Culprit vessel revascularization first with primary use of a dedicated transradial guiding catheter to reduce door to balloon time in primary percutaneous coronary intervention
- 1Division of Cardiology, Beijing Luhe Hospital, Capital Medical University, Beijing, China
- 2Division of Cardiology, Beijing Xuanwu Hospital, Capital Medical University, Beijing, China
Background: The effect of a single transradial guiding catheter (STGC) for culprit vessel percutaneous coronary intervention (PCI) first on door-to-balloon (D2B) time remains unclear.
Materials and methods: Between February 2017 and July 2019, 560 patients with ST-elevation myocardial infarction (STEMI) were randomized into either the STGC group (n = 280) or the control group (n = 280) according to direct culprit vessel PCI with a STGC. In the STGC group, a dedicated transraidal guiding catheter (6F either MAC3.5 or JL3.5) was used for the treatment of electrocardiogram (ECG)-guided culprit vessel first and later contralateral angiography. In the control group, a universal diagnostic catheter (5F Tiger II) was used for complete coronary angiography, followed by guiding catheter selection for culprit vessel PCI. The primary endpoint was D2B time, and the secondary endpoint included catheterization laboratory door-to-balloon (C2B), procedural, fluoroscopy times, and major adverse cardiac events (MACE) at 30 days.
Results: The median D2B time was significantly shorter in the STGC group compared to the control group (53.9 vs. 58.4 min; p = 0.003). The C2B, procedural, and fluoroscopy times were also shorter in the STGC group (C2B: 17.3 vs. 24.5 min, p < 0.001; procedural: 45.2 vs. 49.0 min, p = 0.012; and fluoroscopy: 9.7 vs. 11.3 min, p = 0.025). More patients achieved the goal of D2B time within 90 min (93.9% vs. 87.1%, p = 0.006) and 60 min (61.4% vs. 51.1%, p = 0.013) in the STGC group. Radial artery perforation (RAP) was significantly reduced in the STGC group compared with the control group (0.7% vs. 3.2%, P = 0.033). MACE at 30 days was similar (2.5% vs. 4.6%, P = 0.172) between the two groups.
Conclusion: ECG-guided immediate intervention on culprit vessel with a STGC can reduce D2B, C2B, procedural, and fluoroscopy times (ECG-guided Immediate Primary PCI for Culprit Vessel to Reduce Door to Device Time; NCT03272451).
Introduction
Primary percutaneous coronary intervention (PCI) is currently the preferred method of treatment for acute ST-elevation myocardial infarction (STEMI) when therapy can be performed in a timely fashion (1). Door-to-balloon (D2B) time is a well-established metric of primary PCI care quality. A shorter D2B time is associated with lower mortality in patients with STEMI undergoing primary PCI (2, 3). Many strategies have been proposed to reduce the time from symptom onset to arrival at the catheterization laboratory (4), but few studies have focused on catheterization laboratory strategies.
Compared to femoral access, radial access reduces mortality, major adverse cardiovascular events, and major bleeding in patients with STEMI undergoing PCI (5). However, some studies have shown that radial access is associated with a longer D2B time or lidocaine administration to the first device time (6–8), which offsets part of the radial access advantage.
One way to improve D2B time is to use a single transradial guiding catheter (STGC) for both coronary angiography and culprit vessel PCI, but there have been few studies regarding using a STGC to reduce D2B time (9–11). Another way to shorten the D2B time is by direct PCI without diagnostic angiography of non-culprit vessels (12); however, these were not randomized studies. It is unknown whether the combined STGC and culprit vessel PCI first strategy affects the D2B time.
In this single-center, prospective, randomized study, we compared two strategies: electrocardiogram (ECG)-guided immediate intervention on culprit vessel with a STGC versus complete diagnostic coronary angiography followed by culprit vessel PCI, to assess D2B time.
