Patterns of distribution of mollusc fauna associated with Halopteris scoparia (Linnaeus) Sauvageau: a baseline study in the Azores archipelago helps understanding the impact of climate change/invasive species on biodiversity
- 1MPB – Marine Palaeontology and Biogeography Lab, University of the Azores, Ponta Delgada, Açores, Portugal
- 2CIBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos, InBIO Laboratório Associado, Ponta Delgada, Portugal
- 3UNESCO Chair – Land Within Sea: Biodiversity & Sustainability in Atlantic Islands, Ponta Delgada, Portugal
- 4BIOPOLIS Program in Genomics, Biodiversity and Land Planning, CIBIO, Vairão, Portugal
This work was done on 1996 and 1997, and presents an extremely valuable baseline to compare the recent and future changes on the insular shallow habitats of the Azorean islands. We examined the structure of the molluscan communities of the macroalgae Halopteris scoparia in São Miguel Island (Azores, Portugal). This island was chosen because it is the largest and the most populated of the archipelago, with polluted sites which are not common in the Azores. The relationship between the epifaunal assemblages and a set of environmental factors – geographical location (orientation), seawater temperature, depth, algal volume, degree of disturbance, and degree of exposure to the wave action – was investigated using distance-based redundancy analysis and significant variation in the distribution of richness of assemblages was found. Four environmental predictors were common to all the four analyses implemented (richness and assemblage structure using both AIC and BIC): algal volume (that correlates with algal dry weight), seawater temperature, coastal orientation and depth. Finally, the application in the Azores of this methodology favours a sampling program in Spring-Summer (when disturbance seems to be more susceptible to detection), and the use of H. scoparia in the subtidal zone, as the target alga is recommended due to its large covering of rocky shore substrates.
1 Introduction
Since 1970, there has been much interest in the macrofauna that inhabit marine plants. Most work has been done on seagrasses epifauna (Stoner, 1980; Whippo et al., 2018 and references therein). However, some descriptions of algal macrofauna communities have been made (e.g., Gunnill, 1982, 1983, 1984, 1985) with more detailed studies on population dynamics (Borja, 1986a, b, c), on the effect of certain environmental parameters (Fretter and Graham, 1977; Gunnill, 1983) or on plant-animal interactions (Nelson, 1979a, b; Leber, 1985; Chemello and Milazzo, 2002). In recent years, mollusc communities of seaweeds have been also widely used as surrogate measurements of environmental health/stress (Sánchez-Moyano et al., 2000a; Sánchez-Moyano et al., 2000b). The low representativeness of soft bottoms in oceanic islands such as the Azores enhances the importance of rocky shore algal dominated subtidal biotopes. Therefore, the algal epifauna assemblages emerge as the target communities to be studied under a perspective of possible usage as environmental bioindicators.
In the Azores archipelago, subtidal communities are dominated by foliose brown algae (Tittley and Neto, 2000; Neto, 2001; Martins et al., 2013; Sangil et al., 2018) but Halopteris scoparia (Linnaeus) Sauvageau, 1904 is one of the most abundant perennials, widely distributed across the archipelago, and thus a general representative habitat for macrobenthic subtidal communities in these oceanic islands (Neto et al., 2000). Additionally, this much-ramified alga provides a microhabitat and enhances sediment trapping. Its morphological characteristics, wide distribution and abundance make it a good substrate for an abundant and interesting epiphytic community (Sánchez-Moyano et al., 2000a; Costa and Ávila, 2001).
Of the phytal-associated fauna, the molluscs (Costa and Ávila, 2001), together with the crustaceans (Costa, 2003) and polychaetes (Cordeiro et al., 2019), comprise one of the predominant groups. Chapman (1955) carried out the first ecological study on the marine molluscs inhabiting the shallow-waters of the Azores. This approach was followed by several authors, namely: Morton (1967), Bullock et al. (1990), Hawkins et al. (1990), Azevedo (1991; 1992), Bullock (1995), Ávila (1998, 2000a, 2000b, 2003), Costa and Ávila (2001) and Ávila et al. (2005, 2007, 2015).
This work was done on 1996 and 1997, and presents an extremely valuable baseline to compare the recent and future changes on the insular shallow habitats of the Azorean islands. For instance, one of the most impacting events regarding sessile marine communities in the Azores is the recent arrival and spread of the marine brown macroalgae Rugulopteryx okamurae (E.Y.Dawson) I.K.Hwang, W.J.Lee & H.S.Kim, 2009 in the region (Faria et al., 2022a). Since its initial detection, this species has swiftly become a dominant force, extensively covering the rocky substrates up to 20 m depth. Within just two years, R. okamurae has not only established itself as the predominant species but has also induced substantial structural changes in benthic communities. These changes have resulted in significantly impoverished and more homogenized assemblages, highlighting the profound impact of this invasive alga on local ecosystems (Faria et al., 2022b). Such a rapid transformation underscores the critical role of historical data in understanding the pre-invasion state of marine communities. By analyzing the original state of these ecosystems, we gain invaluable insights into the ecological shifts induced by an invader such as R. okamurae and other anthropogenic pressures. This approach not only facilitates a better assessment of the changes but also provides essential data for evaluating the extent of ecological disturbances caused by biological invasions and human activities.
Herein, we investigate the temporal and spatial patterns of distribution of molluscan assemblages associated with Halopteris scoparia in relation to several environmental factors to gain a better understanding of the underlying processes driving community structure in these assemblages. In addition, we also test if these assemblages can be used as environmental indicators in the Azores.
2 Materials and methods
2.1 Sampling design
The island of São Miguel (Figure 1), with approximately 750 km2, is the largest of the archipelago of the Azores. The coastline is about 155 km in length and seashore is generally steeply sloping. The wave action is known to be generally stronger on north coast and responsible for its higher erosion rate (Borges, 2003). Before being invaded by the Rugulopteryx okamurae (E.Y.Dawson) I.K.Hwang, W.J.Lee & H.S.Kim 2009 (Dictyotales, Heterokontophyta) alga (Faria et al., 2022a, b), the seascape in São Miguel was characterized by rocky substrata mainly covered with other species of brown algae (see Neto, 2001, Martins et al., 2013; Sangil et al., 2018 for further details on the former habitat).
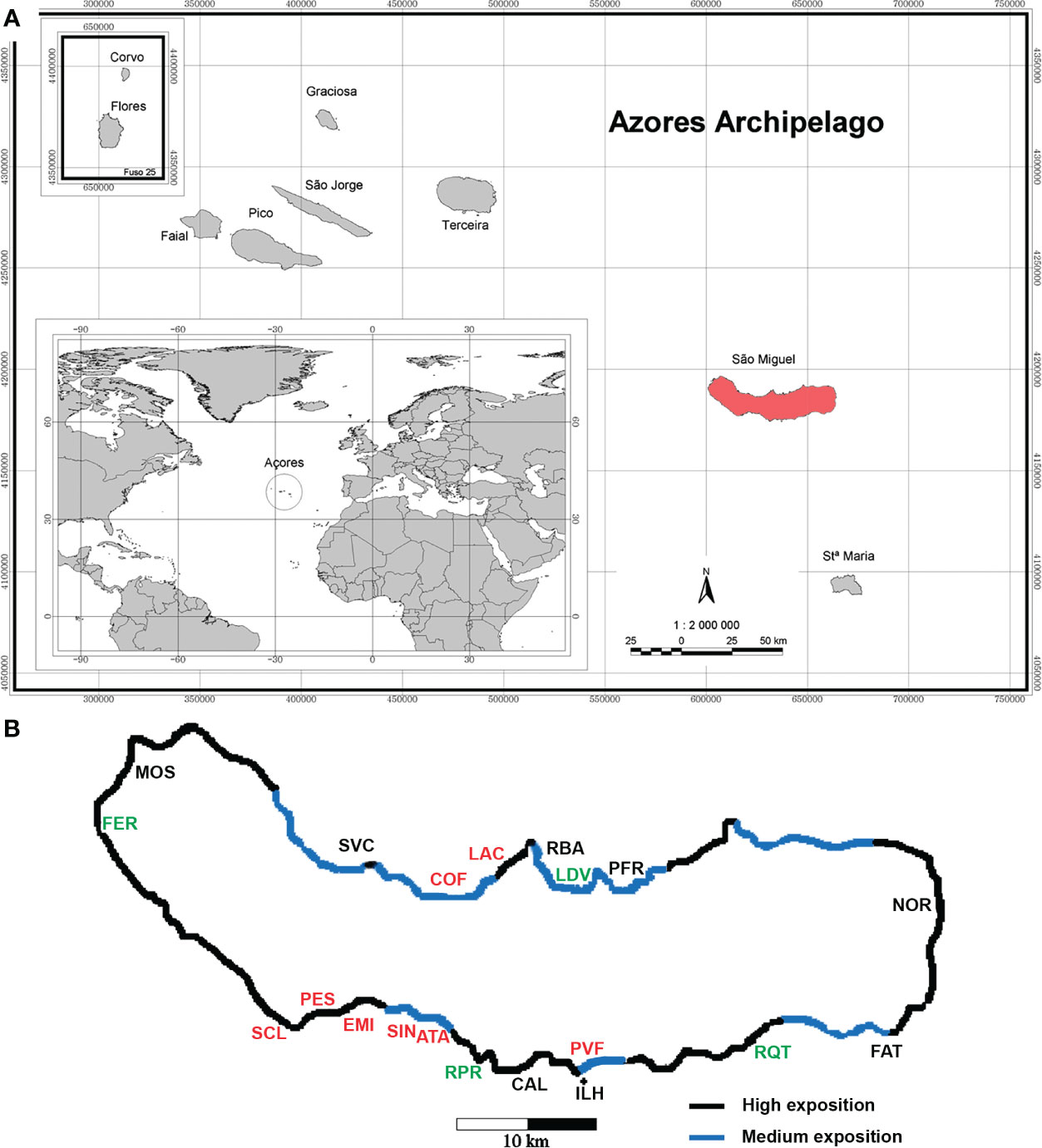
Figure 1 (A) Azores archipelago. (B) São Miguel Island with location of the sampling sites. Polluted sites (in red), disturbed sites (in green) and non-polluted sites (in black). For the abbreviations of the sampling sites, see Table 1.
