0.001) compared with those at hospital discharge, but the incidence of renal injury and hyperkalemia was 2.2 and 4.3%, respectively (Supplementary Table S2). There was no case of discontinuing spironolactone due to gynecomastia in the transition period (Supplementary Table S3).
Effect of Spironolactone According to LVEF
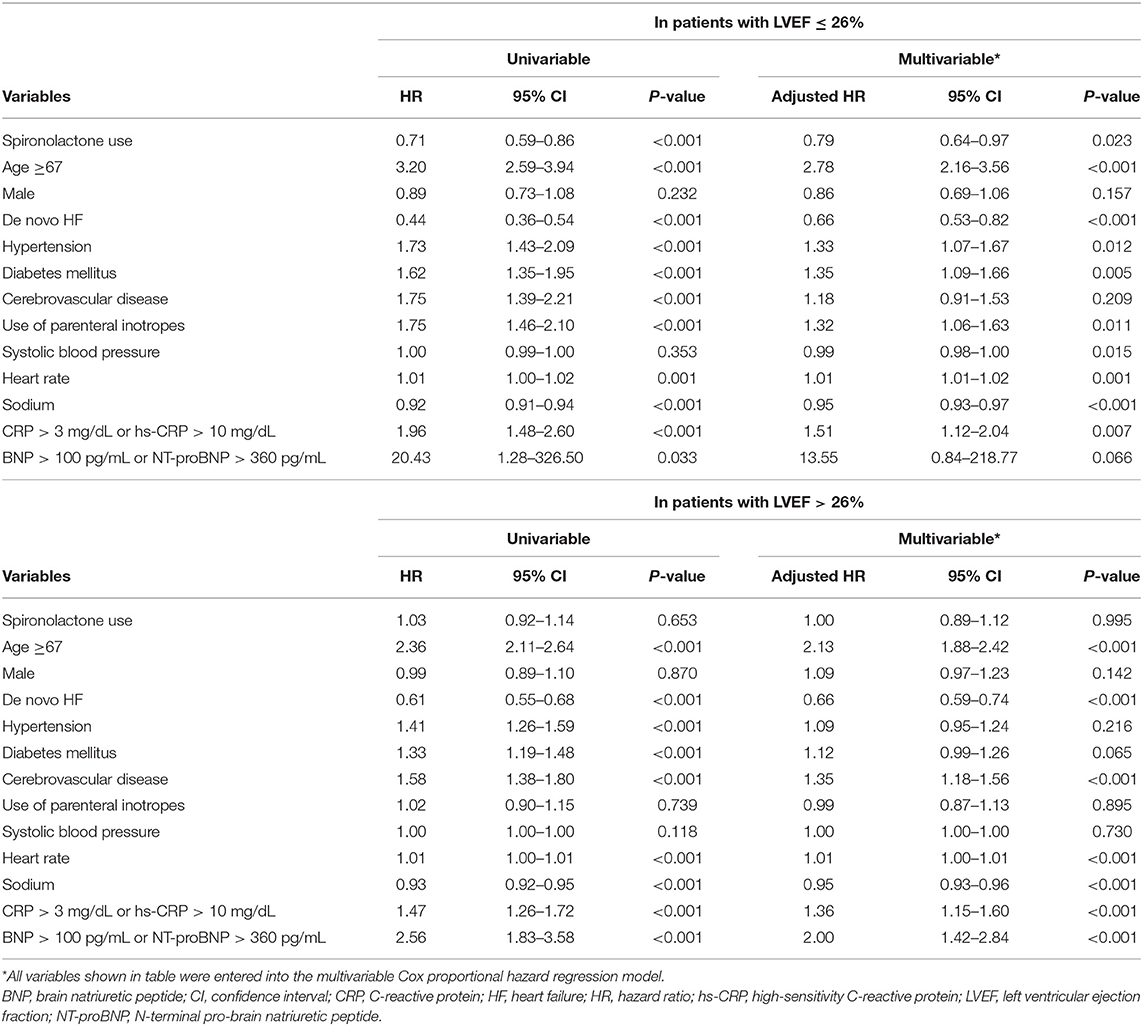
STEPP analysis of the treatment effect of spironolactone across subpopulations according to LVEF showed that the spironolactone treatment was associated with lower 3-year mortality only in subpopulations where LVEF was less than 28% (Figure 2 and Supplementary Table S4). The most significant cut-off value of LVEF by Contal and O'Quigley method was 26.1%, which discriminated patients with different survival according to use of spironolactone. In patients with LVEF < 26.1%, the spironolactone group had significantly lower 3-year (33.8 vs. 44.3%, P < 0.001) mortality than the no spironolactone group, but there was no difference in mortality between the two groups in patients with LVEF > 26% (Figure 3). Cox regression analysis revealed that spironolactone treatment was independently associated with a reduction in 3-year mortality in patients with LVEF ≤ 26% (HR 0.71, adjusted HR 0.79, 95% CI 0.64–0.97, P = 0.023) (Table 2).