Materials and methods
Population and study protocol
The RAPID II (ECG-guided Immediate primary PCI for Culprit vessel to Reduce Door to Device Time) trial was a prospective, randomized, controlled, single-center study carried out at the Beijing Luhe Hospital between February 2017 and July 2019, wherein 751 patients were screened for participation in the study. Of these, 560 (74.6%) patients with STEMI who underwent primary PCI were enrolled in the study. The inclusion criteria were medical consultation <12 h after symptom onset, chest pain lasting ≥ 30 min, ST-elevation ≥ 1 mm in ≥ 2 adjacent ECG leads, or a new left bundle branch block. Patients were ineligible if they were on dialysis, had previous coronary bypass surgery, had received fibrinolytic therapy, or had an absent radial pulse. This study was approved by the ethics committee of our hospital, and all patients provided written informed consent before randomization.
Patients were randomized into two groups in a 1:1 ratio in blocks of four (with sealed and opaque envelopes). The STGC group (n = 280) underwent ECG-guided culprit vessel PCI using a STGC, either 6F MAC 3.5 (Medtronic Corporation, Minneapolis, MN, USA), or JL 3.5 (Medtronic Corporation, Minneapolis, MN, USA), followed by contralateral angiography using the same guiding catheter. JL3.5 guiding catheter manipulation technique for RCA (Supplementary Video 1) and MAC 3.5 guiding catheter for both LCA and RCA (Supplementary Videos 2A,B). If the initial STGC failed to engage the culprit vessel, contralateral angiography with the same STGC was allowed before changing the guiding catheter for the culprit vessel PCI. The control group (n = 280) underwent complete coronary angiography with a 5F Tiger II (Terumo Corporation, Tokyo, Japan) diagnostic catheter, followed by culprit vessel PCI (Figure 1).
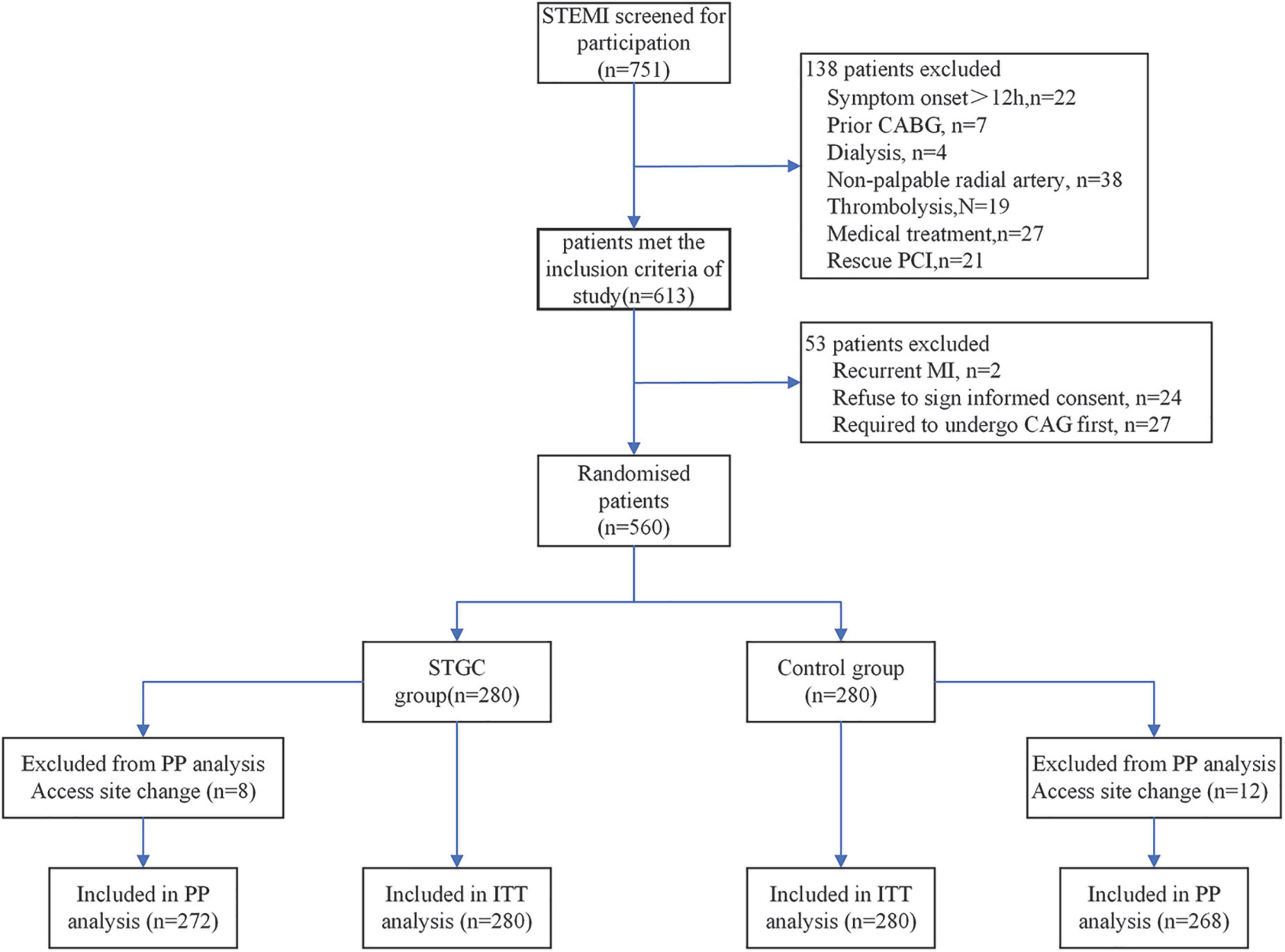
Figure 1. Trial flowchart. CABG, coronary artery bypass graft; ITT, intention-to-treat; PP, per-protocol.
Percutaneous coronary intervention procedure
All patients were pretreated with a loading dose of 300 mg aspirin and a P2Y12 inhibitor (clopidogrel 600 mg or ticagrelor 180 mg) before PCI. The right radial artery was the default access. After successful 6-Fr sheath insertion, Heparin and bivalirudin are acceptable anticoagulants. Unfractionated heparin (70–100 IU/kg body weight) was administered intravenously and then guided by activated clotting time results, aiming