Sampling sites were chosen around the island of São Miguel, with different environmental conditions: geographical location (north/south/east/west sites), degree of disturbance (non-polluted sites/disturbed/polluted sites) and degree of exposure to the wave action (Wallenstein, 2002) (H-high exposure/M-medium exposure/S-sheltered sites). A total of 11 sites (26 replicates) were sampled during Autumn, and 20 sites (79 replicates) were sampled during two consecutive years, 1996 and 1997, at similar depths, ranging from 5.6 to 15.0 m (Table 1). Ribeira Quente (RQT), Ladeira da Velha (LDV) and Ponta da Ferraria (FER) were considered as naturally disturbed sites, because of shallow-water subtidal vents nearby (see Ávila et al., 2007; Couto et al., 2015; Martins et al., 2021). Ribeira da Praia (RPR) was also considered as a naturally disturbed site because of the freshwater influence from stream mouth. Polluted sites were those located near factory outlets (e.g.: Atalhada (ATA), Cofaco (COF), Santa Clara (SCL), Lactoaçoreana (LAC) and Sinaga (SIN)), those located inside harbors (Porto de Vila Franca do Campo (PVF) and Pesqueiro (PES)) and the submarine outlet of the Ponta Delgada water treatment station (EMI). The remaining sites were classified as non-polluted: São Vicente Ferreira (SVC), Ribeirinha (RBA), Caloura (CAL), Ilhéu de Vila Franca do Campo (ILH), Faial da Terra (FAT), Mosteiros (MOS), Porto Formoso (PFR) and Nordeste (NOR).
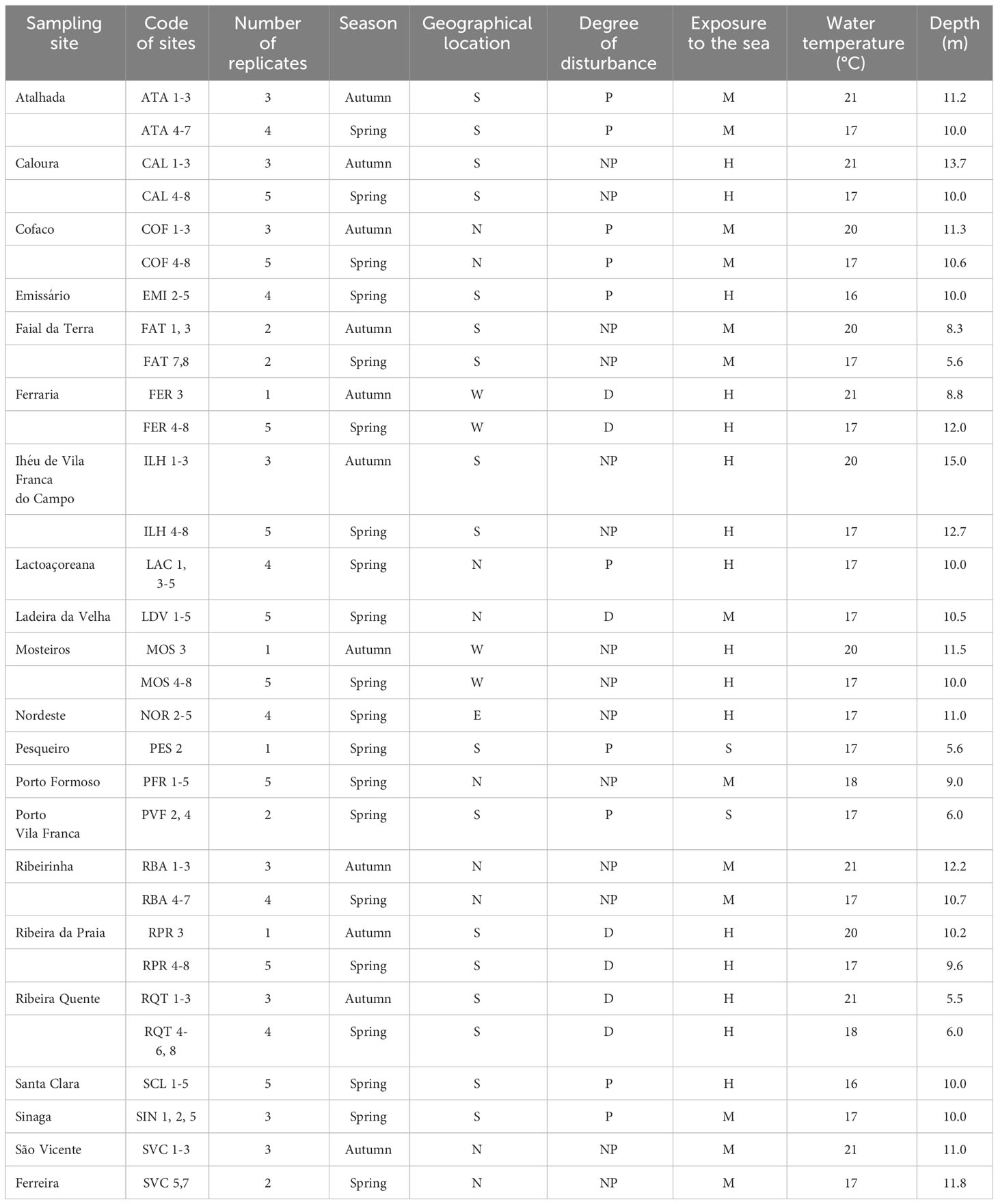
Table 1 Sampling sites, number of replicates by site, date of collection, geographical location (N, North; S, South; E, East; W, West), degree of disturbance (P, polluted; NP, not polluted; D, naturally disturbed), wave exposure (H, high; M, medium; S, sheltered), water temperature (°C) and depth of the samplings (m).
Samples were collected by SCUBA divers. At each site, three replicates (in the Autumn campaign) and 5 replicates (in the Spring campaign) were taken. Sea water temperature was recorded during each sampling. The sampling area was located using a two-digit random method adapted from Fenwick (1984) (see also Costa and Ávila, 2001). At each replicate, ten plants were gently pulled off of the rock by hand and put in a 0.45 mm mesh labelled cotton drawstring bag. In the laboratory, the samples were washed several times and the animals were removed by pouring the washing water through a 0.50 mm mesh sieve. All samples were labelled and preserved in 70% ethanol. After draining for about 30 minutes, the wet weight of the algae (AWW) was determined ( ± 0.01 g). The algae were then dried for 48 hours at 60°C and re-weighted ( ± 0.01 g) (algal dry weight, ADW). The samples were sorted under a binocular dissecting microscope and separated into major invertebrate groups. The live-collected molluscs were then sorted at the species level, identified and counted. Species authorities and synonymy of mollusc species follow the WoRMS database (WoRMS Editorial Board, 2023). All samples received a code and were deposited in the DBUA (Department of Biology of the University of the Azores) marine molluscs reference collection (access numbers DBUA 896-1001).
2.2 Statistical analysis
Species diversity was calculated for each replicate using species richness (S), total number of molluscs per sample (N), species diversity indices of Margalef (d) (Schoch and Dethier, 1996), Pielou (J’) (Warwick and Clarke, 1993) and Shannon-Wiener (H’) (Pearson and Rosenberg, 1978), and Simpson’s dominance index (1-λ’=1-Σ(Ni.(Ni-1)/(N.(N-1))) (Carr, 1996). Mollusc density was also calculated for both algal wet weight (total number of specimens of a species (ni) per 100 g of algal wet weight; ni/100 g AWW) and algal dry weight (ni/100 g ADW) and compared with previous studies.
The relationship between the epifaunal assemblages and a set of environmental factors was investigated using distance-based redundancy analysis (dbRDA, Legendre and Anderson, 1999). Multivariate multiple regression, using the DISTLM routine, tested the significance of these relationships by fitting a linear model based on Bray-Curtis dissimilarities on the square-root-transformed data. The number of species for each samples was calculated and similarly analysed (multiple regression), but based on Euclidean distances of untransformed data. To retain predictor variables with good explanatory power, both the AIC (An Information Criterion; Akaike, 1973) and BIC (Bayesian Information Criterion; Schwarz, 1978) routines were used and compared as selection criteria, the rationale being that AIC tends to be rather generous criterion, whilst BIC tends to be rather severe. All analyses were based on a “forward” selection procedure.
The discrepancy in sampling efforts between seasons (3 vs. 5 replicates), does not bias the comparisons for the following reasons: 1) like PERMANOVA, dbRDA can be used to analyse any balanced or unbalanced design, either with or without covariables, for fixed, random or mixed models, either with or without hierarchical nesting. The procedures (permutation test) implemented in these tests were made so that they cater for unbalanced designs. In these cases, the differences in weights (replicates) between groups are balanced by adjusting the corresponding probabilities; and 2) prior to analysis, predictor variables were examined for skewness and multi-collinearity. No transformations were deemed as necessary, but the variable season was removed from the model as it was highly correlated with seawater temperature. Seawater temperature was retained in the analyses which, does not suffer for such notable differences in replicates.
All analyses were run on the statistical package PRIMER 6 + PERMANOVA add-on (Plymouth Routines in Multivariate Ecological Research – Plymouth Marine Laboratory) (Anderson et al., 2008).
3 Results
The number of molluscan species (S) ranged from 3 (RQT2) to 24 (ATA4), while the total number of individuals ranged from 11 (RQT1) to 2,725 (RBA1). A total of 34,957 specimens of molluscs were found, belonging to 50 species (43 species of Gastropoda and 7 of Bivalvia) (see Supplementary Table 1). Shannon–Wiener diversity (H’) and species richness (Margalef’s index, d) showed similar trends, with higher values at RIB7 (H’=3.456) and ATA4 (H’=3.428), and at ILH6 (d=2.099) and FER (d=2.058) (Supplementary Table 1). The values of the Simpson’s dominance index are very similar in the Autumn replicates of RBA, ILH and FAT, and in the Spring replicates of FAT, MOS, SCL, LAC and EMI. The differences between the maximum and minimum values of the Autumn replicates of SVC and Spring replicates of PVF, are very high (Supplementary Table 1, Supplementary Figure 1).