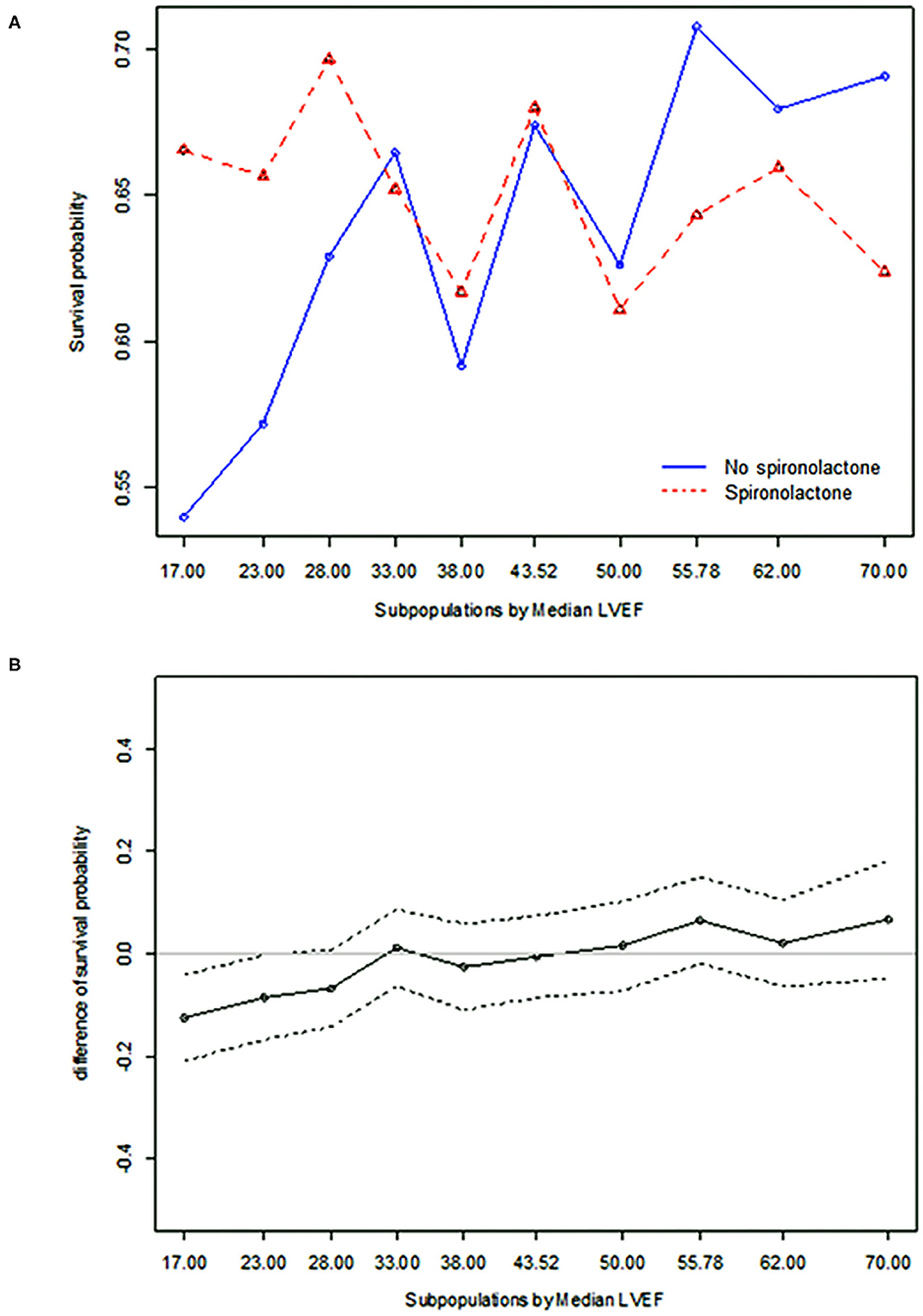
Figure 2. Subpopulation treatment effect pattern plot analysis of the treatment effect of spironolactone as measured by (A) 3-year all-cause mortality, (B) difference in 3-year all-cause mortality.