to maintain an activated clotting time of >250 s or 200–250 s with bailout use of a GP IIb/IIIa inhibitor. For patients who received bivalirudin, an intravenous bolus of 0.75 mg/kg followed by an infusion of 1.75 mg/kg/h for 4 h after the procedure was recommended. The choice of the PCI device and use of GP IIb/IIIa inhibitor was left to the discretion of the operator. The arterial sheath was removed immediately after PCI, and hemostasis was achieved with external compression using a TR band (Terumo, Tokyo, Japan) for 6 h. The procedures were performed by four experienced interventional cardiologists who performed transradial PCI in more than 200 cases annually. Radial artery angiography should be performed immediately if unexpected resistance is felt during the advancement of the guidewire or catheter for the early detection of radial artery perforation (RAP). The patients were discharged on dual antiplatelet therapy for at least 12 months.
Definitions and outcome measurement
The procedural time was defined as the time from local anesthesia to the withdrawal of the last catheter. The D2B time refers to the interval between hospital arrival and the first device (balloon, aspiration catheter, or direct stenting) used in the culprit artery. The C2B time was the interval between catheterization laboratory arrival and the first device used in the culprit artery. Puncture-to-balloon time was the interval between the access puncture and the first device used in the culprit artery. New onset atrial fibrillation (AF) was considered in patients with AF during hospitalization but not documented in previous medical history. Procedural success was defined as TIMI 3 flow in the culprit vessel and residual stenosis of <20% in the treated segment at the end of the procedure, without major clinical complications (e.g., death, myocardial infarction, and emergency coronary artery bypass graft) during hospitalization. The STGC group underwent coronary angiography for both the left coronary artery, including the left anterior descending artery (LAD), left circumflex artery (LCX), and right coronary artery (RCA), and complete culprit vessel PCI was defined as STGC success. Major adverse cardiac events (MACE) included cardiac death, reinfarction, and ischemia-driven target vessel revascularization. RAP was defined as persistent extravascular loss and accumulation of contrast medium through the vessel wall, as demonstrated by angiography.