Rissoidae was the best represented family, with 11 species. Bittium nanum (48.31%), Tricolia azorica (13.92%), the bivalve Parvicardium vroomi (8.44%) and the endemic rissoids Setia subvaricosa (13.58%), Alvania angioyi van Aartsen, 1982 (6.39%), Rissoa guernei (3.13%) and Setia ermelindoi (2,09%) were the most abundant species, comprising 95.86% of the total number of molluscs sampled (Figures 2, 3). Tricolia azorica was present in all but two replicates, whereas Bittium nanum and Setia subvaricosa were not present in just 3 and 10 replicates, respectively (Supplementary Table 2). A large percentage of the 50 species was rare; only 7 species contributing each with more than 1% to the total fauna, and 24% of the species occurred with only one or two individuals.

Figure 2 Apertural view of the most common gastropods (A) Alvania angioyi van Aartsen, 1982; (B) Rissoa guernei Dautzenberg, 1889; (C) Setia subvaricosa Gofas, 1990; (D) Setia ermelindoi S.P. Ávila & Cordeiro, 2015; (E) Bittium nanum (Mayer, 1864); (F) Tricolia azorica (Dautzenberg, 1889); (G) Parvicardium vroomi van Aartsen, Menkhorst and Gittenberger, 1984.
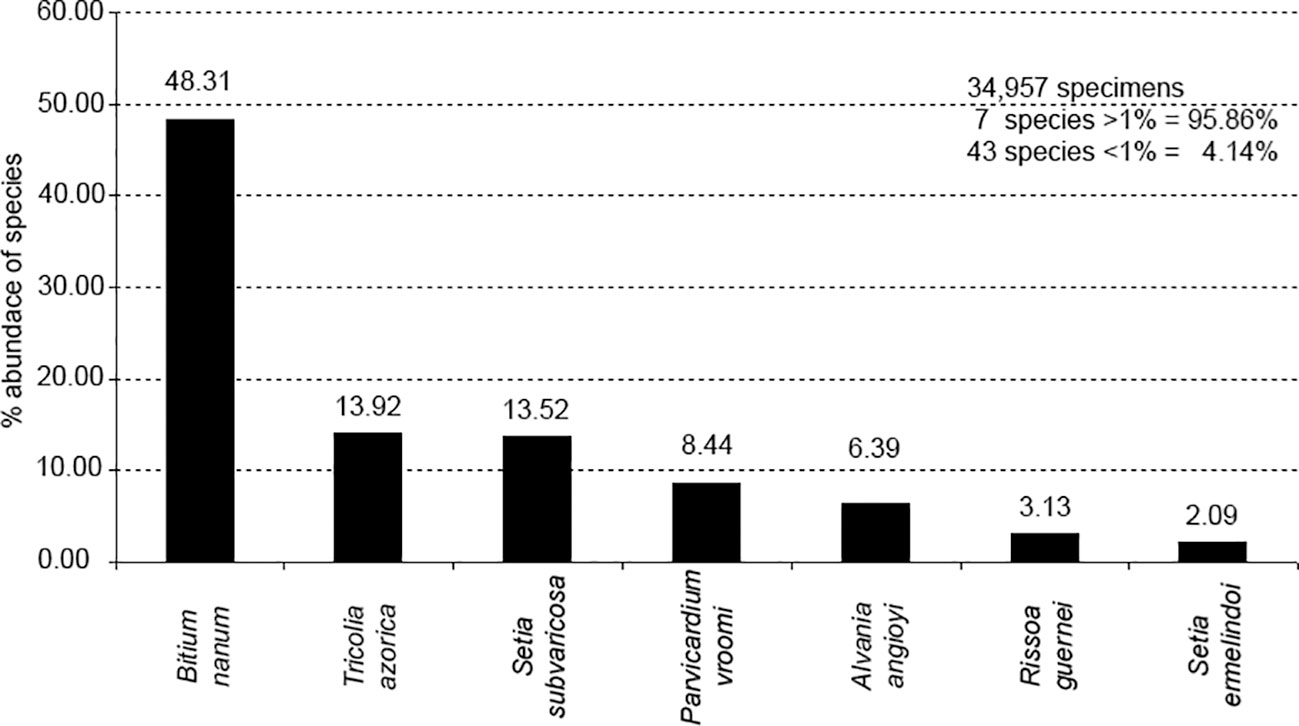
Figure 3 Percentage abundance of quantitatively important mollusc gastropod species in the total assemblage.
There was significant variation in the distribution of richness of assemblages (Table 2; Supplementary Table 1). When examining the influence of each predictor variable alone on the numbers of species (marginal tests in Table 2, Figure 4) there was a significant association with seawater temperature, algal volume, coastal orientation and disturbance, each explaining 11-13% of the variability observed. Unlike these, there was no significant effect of depth or wave exposure on species richness (marginal tests in Table 2). When considering all predictor variables (sequential tests in Table 2), both the AIC and BIC routines identified the most parsimonious model as having five predictor variables (coastal orientation, algal volume, seawater temperature, depth and wave exposure) (Table 2). These models accounted for 53% of total variation in species richness.

Table 2 Results of univariate multiple regression testing the effect of different predictor variables on the richness of epifaunal assemblages inhabiting Halopteris scoparia using both (a) AIC and (b) BIC information criteria.
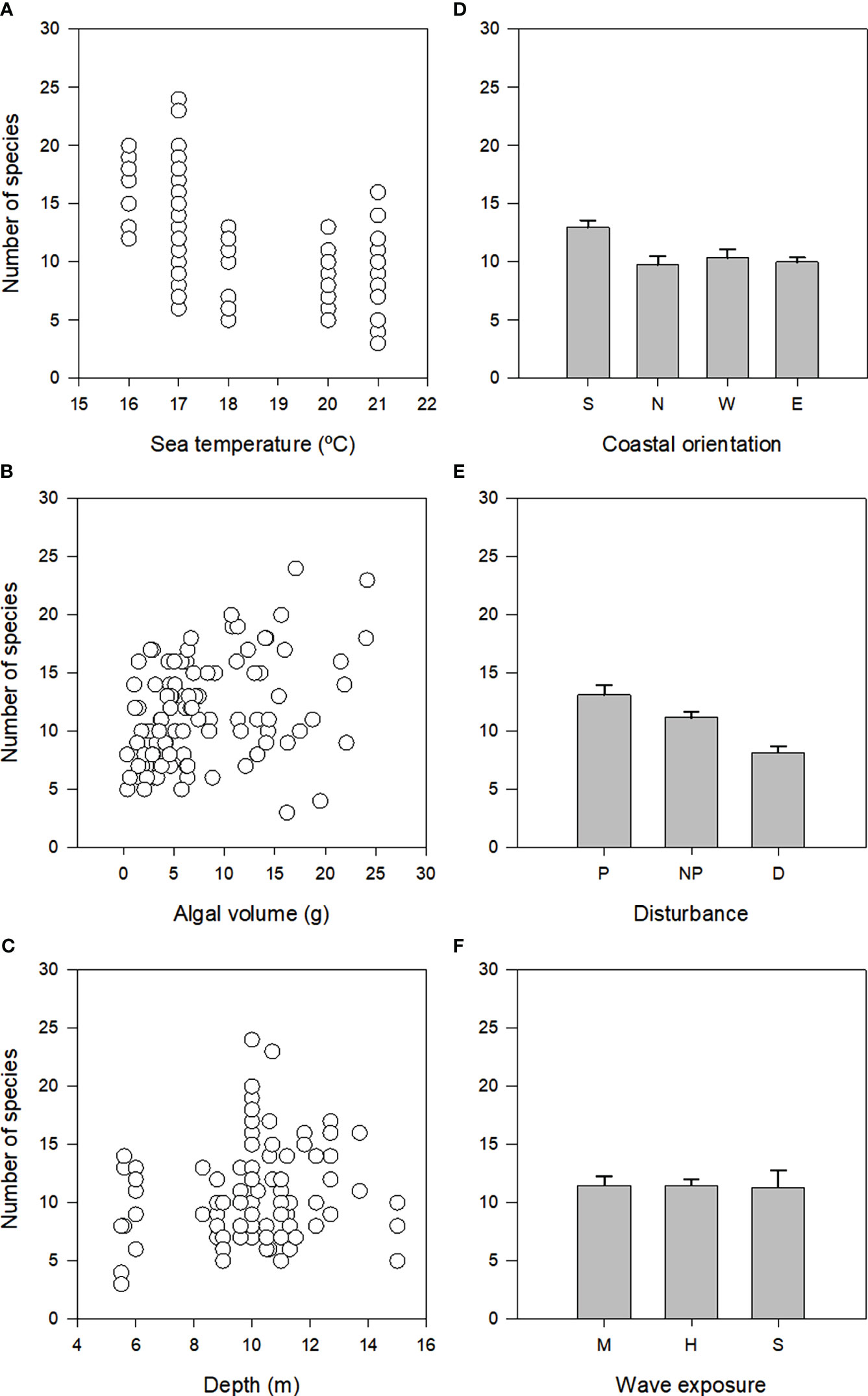
Figure 4 Relationship between number of species and seawater temperature, algal volume and depth (A-C), and mean (+SE) number of species among coastal orientation, disturbance and wave exposure (D-F).
When considering the whole assemblage, there was a significant variation in the assemblage structure (Table 3). This variation is associated with five (all but wave exposure) predictor variables, when considering their effects alone (marginal tests, Table 3, Figure 5), with each predictor variable explaining 3-11% of the variability observed. When considering all predictor variables (sequential tests in Table 3), the AIC routine identified the most parsimonious model as having all the six predictor variables, whilst the BIC routine only considered four of the six predictor variables: coastal orientation, seawater temperature, algal volume, and depth (Table 3). The AIC and BIC based models accounted to 41% and 49% of total variation, respectively.