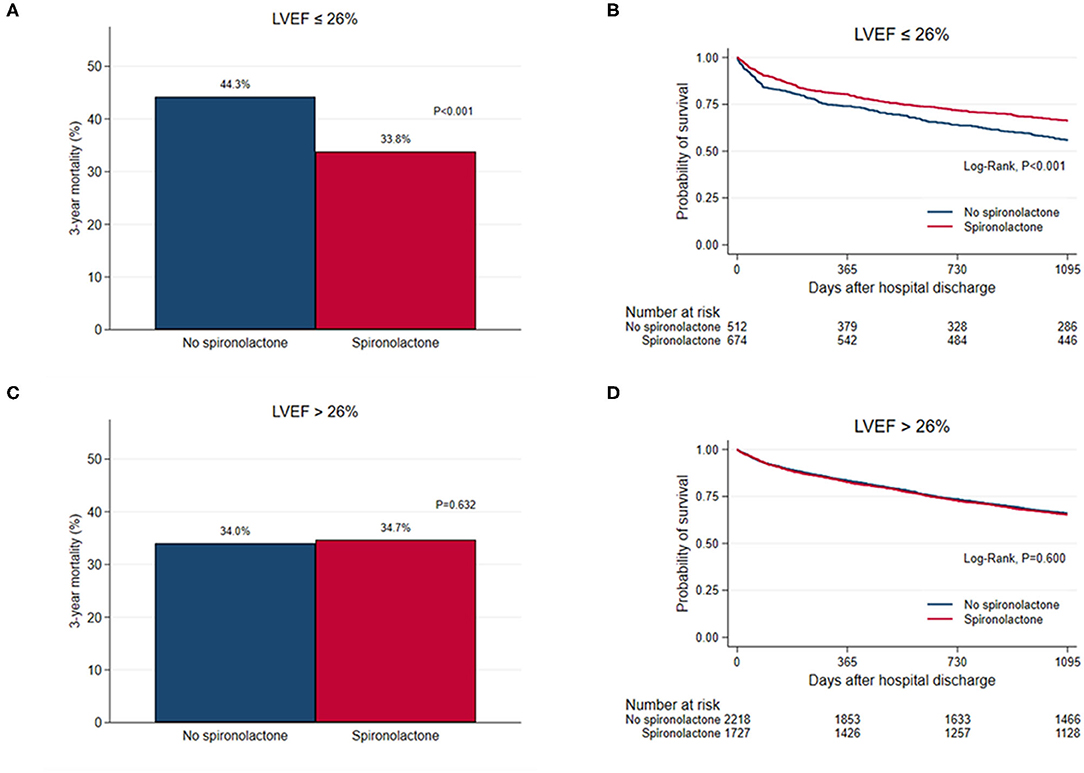
Figure 3. (A,C) Bar graph and (B,D) Kaplan–Meier curves of the 3-year all-cause mortality after hospital discharge according to spironolactone treatment in patients with left ventricular ejection fraction (LVEF) ≤ 26% and in patients with LVEF > 26%.
When overall patients were divided into three classifications of HF based on LVEF, there was no significant difference in 3-year mortality between the two groups among patients with HFmrEF (32.9 vs. 33.1%, P = 0.945) and HFpEF (32.3 vs. 36.3%, P = 0.109) (Figure 4). In patients with HFrEF, the 3-year mortality rate was significantly reduced in the spironolactone group (34.0 vs. 39.6%, P = 0.002).