The primary endpoint was the D2B time. The secondary endpoints included C2B, procedural, fluoroscopy times, and MACE at 30 days follow-up.
Statistical analysis
All statistical tests were performed using SPSS (version 17.0; Chicago, IL, USA). Continuous variables are expressed as mean ± standard deviation or median [interquartile range (IQR)] and were compared using the Student’s t-test or Mann–Whitney test, where appropriate. Categorical variables were indicated as absolute numbers and percentages and were compared using the Pearson χ2 test or, if the number expected was <5, using the Fisher exact test. A 2-tailed p < 0.05 was considered significant. The primary analysis was based on the intention-to-treat principle. A per-protocol analysis (assessment only in cases without access site changes) was performed.
The sample size calculation was performed using a superiority design with an assumed D2B time difference (5 min) between the STGC group and the control group (9, 13). To detect a difference of 5 min in the D2B time with a power of 90% and an α error of 0.5, considering a dropout rate of 10%, the number required was calculated to be at least 560 patients.
Results
Baseline characteristics
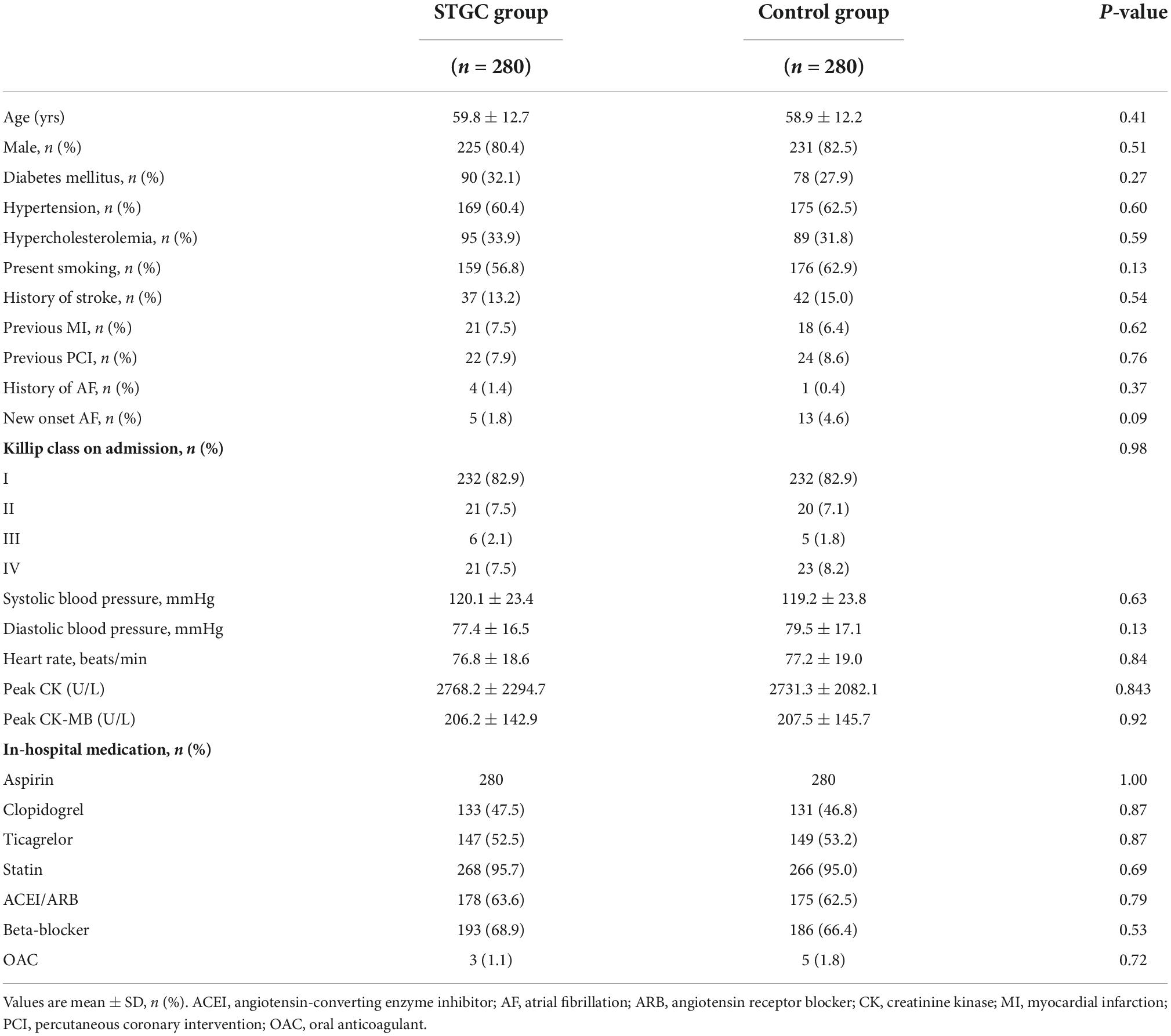