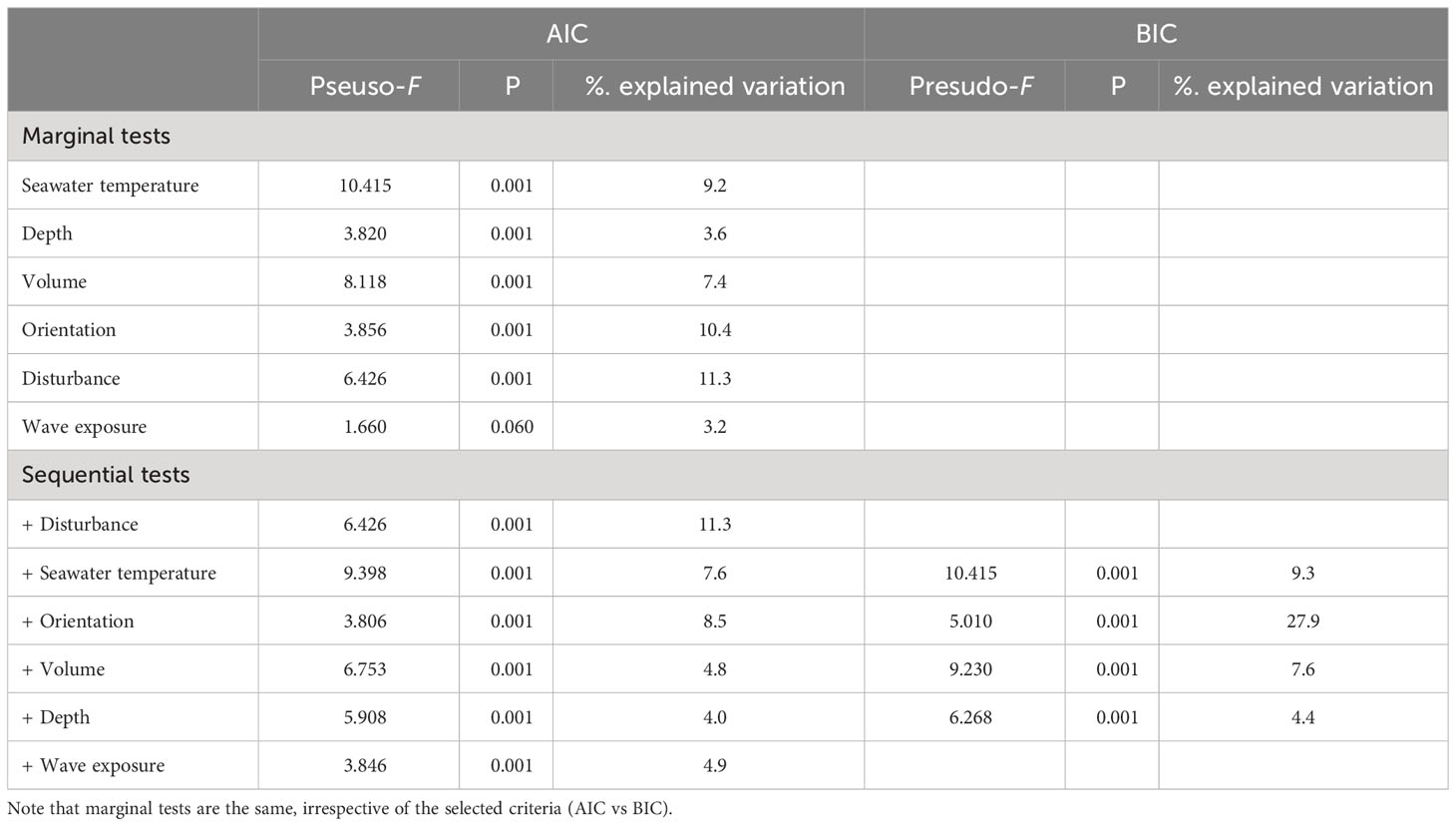
Table 3 Results of multivariate multiple regression testing the effect of different predictor variables on the structure of epifaunal assemblages inhabiting Halopteris scoparia using both (a) AIC and (b) BIC information criteria.
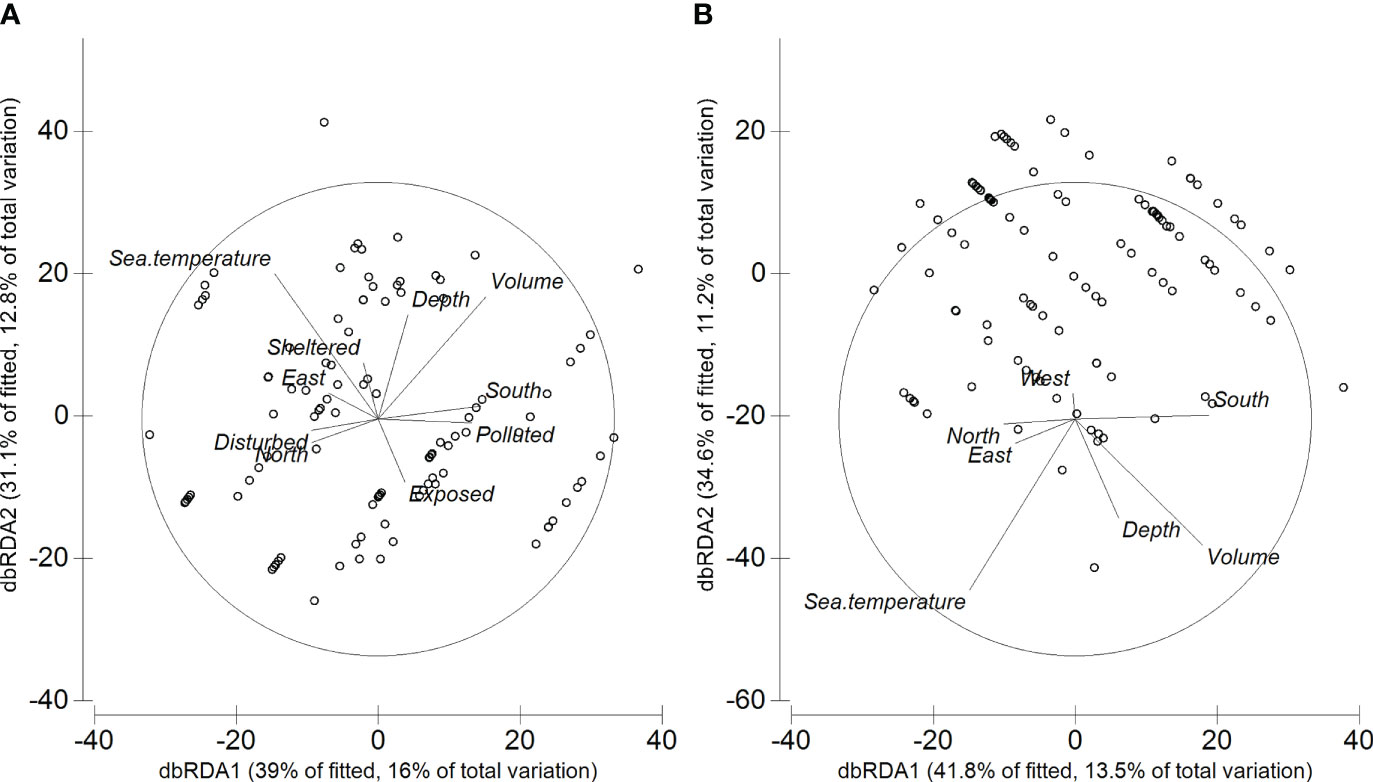
Figure 5 dbRDA plots based on (A) AIC and (B) BIC routines using Bray-Curtis dissimilarities on the square-root transformed data.
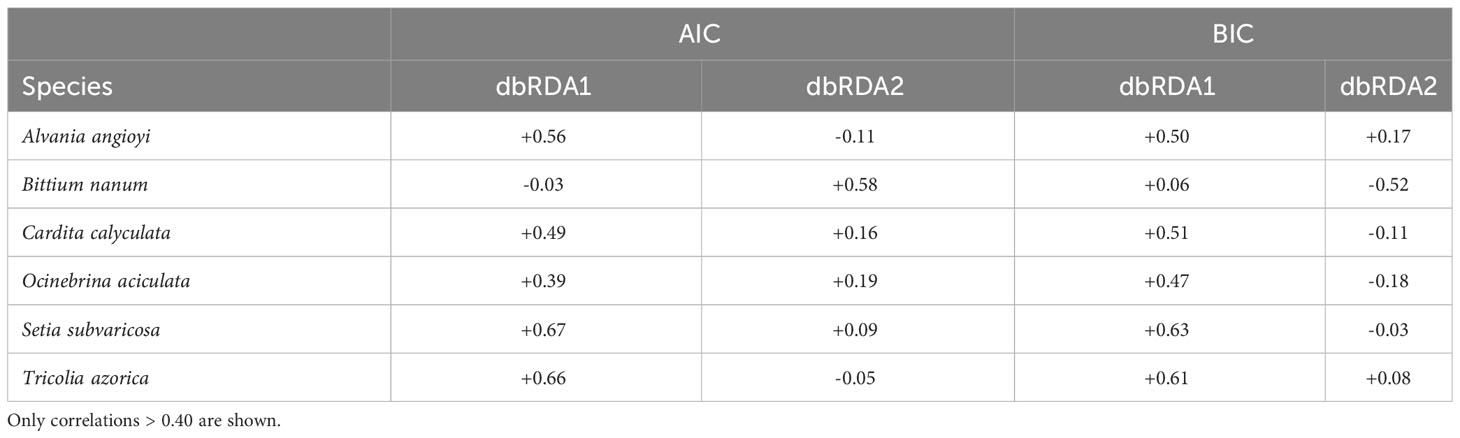
In terms of species, when using both the AIC and BIC routines, six species were strongly (r > 0.40) correlated with either dbRDA1 or dbRDA2 (Table 4): Bittium nanum explained most of the variation along the dbRDA2 axis, whilst Setia subvaricosa, Tricolia azorica, Alvania angioyi, Cardita calyculata and Ocinebrina aciculata explained most of the variation along the dbRDA 1 axis.