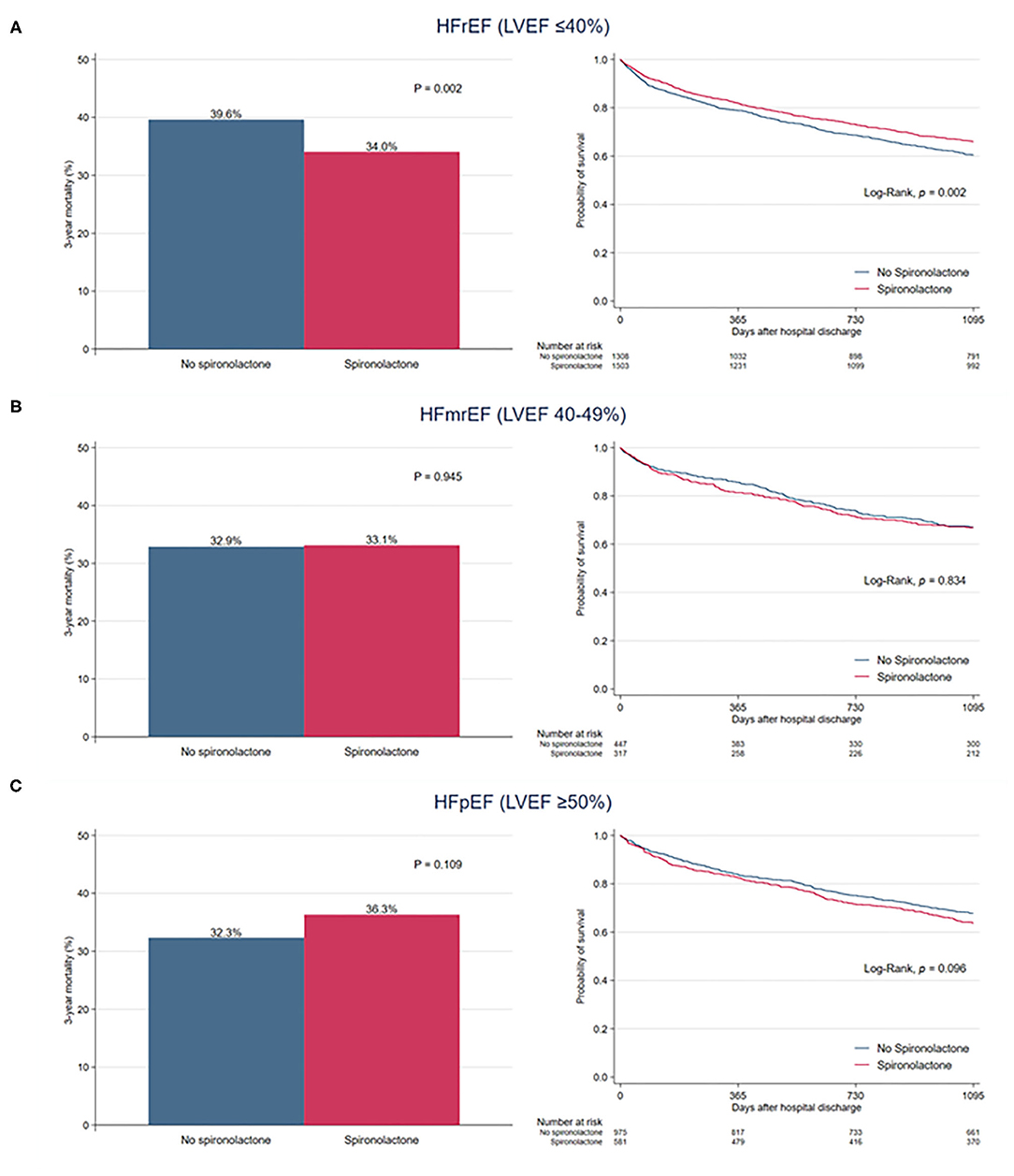
Figure 4. Bar graph and Kaplan–Meier curves of the 3-year all-cause mortality after hospital discharge according to spironolactone treatment in patients with (A) heart failure with reduced ejection fraction (HFrEF), (B) heart failure with mid-range ejection fraction (HFmrEF), and (C) heart failure with preserved ejection fraction (HFpEF).