A total of 560 patients who presented with STEMI during the study period were analyzed, with 280 in each group (STGC group vs. control group). There were 456 men and 104 women, with an average age of 59.4 ± 12.5 years. The baseline characteristics of the patients who underwent randomization are shown in Table 1. There were no significant differences in baseline clinical characteristics between the two groups.
Procedural characteristics and angiographic results
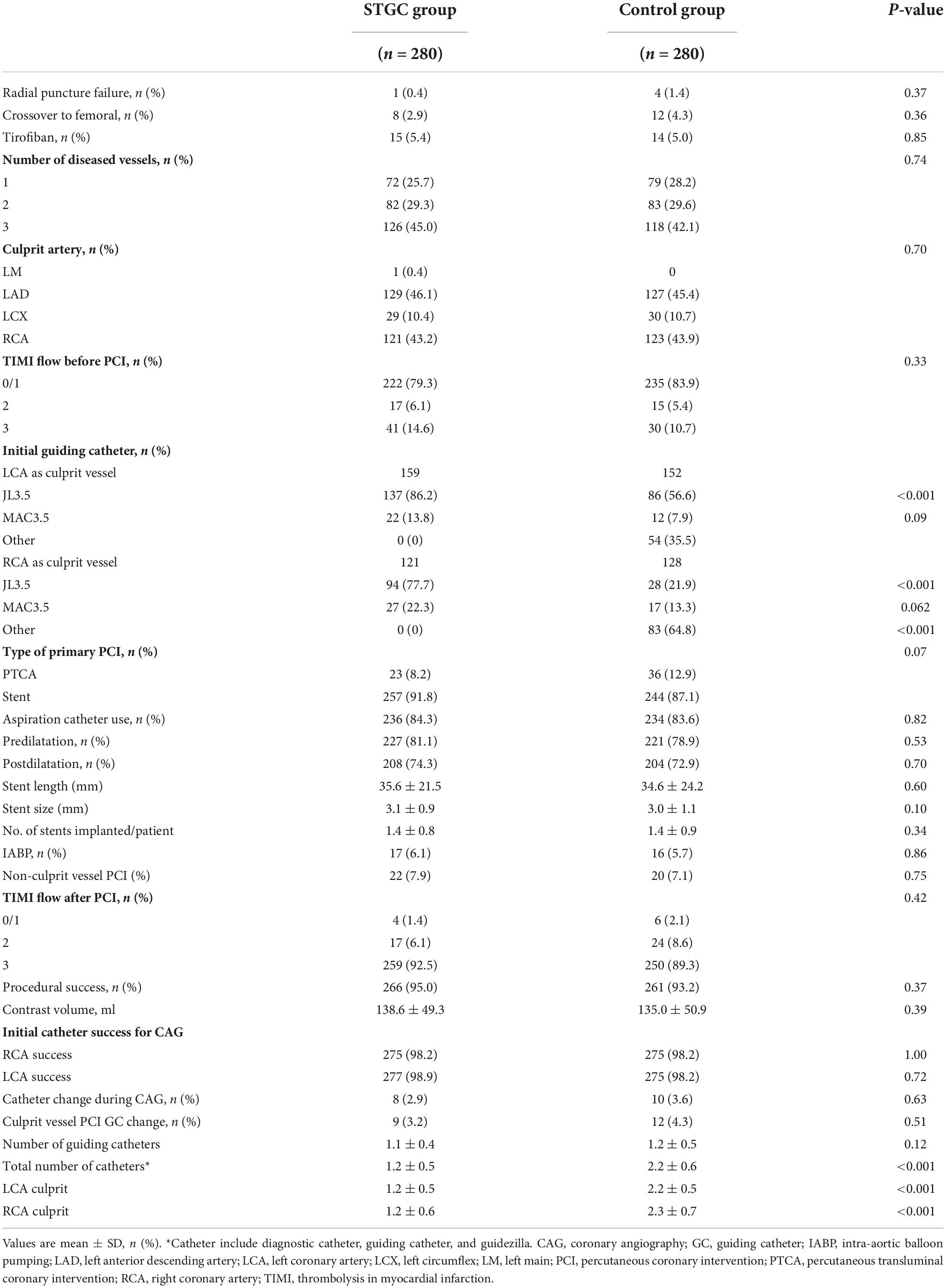
No significant differences were noted between the two groups with regard to the number of diseased vessels, distribution of culprit vessels, TIMI flow before PCI, aspiration catheter use, administration of GP IIb/IIIa inhibitor, and contrast medium volume. Compared with the control group, the STGC group had less total number of catheters (1.2 ± 0.5 vs. 2.2 ± 0.6, p < 0.001).
During diagnostic coronary angiography, in the STGC group, initial guiding catheter engagement failed in eight cases (3 LCA and 5 RCA), of which three cases with culprit vessels failed. In the control group, the Tiger II diagnostic catheter failed in 10 cases (5 LCA and 5 RCA), and the success rate of bilateral coronary angiography in the STGC group was similar to that in the control group (97.1% vs. 96.4%, p = 0.34).