4 Discussion
4.1 Epifauna associated with Halopteris scoparia
At the time when this study was conducted (1996-1997), the invertebrates inhabiting Halopteris scoparia fronds were an abundant and taxonomically diversified group, dominated by peracarid crustaceans, but with a large proportion of molluscs (Costa, 2003). Prior to the recent invasion of the Azorean shores by the now dominant alga Rugulopteryx okamurae (Dictyotales, Ochrophyta), H. scoparia was a perennial and common alga in São Miguel Island, being very important for the local epiphytic fauna, since its biomass presented a low seasonal variation (Neto, 1997). Thus, this alga constituted a stable habitat for the establishment of assemblages of invertebrates and its dense ramification enabled the proliferation of a large epiphytic community that profited from all the microhabitats available in the algal fronds (Sánchez-Moyano et al., 2000a) and on which other invertebrates feed.
4.2 Comparisons with other studies
Most of the mollusc species found in algal fronds in the Azores belong to the family Rissoidae (e.g. Bullock et al., 1990; Gofas, 1990; Azevedo, 1991; Ávila, 1998; 2000a, 2003, 2005; Costa and Ávila, 2001; Ávila et al., 2005; Cordeiro and Ávila, 2015; Baptista et al., 2021). However, Bittium nanum (Mayer, 1864) [formerly misidentified by authors as Bittium latreillii (Payraudeau, 1826)] usually dominates these communities, with very high densities (Ávila, 2000b, 2003). Tricolia azorica (Dautzenberg, 1889) (see Baptista et al., 2023) is another very common gastropod, as well as Jujubinus pseudogravinae Nordsieck, 1973 (cf. Ávila et al., 2011), Manzonia unifasciata Dautzenberg, 1889 and Alvania sleursi (Amati, 1987). Besides T. azorica, endemics as Rissoa guernei Dautzenberg, 1889, Setia subvaricosa Gofas, 1990 and Setia ermelindoi S.P. Ávila & Cordeiro, 2015, are also found among algae (Costa and Ávila, 2001; Cordeiro and Ávila, 2015). Among the bivalves, Parvicardium vroomi van Aartsen, Menkhorst & Gittenberger, 1984 seems to be the most important species in these epifaunistic communities (Ávila, 2000b; Costa and Ávila, 2001), but Talochlamys multistriata (Poli, 1795) is also quite common.
The densities of molluscs found by us in this work are relatively high and some of them are the highest ever reported to the Azores: Bittium nanum [59,474 individuals/100 g ADW (Algal Dry Weigh)]; Parvicardium vroomi (4,867 individuals/100 g ADW); Setia subvaricosa (2,949 individuals/100 g ADW) and S. ermelindoi (762 individuals/100 g ADW) (Table 5). Our findings also indicate that within the malacofauna associated with H. scoparia in the Azores, the gastropod family Rissoidae was the most prevalent, as evidenced by the identification of 11 species. The endemic Setia subvaricosa was generally the most abundant rissoid species in the samples, and the also endemics Alvania angioyi, Rissoa guernei and Setia ermelindoi were relatively well represented (Supplementary Table 2).

Table 5 Maximum density of molluscs/100 g ADW (algal dry weight) in this and previous works in the Azores.
In the malacological communities of H. scoparia in the Mediterranean (South of Spain), Rissoa guerinii Récluz, 1843 was very abundant (Sánchez-Moyano et al., 2000a) and in the coast of the Basque country, Rissoa parva (da Costa, 1778) was the dominant species (Borja, 1986a, b, c). In spite of being well represented in H. scoparia in São Miguel, Rissoa guernei was not very abundant. Barleeia unifasciata (Montagu, 1803) a highly abundant species in H. scoparia in the Mediterranean (Borja, 1986a, b, c) does not occur in the Azores (Ávila, 2005; Freitas et al., 2019). The most frequent species of molluscs found on this alga in the Mediterranean by Chemello and Milazzo (2002) were the rissoids Alvania lineata Risso, 1826, Alvania scabra (Phillippi, 1844) [reported as A. oranica (Philippi, 1844)], Rissoa similis Scacchi, 1836 and Setia ambigua (Brugnone, 1873), although some species belonging to other families, such as Cerithiidae (Bittium spp.), Tricoliidae (Tricolia spp.) and Columbellidae (Collumbela sp.) were also relatively abundant and frequent. The Azorean congenerics Bittium nanum and Tricolia azorica were also very abundant in the samples analysed by us and indeed, B. nanum is the dominant species in almost all the samples, making almost 50% of all the counted specimens (Figure 3).
Bittium reticulatum (da Costa, 1778), Rissoa guerinii and Alvania discors (Allan, 1818) [reported as Alvania montagui Payraudeau, 1826] were found to be dominant in southern Spain locations studied by Sánchez-Moyano et al. (2000b), in conditions of high sedimentation, in communities where the diversity of molluscs was also high. In the same locations, Jujubinus ruscurianus (Weinkauff, 1868) and Skeneopsis planorbis (Fabricius O., 1780) were also abundant. The latter species and the Azorean endemism Jujubinus pseudogravinae were also frequent in H. scoparia samples from São Miguel Island. All these species and their congeneric seem to be characteristic from these algal communities (Borja, 1986a, b, c; Russo, 1997; Sánchez-Moyano et al., 2000a; Chemello and Milazzo, 2002). It is important to stress the endemic character of many mollusc epiphytic species in H. scoparia at São Miguel Island, especially the gastropods A. angioyi, R. guernei, Setia ermelindoi and S. subvaricosa.
Bivalves as Cardita calyculata (Linnaeus, 1758), Gregariella semigranata (Reeve, 1858) and Parvicardium vroomi had already been reported as algal turf inhabitants in São Miguel by Morton et al. (1998) and Ávila et al. (2005). Cardita seems to be a frequent genus in this type of community in the Mediterranean (Russo, 1997), as well as P. vroomi that was found there by Sánchez-Moyano et al. (2000b) on H. scoparia. P. vroomi was the most abundant bivalve in the samples of H. scoparia from São Miguel, similarly to what was reported by Ávila (2000b) for the multispecific algal samples collected on the north shore of this island at São Vicente Ferreira.
Borja (1986b) claimed that B. reticulatum is more abundant in November and that may be the reason why our Autumn samples are much richer in B. nanum than the Spring samples. However, and at least for the infralittoral communities studied by Azevedo (1991), the seasonal variation of B. nanum abundance was not very high, in spite of the higher abundance observed in the winter, especially in the most exposed locations on the north coast of São Miguel Island.
4.3 Patterns of distribution of the mollusc epifauna of Halopteris scoparia
The choice of a single substrate such as H. scoparia for spatio-temporal comparisons of the mollusc community minimizes structural variability. However, it is possible that the environmental parameters may contribute to changes in the epifaunal populations by affecting the structure of biogenic habitats themselves. For example, hydrodynamics can influence the size and morphological structure of algae (Neto, 1997, 2001; Neto et al., 2000), which in turn may contribute to variation in the distribution of associated biota (Boaden et al., 1975).
Despite some variation among models, there were four environmental predictors that were common to all the four analyses (richness and assemblage structure using both AIC and BIC; cf. Tables 2, 3): algal volume (that correlates with algal dry weight), seawater temperature, coastal orientation and depth.
The observation by Edgar (1983a), and many other subsequent papers (e.g., Rueda and Salas, 2003; Rosenfeld et al., 2017), that the abundance of molluscs is related to the algal biomass was corroborated in São Miguel by Azevedo (1992), who also noted that the algal biomass had a negative influence on diversity indices, due to increases in dominance. As such, it is not surprising that in the present study, there was also an important and consistent association between richness and assemblage structure with algal biomass (Figure 4B). This is most likely the result of a simple species-area relationship (cf. Ávila et al., 2018 and references therein), with greater amounts of habitat (the alga) supporting a richer assemblage of molluscs, although the influence of other factors acting on host size or morphologies, cannot be discarded without further examination.
Along with algal volume, seawater temperature (or seasons, which was discarded from the model due to collinearity) was also one of the most important factors associated with variation in richness and structure of mollusc assemblages (Figure 4A). Seasonality of abiotic factors (e.g., temperature and photoperiod) is mentioned by Edgar (1983b) as having a marked influence in the epifaunistic community and may be directly related to the life cycle of epifaunal species. Moreover, the great number of ovigerous females and juveniles of several invertebrate taxa other than the molluscs that were observed in the Spring samples by Costa (2003), and the fact that, in the malacological analysis, Bittium nanum was the species that most contributed for the observed separation due to high abundances related to reproduction, in the Autumn samples, reinforces the idea of a seasonal rather than annual pattern. The photoperiod is related to the onset of reproduction of many invertebrate species in Spring. The increased photoperiod in Spring will also result in an increased productivity of epiphytic filamentous algae. The number of animals may increase as a consequence of the increased heterogeneity of the habitat (provided by the growth of the host plant) and of the increase in epiphytes’ density. These fluctuations are particularly expected in temperate environments such as the Azorean islands, sheltered enough to allow a considerable growth of epiphytic algae (Edgar, 1983b). Also, the greatest values of algal biomass occur in Spring and Summer (Neto, 1997), which is the period when our second sampling campaign was carried out. Thus, the higher abundance of animals on Halopteris scoparia observed in the samples collected in the Spring may be related to an increased biomass of that alga in that period and also to an increase in the density of epiphytes. Similar seasonal fluctuations have been reported for epiphytic communities of Bryozoa (Conradi et al., 2000) and for epiphytic fauna of macrophytes all over the world (Edgar and Aoki, 1993). These fluctuations are related to variations in reproduction and recruiting associated with variations in food and substrate availability, factors which are not independent. Seasonal fluctuations in abundance related to population cycles of many species may be important for the detected variation. Finally, the space availability in the host plant also limits the number of organisms (Robertson and Mann, 1982). Therefore the decrease in biomass that H. scoparia suffers in winter (Neto, 1997) may result in reduced epifaunistic populations due both to a decreased microhabitat (Schneider and Mann, 1991) and to a decrease in food availability, which is not so easily retained in the plant ramifications (Borja, 1986b).