Sensitivity Analysis
Clinical and in-hospital treatment characteristics of the two groups in propensity score matched cohort are shown in the Supplementary Table S5. The no spironolactone group had a higher proportion of chronic renal failure and renal replacement therapy during hospitalization than the spironolactone group. Potassium and creatinine levels and LVEF at discharge were also slightly higher in the no spironolactone group. Similar to the results of our main analysis, the Kaplan-Meier survival curve showed a significant difference between the survival rates of the two groups during a 3-year follow up in patients with LVEF ≤ 26% (log rank test, P = 0.043), but the survival rates were similar between the two groups in patients with LVEF > 26% (log rank test, P = 0.824) (Supplementary Figure S4). In Cox regression analysis, spironolactone was related to 3-year mortality in patients with LVEF ≤ 26% (adjusted HR 0.78, 95% CI 0.62–0.99, P = 0.044), but not in patients with LVEF > 26% (adjusted HR 1.01, 95% CI 0.89–1.15, P = 0.824).
Discussion
In the present study, we identified the prescription pattern of spironolactone and evaluated the efficacy and safety of spironolactone in patients with AFHS using a large prospective nationwide cohort in Korea. We found that (1) spironolactone was used in 46.8% of Korean patients with AHFS and spironolactone was often used at a dose lower than the dose recommended in the guidelines; (2) use of spironolactone was not associated with reduction of 3-year all-cause mortality and occurrence of significant renal injury or hyperkalemia in overall patients; and (3) the effect of spironolactone on mortality was different depending on the LVEF, and the survival benefit was particularly remarkable in patients with severely reduced LVEF.
Mineralocorticoid antagonists, including spironolactone, are commonly prescribed in HFrEF patients, which ranges from about 50 to 70% in recent studies (18–21). Spironolactone has emerged as an important treatment option for HF since the 1990s because of its ability to attenuate the neurohormonal signals, which play a central role in the progression of HF, and to reverse remodeling. Spironolactone treatment significantly reduces plasma procollagen type III aminoterminal peptide (PIIINP), a biochemical marker of myocardial fibrosis and/or remodeling, and BNP, a prognostic marker of HF, and improves endothelial function, which is associated with cardiovascular events in patients with HF of varying severity (22–24). Studies evaluating the effect of spironolactone with echocardiographic assessment showed improvement of LV systolic and diastolic function and ventricular-arterial coupling, as well as reduction of LV volume and mass in patients treated with spironolactone (25, 26). In addition to the improvement of these laboratory and echocardiographic parameters, positive results from three large-scale, multi-center, placebo-controlled clinical trials, RALES, the Eplerenone Post–Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS), and the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF), empowered MRA treatment in HFrEF (2, 27, 28). Furthermore, the TOPCAT trial suggested that the effect of spironolactone are not limited to HFrEF, but may extend to patients with HFpEF (6).
Contrary to the favorable results of the previous studies, spironolactone was not associated with a reduced mortality in our entire cohort. Our findings are consistent with a study of Lund et al. who failed to show differences in mortality according to mineralocorticoid antagonist treatment in a large general HF population of the Swedish Heart Failure Registry (8). It suggests that there may be a gap between the randomized clinical trials and real-world practice. In our study, patients who received spironolactone treatment had substantially different characteristics from those who did not, suggesting that spironolactone was selectively prescribed. Considering the post-hoc analysis of the TOPCAT trial, which demonstrated the the response to spironolactone was significantly different according to clinical phenogroups (6, 29), differences in patient selection and patient characteristics may be one explanation. In particular, we did not limit our analysis to patients with HFrEF or HFpEF because our study was interested in evaluating the efficacy of spironolactone in real-world practice, but the efficacy of spironolactone was different depending on the LVEF. The survival benefit of spironolactone was significant only in patient with severely reduced LVEF. Although our findings cannot explain the underlying mechanism of the relationship between spironolactone and LVEF, it supports the current guidelines recommending the use of spironolactone in patients with LVEF ≤ 35% (17, 30).