To a lesser extent, coastal orientation (Figure 4D) and depth (Figure 4C) were also important factors associated with variation of the structure of mollusc assemblages by all models. Coastal orientation can determine a number of environmental conditions, which in turn may influence the distribution of organisms. For instance, coastal orientation can determine the exposure to predominant winds and oceanic swells (e.g. leeward versus windward coasts of islands). Wave-action has profound effects on nearly all aspects of an organism’s life (Denny, 1988), such as recruitment and dislodgment of organisms (e.g. Vadas et al., 1990; Blanchette, 1997), supply of food and nutrients (e.g. McQuaid and Lindsay, 2007) and foraging activities of consumers (e.g. Vergés et al., 2009; Taylor and Schiel, 2010). It is thus not surprising that differences in community structure have been found between the leeward and windward coasts of islands (e.g. Hassett and Boehlert, 1999; Tuya and Haroun, 2006; Wernberg and Connell, 2008), even though Martins et al. (2013) found no consistent differences in the structure of macroalgae between the northern and southern coasts of São Miguel. In this study, the high number of sites in the North (30) and South (58) and only 4 in the East and 12 in the West prevents a more accurate analysis regarding the influence of geographical location in mollusc diversity. Still, it is obvious that both the bivalve Parvicardium vroomi and the gastropod Rissoa guernei are rare in these sites (E, W), when compared with North and South sites (Supplementary Table 2). In spite of the scarcity of sheltered sites used in our analysis (only 3 sites) when compared with medium or highly exposed sites (43 and 58 sites, respectively) some considerations can be made. Tricolia azorica and Setia subvaricosa were more abundant in sheltered sites in contrast with Parvicardium vroomi that was rare in these locations. Sánchez-Moyano et al. (2000a) observed, in Algeciras bay (Spain), that when wave exposure increased, there was a tendency to an increase in diversity and a decrease in the abundance of organisms living in H. scoparia. This fact was also detected in the same bay by Conradi et al. (2000) in peracarids inhabiting the bryozoan Bugula neritina, as a consequence of a higher number of taxa represented in the more exposed localities. Kluijver (1997) reported that the distribution of vagile organisms in infralittoral communities of the North Sea was influenced by water movements, with a lower abundance of organisms in the more sheltered zones. In contrast, Tararam et al. (1981) in a study on Sargassum algae in Brazil did not find a relation between different wave exposure levels and diversity even though density would be higher in sheltered conditions. These apparently contradictory findings could only show different patterns in sedimentation, a factor that is strongly dependent on hydrodynamics (Moore, 1972). In very exposed sites there is no sediment deposition; in moderately exposed sites deposition of coarser sediments is allowed whereas in sheltered areas fine particulated matter will settle down. More detritus usually promote diversity or at least density of organisms, possibly by raising the number of detritivorous (Soughtgate, 1982). However, the deposition of fine sediments that occur at less exposed sites covers epifauna (Gibbons, 1988) and can decrease abundance and diversity by collapsing the spaces between branches and interfering with the feeding structures of the organisms (Hicks, 1980).
Similarly to coastal orientation, depth is another key environmental factor in subtidal habitats, which influence a number of environmental conditions. For instance, light intensity and water motion decline with depth affecting photosynthetic rate and nutrient uptake (see Hurd (2000) for a review). Even though the range of depth sampled was relatively small (5.5 to 15 m; Table 1), the first few metres below the surface are also where, proportionally, the largest changes in environmental conditions associated with depth occur, which may explain the observed association of the biota with depth (Figure 4C; Tables 2, 3). Moreover, the relationship between depth and sample richness seems to be hump-shaped rather than linear. This could in theory be associated with disturbance caused by water motion, which decreases with depth.
Despite its significant effect when examined in isolation (cf. Figure 4E), disturbance was seldom selected by models when considering all predictor variables together. This suggests that variation associated with disturbance is proportionally small (see Tables 2, 3) and/or is already accounted for in terms of intensity and direction by previous predictor variables in the model (Anderson et al., 2008). We note that in some of the disturbed sites, Parvicardium vroomi and Setia subvaricosa, along with Alvania angioyi, are the most abundant species, in contrast with non-poluted and polluted sites, where usually Bittium nanum is, by far, the most abundant species.
4.4 Molluscs as bioindicators of environmental stress
Analysis of species abundance demonstrated that the endemic rissoid Setia subvaricosa, notably scarce on the north shore, was significantly more abundant in the south, especially in areas considered as naturally disturbed or polluted. This pattern suggest that S. subvaricosa could be used as an indicator species for disturbances within the Azores. Thus, the application in the Azores of this methodology aimed to detect disturbance in marine communities induced by effluents, favours a sampling program in Spring-Summer (when disturbance seems to be more susceptible to detection), and the use of H. scoparia in the subtidal zone, as the target alga is recommended due to its large covering of rocky shore substrates.
5 Conclusions
In the Azores, Halopteris scoparia is a general representative habitat for macrobenthic subtidal communities. Our study shows that a significant variation in the distribution of richness of assemblages characterizes our samples, and that four environmental predictors are common to all the four analyses implemented (richness and assemblage structure using both AIC and BIC): algal volume (that correlates with algal dry weight), seawater temperature, coastal orientation and depth. Finally, we suggest its implementation with a sampling program in Spring-Summer, when disturbance seems to be more susceptible to detection.
The importance of baseline surveys on biodiversity lies in their ability to provide critical reference points for understanding disturbances in ecosystems such as those associated with the impact of current and future climate changes on coastal oceanic island ecosystems. This is particularly crucial in isolated oceanic islands like the Azores, where ecosystems are often unique and sensitive to changes (e.g., habitat destruction, climate change, pollution). These ecological units are particularly vulnerable to the introduction of non-indigenous marine species (NIS), and understanding the biological processes and patterns that lead to such ecological, economic and even human health impacts is crucial to preventing the spread of NIS. Thus, the sound and detailed data collected over the course of this two-year study carried out in 1996-1997 help in identifying changes in species composition and abundance over time, and provides a much useful snapshot of the ecological conditions that prevailed in the Azores before the arrival of many invasive marine species, of which the Rugulopteryx okamurae (Dictyotales, Ochrophyta) alga is, at this moment, the most worrying (Faria et al., 2022a, b). By comparing current ecological data with baseline data, we can better understand the extent and nature of these disturbances and their impact on biodiversity.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
SÁ: Data curation, Formal analysis, Investigation, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AC: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – review & editing. PM: Writing – review & editing. JB: Writing – review & editing. AP: Writing – review & editing. JF: Writing – review & editing. GM: Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. SA and PM acknowledge their contracts by the project M1.1.A/INFRAEST CIENT/A/001/2021 - Base de Dados da PaleoBiodiversidade da Macaronésia, funded by Direção Regional da Ciência e Tecnologia, Governo Regional dos Açores. This work also benefited from FEDER funds, through the Operational Program for Competitiveness Factors – COMPETE, and from National Funds, through FCT (UIDB/50027/2020, POCI-01-0145-FEDER-006821, LA/P/0048/2020), as well as through the Regional Government of the Azores (M1.1.a/005/Funcionamento-C-/2016, CIBIO-A; M3.3.B/ORG.R.C./005/2021).
Acknowledgments
We acknowledge valuable comments and suggestions made by Prof. Malcom Jones that significantly improved the manuscript. We thank to all the people who helped in field sampling and sample sorting.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1361068/full#supplementary-material
References
Akaike H. (1973). “Information theory and an extension of the maximum likelihood principle,” in 2nd International Symposium on Information Theory (Akademia Kiado). Eds. Petrov B. N., Csaki F.(Budapest: Akadémiai Kiadó), 267–281.
Anderson M. J., Gorley R. N., Clarke K. R. (2008). PERMANOVA+ for PRIMER: Guide to software and statistical methods (Plymouth, UK: PRIMER-E).
Ávila S. P. (1998). Zonação intertidal de uma comunidade malacológica numa lagoa costeira localizada na costa Sul da ilha do Pico, Açores. Açoreana 8, 436–486.
Ávila S. P. (2000a). The shallow-water Rissoidae (Mollusca, Gastropoda) of the Azores and some aspects of their ecology. Iberus 18, 51–76.
Ávila S. P. (2000b). Sistemática e ecologia dos Moluscos (Gastropoda, Bivalvia e Polyplacophora) litorais em São Vicente, Capelas (São Miguel, Açores) (Ponta Delgada: Universidade dos Açores), 113 pp. Unpublished MSc thesis.
Ávila S. P. (2003). The littoral molluscs (Gastropoda, Bivalvia and Polyplacophora) of São Vicente, Capelas (São Miguel Island, Azores): ecology and biological associations to algae. Iberus 21, 11–33.
Ávila S. P. (2005). Processos e Padrões de Dispersão e Colonização nos Rissoidae (Mollusca: Gastropoda) dos Açores (Ponta Delgada: Universidade dos Açores), x+329 pp. Unpublished PhD thesis.
Ávila S. P., Borges J. P., Martins A. M. F. (2011). The littoral trochoidea (Mollusca: gastropoda) of the azores. J. Conchol. 40, 408–427.
Ávila S. P., Cardigos F., Santos R. S. (2007). Comparison of the community structure of the marine molluscs of the “Banco D. João de Castro” seamount (Azores, Portugal) with that of typical inshore habitats on the Azores archipelago. Helgoland Mar. Res. 61, 43–53. doi: 10.1007/s10152-006-0052-5
Ávila S. P., Cordeiro R., Madeira P., Silva L., Medeiros A., Rebelo A. C., et al. (2018). Global change impacts on large-scale biogeographic patterns of marine organisms on Atlantic oceanic islands. Mar. pollut. Bull. 126, 101–112. doi: 10.1016/j.marpolbul.2017.10.087
Ávila S. P., Madeira P., Rebelo A. C., Melo C., Hipólito A., Pombo J., et al. (2015). Phorcus sauciatus (Koch 1845) (Mollusca: Gastropoda) in the Archipelago of the Azores (NE Atlantic Ocean): the onset of a biological invasion. J. Molluscan Stud. 81, 516–521. doi: 10.1093/mollus/eyv012
Ávila S. P., Santos A. C., Penteado A. M., Rodrigues A. M., Quintino I., MaChado M. I. (2005). The molluscs of the intertidal algal turf in the Azores. Iberus 23, 67–76.
Azevedo J. M. N. (1991). Estudo das comunidades malacológicas fitais do litoral em São Miguel, Açores (Ponta Delgada: Provas de A.P.C.C. Universidade dos Açores), iv+75 pp.
Azevedo J. M. N. (1992). Algae-associated marine molluscs in the Azores. Biol. J. Linn. Soc. 46, 177–187. doi: 10.1111/j.1095-8312.1992.tb00859.x
Baptista L., Fassio G., Gofas S., Oliverio M., Ávila S. P., Santos A. M. (2023). Evaluating the taxonomic status of the large sized Tricolia Risso 1826 in the Northeast Atlantic and Mediterranean Sea. Mol. Phylogenet. Evol. 186, 107857. doi: 10.1016/j.ympev.2023.107857
Baptista L., Meimberg H., Ávila S. P., Santos A. M., Curto M. (2021). Dispersal ability, habitat characteristics, and sea-surface circulation shape population structure of Cingula trifasciata (Gastropoda: Rissoidae) in the remote Azores Archipelago. BMC Ecol. Evol. (formerly BMC Evol. Biol.) 21, 128. doi: 10.1186/s12862-021-01862-1
Blanchette C. A. (1997). Size and survival of intertidal plants in response to wave action: a case study with Fucus gardneri. Ecology 78, 1563–1578. doi: 10.1890/0012-9658(1997)078[1563:SASOIP]2.0.CO;2
Boaden P., O’connor J. R., Seed R. (1975). The composition and zonation of a Fucus serratus community in Strangford Lough, Co. Down. J. Exp. Mar. Biol. Ecol. 17, 111–136. doi: 10.1016/0022-0981(75)90026-X
Borges P. (2003). Ambientes litorais nos Grupos Central e Oriental do Arquipélago dos Açores. Conteúdos e Dinâmica de Microescala (Ponta Delgada: Universidade dos Açores), xxxiii + 413 pp. Unpublished PhD thesis.
Borja A. (1986a). Biology and ecology of three intertidal gastropods: Rissoa parva, Barleeia unifasciata and Bittium reticulatum. 1. Population structure and dynamics. Cahiers Biologie Mar. 27, 491–507.
Borja A. (1986b). Variacion anual de la abundancia de Rissoa parva (da Costa 1779), Barleeia unifasciata (Montagu 1803) y Bittium reticulatum (da Costa 1778) (Mollusca: Gastropoda) sobre el alga Halopteris scoparium L. Sauv. Iberus 6, 215–227.
Borja A. (1986c). La alimentación y distributión del espacio en tres moluscos gasterópodos: Rissoa parva (da Costa), Barleeia unifasciata (Montagu) y Bittium reticulatum (da Costa). Cahiers Biologie Mar. 27, 69–75.
Bullock R. C. (1995). The distribution of the molluscan fauna associated with the intertidal coralline algal turf of a partially submerged volcanic crater, the Ilhéu de Vila Franca, São Miguel, Azores. Açoreana Suplemento 4, 9–55.
Bullock R. C., Turner R. D., Fralick R. A. (1990). Species richness and diversity of algal-associated micromolluscan communities from São Miguel, Açores. Açoreana Suplemento 2, 39–58.
Carr M. (1996). Primer user manual (Plymouth routines in multivariate ecological research). Plymouth Mar. Lab, 26.
Chapman G. (1955). Aspects of the fauna and flora of the Azores. VI. The density of animal life in the Coralline alga zone. Ann. Magazine Natural History 12, 801–805. doi: 10.1080/00222935508655700
Chemello R., Milazzo M. (2002). Effect of algal architecture on associated fauna: some evidence from phytal molluscs. Mar. Biol. 140, 981–990. doi: 10.1007/s00227-002-0777-x
Conradi M., López-González P., Cervera J., Garía-Gómez J. (2000). Seasonality and spatial distribution of Peracarids associated with the bryozoan Bugula neritina in Algeciras Bay, Spain. J. Crustacean Biol. 20, 334–349. doi: 10.1163/20021975-99990045
Cordeiro R., Ávila S. P. (2015). New species of Rissoidae (Mollusca: Gastropoda) from the Archipelago of the Azores (northeast Atlantic) and a checklist of the family for the region. Zookeys 480, 1–19. doi: 10.3897/zookeys.480.8599
Cordeiro R., Bagaço L., Santos M. A., Ávila S. P. (2019). First record of Nereiphylla paretti (Polychaeta: Phyllodocidae) in the Azores, with a compiled list of the shallow-water marine polychaetes from the archipelago. Cahiers Biologie Mar. 60, 69–79. doi: 10.21411/CBM.A.71730B95
Costa A. C. (2003). Diversidade de invertebrados das comunidades algais do subtidal de São Miguel e perturbação ambiental (Ponta Delgada: Universidade dos Açores), xv+196 pp. Unpublished PhD thesis.
Costa A. C., Ávila S. P. (2001). Macrobenthic mollusc fauna inhabiting Halopteris spp. subtidal fronds in São Miguel island, Azores. Scientia Marina 65, 117–126. doi: 10.3989/scimar.2001.65n2117
Couto R. P., Rodrigues A. S., Neto A. I. (2015). Shallow-water hydrothermal vents in the Azores (Portugal). J. Integrated Coast. Zone Manage. 15, 495–505. doi: 10.5894/rgci584
Denny M. W. (1988). Biology and mechanics of the wave-swept environment (Princeton: PhD Thesis, Princeton University).
Edgar G. (1983a). The ecology of south-east Tasmanian phytal animal communities. I. Spatial organization on a local scale. J. Exp. Mar. Biol. Ecol. 70, 129–157. doi: 10.1016/0022-0981(83)90127-2
Edgar G. (1983b). The ecology of south-east Tasmanian phytal animal communities. II. Seasonal change in plant and animal populations. J. Exp. Mar. Biol. Ecol. 70, 159–179. doi: 10.1016/0022-0981(83)90128-4
Edgar G., Aoki M. (1993). Resource limitation and fish predation: their importance to mobile epifauna associated with Sargassum. Oecologia 95, 122–133. doi: 10.1007/BF00649515
Faria J., Prestes A. C. L., Moreu I., Cacabelos E., Martins G. M. (2022b). Dramatic changes in the structure of shallow-water marine benthic communities following the invasion by Rugulopteryx okamurae (Dictyotales, Ochrophyta) in Azores (NE Atlantic). Mar. pollut. Bull. 175, 113358. doi: 10.1016/j.marpolbul.2022.113358
Faria J., Prestes A., Moreu I., Martins G. M., Neto A. I., Cacabelos E. (2022a). Arrival and proliferation of the invasive seaweed Rugulopteryx okamurae in NE Atlantic islands. Botanica Marina 65, 45–50. doi: 10.1515/bot-2021-0060
Fenwick G. (1984). Life history and population biology of the giant ostracod Leuroleberis zealandica (Baird 1850) (Myodocopida). J. Exp. Mar. Biol. Ecol. 77, 255–289. doi: 10.1016/0022-0981(84)90123-0
Freitas R., Romeiras M., Silva L., Cordeiro R., Madeira P., González J. A., et al. (2019). Restructuring of the “Macaronesia” biogeographic unit: a marine multi-taxon biogeographical approach. Sci. Rep. 9, 15792. doi: 10.1038/s41598-019-51786-6
Fretter V., Graham A. (1977). The prosobranch molluscs of Britain and Denmark. Part 2 - Trochacea. J. Molluscan Stud. Suppl. 3, 39–100.
Gibbons M. J. (1988). The impact of wave exposure on the meiofauna of Gelidium pristoides (Turner) Kuetzing (Gelidiales: Rhodophyta). Estuar. Coast. Shelf Sci. 27, 581–593. doi: 10.1016/0272-7714(88)90070-4
Gofas S. (1990). The littoral rissoidae and anabathridae of São Miguel, Azores. Açoreana Suplemento 2, 97–134.
Gunnill F. (1982). Effects of plant size and distribution on the numbers of invertebrate species and individuals inhabiting the brown alga Pelvetia fastigiata. Mar. Biol. 69, 263–280. doi: 10.1007/BF00397492
Gunnill F. (1983). Seasonal variations in the invertebrate faunas of Pelvetia fastigiata (Fucaceae): Effects of plant size and distribution. Mar. Biol. 73, 115–130. doi: 10.1007/BF00406879
Gunnill F. (1984). Differing distributions of potentially competing amphipods, copepods and gastropods among specimens of the intertidal alga Pelvetia fastigiata. Mar. Biol. 82, 277–291. doi: 10.1007/BF00392408
Gunnill F. (1985). Growth, morphology and microherbivore faunas of Pelvetia fastigiata (Phaeophyta, Fucaceae) at La Jolla, California, U.S.A. Botanica Marina 28, 187–199. doi: 10.1515/botm.1985.28.5.187
Hassett R. P., Boehlert G. W. (1999). Spatial and temporal distributions of copepods to leeward and windward of Oahu, Hawaiian Archipelago. Mar. Biol. 134, 571–584. doi: 10.1007/s002270050572
Hawkins S. J., Burnay L. P., Neto A. I., Tristão Da Cunha R., Martins A.M. De F. (1990). A description of the zonation patterns of molluscs and other important biota on the south coast of São Miguel, Azores. Açoreana Suplemento 2, 21–38.
Hicks G. (1980). Structure of phytal harpacticoid copepod assemblages and the influence of habitat complexity and turbidity. J. Exp. Mar. Biol. Ecol. 44, 157–192. doi: 10.1016/0022-0981(80)90151-3
Hurd C. L. (2000). Water motion, marine macroalgal physiology, and production. J. Phycol. 36, 453–472. doi: 10.1046/j.1529-8817.2000.99139.x
Kluijver M. (1997). Sublittoral communities of North sea hard-substrata (Amsterdam: Academisch proefschrift. Universiteit van Amsterdam), 330 pp.