In our study, spironolactone was prescribed in only about half of overall patients. In particular, the proportion of patients prescribed spironolactone was significantly reduced in patients with severe renal dysfunction. Our findings are similar to a recent study by Patel et al. which demonstrated that spironolactone was infrequently used compared to other guideline recommended, especially in patients with renal dysfunction (31). It is presumably the result of concerns about spironolactone-related complications, such as worsening of renal function and hyperkalemia. However, selective use of spironolactone did not increase the incidence of mortality or adverse events in patients with renal dysfunction in our cohort. Furthermore, Oh et al. showed the survival benefit of spironolactone in patients with stage 3b chronic kidney disease (32). Therefore, further studies should be conducted for the proper use of spironolactone in patients with renal dysfunction.
Also, the that about 40% of patients treated with spironolactone in our study were not prescribed a guideline-recommended dose may have may have influenced the efficacy of spironolactone treatment. Although current guidelines recommend 25 mg spironolactone once daily with titration up to 50 mg once daily for patients with HFrEF based on landmark trials (17, 30), a large number of patients are treated with spironolactone doses of less than 25 mg in real-world practice (19, 33). The dose response relationship between spironolactone and survival has not yet been clearly identified. In the Aldosterone Targeted Neurohormonal Combined with Natriuresis Therapy in Heart Failure (ATHENA-HF) trial, 100 mg of spironolactone was not associated with an improved outcome compared to placebo or 25 mg of spironolactone in patients with AHFS (34). On the other hand, in the ASIAN-HF registry, patients who received at least 100% of guideline-recommended dose had better composite outcomes of all-cause deaths or hospitalization for HF than did those who received lower doses (19). To properly assess the characteristics of patients who are likely to benefit from spironolactone treatment, the effects of under-dosing should be further elucidated.
Although our study provided information regarding the association between spironolactone treatment and long-term survival in a large population of Korean patients with AHFS, there are several limitations that should be considered. First, because of the observational study design, our findings remain prone to various biases and potential confounding factors. Although we used regression modeling and propensity score matching to control for confounders, unmeasured confounders may have been present. In particular, our study did not control the effect of standard medical treatment for HF with reduced EF such as renin-angiotensin system blockade or beta blocker. Second, it is possible that more severe and complex patients were included in this study because only tertiary university-affiliated hospitals participated in the registry. In addition, since risk factors for mortality and the efficacy of medication in patients with HF may vary depending on regional differences, our findings have limitations in their generalizability to other populations (7). Third, we determined whether the patient was treated with spironolactone only by prescription at hospital discharge. Because the total treatment duration and changes in dosage of spironolactone and medication adherence were not evaluated, we could not exclude the possibility that insufficient treatment duration and adherence may have affected our findings. In addition, we could not assess the effect of valvular and right ventricular function on clinical outcomes. Further prospective randomized controlled studies are needed to confirm these findings.
Conclusion
Spironolactone was prescribed in selective patients and under-dosing was common for treatment of AHFS in real-world clinical practice. Although spironolactone was used in patients with a wide range of LVEF, the effect of spironolactone on mortality differed according to the LVEF and spironolactone was associated with a reduction of 3-year mortality only in patients with severely reduced LVEF. Further studies to identify patients who are likely to benefit from spironolactone treatment are necessary for the expansion of the therapeutic field of spironolactone and the optimal use in patient with AHFS.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the Catholic Medical Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
SJN, J-CY, and SHB conceived and designed the study and drafted the manuscript for intellectual content. SJN, J-CY, HSL, SJ, and SHB analyzed and interpreted the data. SJN, J-CY, H-YL, H-JC, J-OC, E-SJ, SEL, M-SK, J-JK, K-KH, M-CC, SCC, S-MK, D-JC, B-SY, KHK, B-HO, and SHB revised the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT (NRF-2021R1F1A1063430), by the Catholic Medical Center Research Foundation (2021) and by Research of Korea Centers for Disease Control and Prevention (2010-E63003-00, 2011-E63002-00, 2012-E63005-00, 2013-E63003-00, 2014-E63003-01, 2015-E63003-02, 2016-ER6303-00, and 2017-ER6303-01). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.791446/full#supplementary-material
References
1. Sztechman D, Czarzasta K, Cudnoch-Jedrzejewska A, Szczepanska-Sadowska E, Zera T. Aldosterone and mineralocorticoid receptors in regulation of the cardiovascular system and pathological remodelling of the heart and arteries. J Physiol Pharmacol. (2018) 69:829–45. doi: 10.26402/jpp.2018.6.01
2. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med. (1999) 341:709–17. doi: 10.1056/nejm199909023411001
3. Maddox TM, Januzzi JL Jr, Allen LA, Breathett K, Butler J, Davis LL, et al. 2021 Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the american college of cardiology solution set oversight committee. J Am Coll Cardiol. (2021) 77:772–810. doi: 10.1016/j.jacc.2020.11.022
4. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
5. FDA. FDA Briefing document cardiovascular and renal drugs advisory committee meeting december 16, 2020: spironolactone for heart failure with preserved ejection fraction (HFpEF). Available online at: https://www.fda.gov/media/144411/download (accessed December 16, 2020).
6. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. (2014) 370:1383–92. doi: 10.1056/NEJMoa1313731
7. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, et al. Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circ. (2015) 131:34–42. doi: 10.1161/circulationaha.114.013255
8. Lund LH, Svennblad B, Melhus H, Hallberg P, Dahlström U, Edner M. Association of spironolactone use with all-cause mortality in heart failure: a propensity scored cohort study. Circ Heart Fail. (2013) 6:174–83. doi: 10.1161/circheartfailure.112.000115
9. Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. (2004) 351:543–51. doi: 10.1056/NEJMoa040135
10. Beldhuis IE, Myhre PL, Claggett B, Damman K, Fang JC, Lewis EF, et al. Efficacy and safety of spironolactone in patients with HFpEF and chronic kidney disease. JACC Heart Fail. (2019) 7:25–32. doi: 10.1016/j.jchf.2018.10.017
11. Lee SE, Cho HJ, Lee HY, Yang HM, Choi JO, Jeon ES, et al. A multicentre cohort study of acute heart failure syndromes in Korea: rationale, design, and interim observations of the Korean acute heart failure (KorAHF) registry. Eur J Heart Fail. (2014) 16:700–8. doi: 10.1002/ejhf.91
12. Youn JC, Han S, Ryu KH. Temporal trends of hospitalized patients with heart failure in Korea. Korean Circ J. (2017) 47:16–24. doi: 10.4070/kcj.2016.0429
13. Nishimoto Y, Kato T, Morimoto T, Yaku H, Inuzuka Y, Tamaki Y, et al. C-reactive protein at discharge and 1-year mortality in hospitalised patients with acute decompensated heart failure: an observational study. BMJ Open. (2020) 10:e041068. doi: 10.1136/bmjopen-2020-041068
14. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO. 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the american heart association. Circ. (2003) 107:499–511. doi: 10.1161/01.cir.0000052939.59093.45
15. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute dialysis quality initiative w. acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI). Group Crit Care. (2004) 8:R204–12. doi: 10.1186/cc2872
16. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2015) 28:1–39. doi: 10.1016/j.echo.2014.10.003
17. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circ. (2013) 128:e240–327. doi: 10.1161/CIR.0b013e31829e8776
18. Crespo-Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, et al. European society of cardiology heart failure long-term registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail. (2016) 18:613–25. doi: 10.1002/ejhf.566
19. Teng TK, Tromp J, Tay WT, Anand I, Ouwerkerk W, Chopra V, et al. Prescribing patterns of evidence-based heart failure pharmacotherapy and outcomes in the ASIAN-HF registry: a cohort study. Lancet Glob Health. (2018) 6:e1008–e18. doi: 10.1016/S2214-109X(18)30306-1
20. McMurray JJV, DeMets DL, Inzucchi SE, Kober L, Kosiborod MN, Langkilde AM, et al. The Dapagliflozin and prevention of adverse-outcomes in heart failure (DAPA-HF) trial: baseline characteristics. Eur J Heart Fail. (2019) 21:1402–11. doi: 10.1002/ejhf.1548
21. Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, et al. Effect of Empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation. (2021) 143:516–25. doi: 10.1161/CIRCULATIONAHA.120.052186
22. Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, et al. Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol. (2001) 37:1228–33. doi: 10.1016/s0735-1097(01)01116-0
23. Rousseau MF, Gurne O, Duprez D, Van Mieghem W, Robert A, Ahn S, et al. Beneficial neurohormonal profile of spironolactone in severe congestive heart failure: results from the RALES neurohormonal substudy. J Am Coll Cardiol. (2002) 40:1596–601. doi: 10.1016/s0735-1097(02)02382-3