Leber K. (1985). The influence of predatory Decapods, refuge, and microhabitat selection on seagrass communities. Ecology 66, 1951–1964. doi: 10.2307/2937391
Legendre P., Anderson M. J. (1999). Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 69, 1–24. doi: 10.1890/0012-9615(1999)069[0001:DBRATM]2.0.CO;2
Martins M., Carreiro-Silva M., Martins G. M., Barcelos E Ramos J., Viveiros F., Couto R. P., et al. (2021). Ervilia castanea (Mollusca, Bivalvia) populations adversely affected at CO2 seeps in the North Atlantic. Sci. Total Environ. 754, 142044. doi: 10.1016/j.scitotenv.2020.142044
Martins G. M., Patarra R. F., Álvaro N. V., Prestes A. C. L., Neto A. I. (2013). Effects of coastal orientation and depth on the distribution of subtidal benthic assemblages. Mar. Ecol. 34, 289–297. doi: 10.1111/maec.12014
McQuaid C. D., Lindsay T. L. (2007). Wave exposure effects on population structure and recruitment in the mussel Perna perna suggest regulation primarily through availability of recruits and food, not space. Mar. Biol. 151, 2123–2131. doi: 10.1007/s00227-007-0645-9
Moore P. G. (1972). Particulate matter in the sublittoral zone of an exposed coast and its ecological significance with special reference to the fauna inhabiting kelp holdfasts. J. Exp. Mar. Biol. Ecol. 10, 59–80. doi: 10.1016/0022-0981(72)90093-7
Morton B. (1967). Malacological report. Chelsea College Azores expedition, July - October 1965. Final Rep., 30–38.
Morton B., Britton J. C., Martins A. M. F. (1998). Ecologia Costeira dos Açores (Ponta Delgada: Sociedade Afonso Chaves), X+249 pp.
Nelson W. (1979a). Experimental studies of selective predation on amphipods: consequences for amphipod distribution and abundance. J. Exp. Mar. Biol. Ecol. 38, 225–245. doi: 10.1016/0022-0981(79)90069-8
Nelson W. (1979b). An analysis of structural patterns in an eelgrass (Zostera marina L.) amphipod community. J. Exp. Mar. Biol. Ecol. 39, 231–264. doi: 10.1016/0022-0981(79)90129-1
Neto A. I. (1997). Studies on algal communities of São Miguel, Azores (Ponta Delgada: Dissertação para a obtenção do Grau de Doutor em Biologia, especialidade de Biologia Marinha. Universidade dos Açores), X+309 pp.
Neto A. I. (2001). Macroalgal species diversity and biomass of subtidal communities of São Miguel (Azores). Helgoland Mar. Res. 55, 101–111. doi: 10.1007/s101520100074
Neto A. I., Tittley I., Levi A., Farnham W. F. (2000). Structure and zonation of algal communities in the bay of São Vicente (São Miguel, Azores). Arquipélago 2, 63–69. Life and Marine Sciences Supplement.
Pearson T., Rosenberg R. (1978). Macrobenthic succession in relation to organic enrichment and pollution of the marine environments. Oceanogr. Mar. Biol. Annu. Rev. 16, 229–311.
Robertson A. I., Mann K. H. (1982). Population dynamics and life history adaptations of Littorina neglecta Bean in an eelgrass meadow (Zostera marina L.) in Nova Scotia. J. Exp. Mar. Biol. Ecol. 63, 151–171. doi: 10.1016/0022-0981(82)90029-6
Rosenfeld S., Aldea C., Ojeda J., Marambio J., Hüne M., Troncoso J. S., et al. (2017). Molluscan assemblages associated with Gigartina beds in the Strait of Magellan and the South Shetland Islands (Antarctica): a comparison of composition and abundance. Polar Res. 36, 1297915. doi: 10.1080/17518369.2017.1297915
Rueda J. L., Salas C. (2003). Seasonal variation of a molluscan assemblage living in a Caulerpa prolifera meadow within the inner Bay of Cádiz (SW Spain). Estuar. Coast. Shelf Sci. 57, 909–918. doi: 10.1016/S0272-7714(02)00421-3
Russo A. (1997). Epifauna living on sublittoral seaweeds around Cyprus. Hydrobiologia 344, 169–179. doi: 10.1023/A:1002970714963
Sánchez-Moyano J., Estacio F., García-Adiego E., García-Gómez J. (2000b). The molluscan epifauna of the alga Halopteris scoparium in southern Spain as a bioindicator of coastal environmental conditions. J. Molluscan Stud. 66, 431–448. doi: 10.1093/mollus/66.4.431
Sánchez-Moyano J., García-Adiego E., Estacio F., García-Gómez J. (2000a). Effect of environmental factors on the spatial distribution of the epifauna of the alga Halopteris scoparium in Algeciras Bay, Southern Spain. Aquat. Ecol. 34, 355–367.
Sangil C., Martins G. M., Hernández J. C., Alves F., Neto A. I., Ribeiro C., et al. (2018). Shallow subtidal macroalgae in the North-eastern Atlantic archipelagos (Macaronesian region): a spatial approach to community structure. Eur. J. Phyciol. 53, 83–98. doi: 10.1080/09670262.2017.1385098
Schneider F. I., Mann K. H. (1991). Species specific relationships of invertebrates to begetation in a seagrass bed. II. Experiments on the importance of macrophyte shape, epiphyte cover and predation. J. Exp. Mar. Biol. Ecol. 145, 119–139. doi: 10.1016/0022-0981(91)90009-L
Schoch G., Dethier M. (1996). Scaling up: the statistical linkage between abundance and geomorphology on rocky intertidal shorelines. J. Exp. Mar. Biol. Ecol. 201, 37–72. doi: 10.1016/0022-0981(95)00167-0
Schwarz G. (1978). Estimating the dimension of a model. Ann. Stat 6, 461–464. doi: 10.1214/aos/1176344136
Soughtgate T. (1982). Studies on an intertidal population of Rissoa parva (Gastropoda: Prosobranchia) in south-west Ireland. J. Natural History 16, 183–194. doi: 10.1080/00222938200770141
Stoner A. (1980). Abundance, reproductive seasonality and habitat preferences of amphipod crustaceans in seagrass meadows of Apalachee Bay, Florida. Contrib. Mar. Sci. 23, 64–77.
Tararam A., Wakabara Y., Takeda A. (1981). Seasonal variations of amphipoda species living on Sargassum (São Paulo: Contribution n° 501 Instituto de Oceanografia, Univ), 305–321.
Taylor D. I., Schiel D. R. (2010). Algal populations controlled by fish herbivory across a wave exposure gradient on southern temperate shores. Ecology 91, 201–211. doi: 10.1890/08-1512.1
Tittley I., Neto A. I. (2000). A provisional classification of algal characterized rocky shore biotopes in the Azores. Hydrobiologia 440, 19–25. doi: 10.1023/A:1004172321900
Tuya F., Haroun R. J. (2006). Spatial patterns and response to wave exposure of shallow water algal assemblages across the Canarian Archipelago: a multi-scaled approach. Mar. Ecol. Prog. Ser. 311, 15–28. doi: 10.3354/meps311015
Vadas R. L., Wright W. A., Miller S. L. (1990). Recruitment of Ascophyllum nodosum: wave action as a source of mortality. Mar. Ecol. Prog. Ser. 61, 263–272. doi: 10.3354/meps061263
Vergés A., Alcoverro T., Ballesteros E. (2009). Role of fish herbivory in structuring the vertical distribution of canopy algae Cystoseira spp. in the Mediterranean Sea. Mar. Ecol. Prog. Ser. 375, 1–11. doi: 10.3354/meps07778
Wallenstein F. M. (2002). Biótopos do intertidal rochoso da ilha de São Miguel (Açores). Relatório de estágio para conclusão da Licenciatura em Biologia – Ramo de Biologia Marinha (Ponta Delgada: Universidade dos Açores).
Warwick R., Clarke K. (1993). Comparing the severity of disturbance: a meta-analysis of marine macrobenthic community data. Mar. Ecol. Prog. Ser. 92, 224–231. doi: 10.3354/meps092221
Wernberg T., Connell S. D. (2008). Physical disturbance and subtidal habitat structure on open rocky coasts: effects of wave exposure, extent and intensity. J. Sea Res. 59, 237–248. doi: 10.1016/j.seares.2008.02.005
Whippo R., Knight N. S., Prentice C., Cristiani J., Siegle M. R., O'Connor M. I. (2018). Epifaunal diversity patterns within and among seagrass meadows suggest landscape-scale biodiversity processes. Ecosphere 9, e02490. doi: 10.1002/ecs2.2490
WoRMS Editorial Board (2023) World Register of Marine Species. Available online at: https://www.marinespecies.org (Accessed 2023-11-25).
Keywords: molluscs, biodiversity, patterns of distribution, Halopteris scoparia, Azores, oceanic islands, environmental indicators
Citation: Ávila SP, Costa AC, Madeira P, Brum J, Prestes ACL, Faria J and Martins GM (2024) Patterns of distribution of mollusc fauna associated with Halopteris scoparia (Linnaeus) Sauvageau: a baseline study in the Azores archipelago helps understanding the impact of climate change/invasive species on biodiversity. Front. Mar. Sci. 11:1361068. doi: 10.3389/fmars.2024.1361068
Received: 27 December 2023; Accepted: 29 February 2024;
Published: 13 March 2024.
Edited by:
Irini Tsikopoulou, Hellenic Center for Marine Research, GreeceReviewed by:
Salvatore Giacobbe, University of Messina, ItalyFrancesco Tiralongo, University of Catania, Italy
Nikolaos Katsiaras, Hellenic Centre for Marine Research (HCMR), Greece
Copyright © 2024 Ávila, Costa, Madeira, Brum, Prestes, Faria and Martins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sérgio P. Ávila, avila@uac.pt
 Sérgio P. Ávila
Sérgio P. Ávila Ana Cristina Costa
Ana Cristina Costa Patrícia Madeira1,2,3,4
Patrícia Madeira1,2,3,4  João Faria
João Faria Gustavo M. Martins
Gustavo M. Martins