24. Macdonald JE, Kennedy N, Struthers AD. Effects of spironolactone on endothelial function, vascular angiotensin converting enzyme activity, and other prognostic markers in patients with mild heart failure already taking optimal treatment. Heart. (2004) 90:765–70. doi: 10.1136/hrt.2003.017368
25. Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Brighetti G, et al. Long-term, dose-dependent effects of spironolactone on left ventricular function and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol. (2002) 40:304–10. doi: 10.1016/s0735-1097(02)01965-4
26. Vizzardi E, Sciatti E, Bonadei I, D'Aloia A, Tartiere-Kesri L, Tartiere JM, et al. Effects of spironolactone on ventricular-arterial coupling in patients with chronic systolic heart failure and mild symptoms. Clin Res Cardiol. (2015) 104:1078–87. doi: 10.1007/s00392-015-0877-5
27. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. (2003) 348:1309–21. doi: 10.1056/NEJMoa030207
28. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. (2011) 364:11–21. doi: 10.1056/NEJMoa1009492
29. Cohen JB, Schrauben SJ, Zhao L, Basso MD, Cvijic ME Li Z, et al. Clinical Phenogroups in Heart Failure With Preserved Ejection Fraction: Detailed Phenotypes, Prognosis, and Response to Spironolactone. JACC Heart Fail. (2020) 8:172–84. doi: 10.1016/j.jchf.2019.09.009
30. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
31. Patel RB, Fonarow GC, Greene SJ, Zhang S, Alhanti B, DeVore AD, et al. Kidney function and outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. (2021) 78:330–43. doi: 10.1016/j.jacc.2021.05.002
32. Oh J, Kang SM, Song MK, Hong N, Youn JC, Han S, et al. Clinical benefit of spironolactone in patients with acute decompensated heart failure and severe renal dysfunction: data from the Korean heart failure registry. Am Heart J. (2015) 169:713–20 e3. doi: 10.1016/j.ahj.2015.01.014
33. Linssen GCM, Veenis JF, Brunner-La Rocca HP, van Pol PEJ, Engelen DJM, van Tooren RM, et al. Differences in guideline-recommended heart failure medication between Dutch heart failure clinics: an analysis of the CHECK-HF registry. Neth Heart J. (2020) 28:334–44. doi: 10.1007/s12471-020-01421-1
Keywords: acute heart failure syndrome, spironolactone, mineralocorticoid receptor antagonists, drug therapy, outcome
Citation: Na SJ, Youn J-C, Lee HS, Jeon S, Lee H-Y, Cho H-J, Choi J-O, Jeon E-S, Lee SE, Kim M-S, Kim J-J, Hwang K-K, Cho M-C, Chae SC, Kang S-M, Choi D-J, Yoo B-S, Kim KH, Oh B-H and Baek SH (2022) The Prescription Characteristics, Efficacy and Safety of Spironolactone in Real-World Patients With Acute Heart Failure Syndrome: A Prospective Nationwide Cohort Study. Front. Cardiovasc. Med. 9:791446. doi: 10.3389/fcvm.2022.791446
Received: 08 October 2021; Accepted: 31 January 2022;
Published: 22 February 2022.
Edited by:
Hendrik Tevaearai Stahel, Bern University Hospital, SwitzerlandReviewed by:
Ali Javaheri, Washington University School of Medicine in St. Louis, United StatesBertram Pitt, University of Michigan, United States
Copyright © 2022 Na, Youn, Lee, Jeon, Lee, Cho, Choi, Jeon, Lee, Kim, Kim, Hwang, Cho, Chae, Kang, Choi, Yoo, Kim, Oh and Baek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jong-Chan Youn, jong.chan.youn@gmail.com; Sang Hong Baek, whitesh@catholic.ac.kr
†These authors have contributed equally to this work
 Soo Jin Na
Soo Jin Na Jong-Chan Youn
Jong-Chan Youn Hye Sun Lee
Hye Sun Lee Soyoung Jeon4
Soyoung Jeon4  Seok-Min Kang
Seok-Min Kang