During the PCI procedure, nine patients required guiding catheter exchange (6 LCA and 3 RCA) in the STGC group, while 12 patients required guiding catheter exchange (5 LCA, 12 RCA) in the control group. The success rate of the initial guiding catheter for culprit vessel PCI was similar between the STGC and control groups (95.4% vs. 93.9%, p = 0.45). The total STGC success rate (both coronary angiography and culprit vessel PCI) in the STGC group was 92.5% (Supplementary Figure 1).
Eight patients in the STGC group and 12 patients in the control group crossed to the femoral route (p = 0.362). The causes of crossover in the STGC group were radial puncture failure (n = 1), radial/brachial tortuosity (n = 2), and tortuous innominate artery/subclavian artery (n = 5), while the causes in the control group were radial artery puncture failure (n = 4), radial/brachial tortuosity (n = 3), and right subclavian/aortic arch tortuosity (n = 5) (Supplementary Figure 1).
An ECG can identify LAD as the culprit vessel in anterior STEMI (100%). However, 13 patients (4.6%) in the STGC group with inferior STEMI failed to identify the culprit vessel by ECG (11 LCX and 1 LAD). Conversely, one patient was found to have an LCX as the culprit vessel that was missed on the ECG.
There were 22 (7.9%) patients in the STGC group and 20 (7.1%) patients in the control group who underwent non-culprit vessel PCI at the time of primary PCI. No emergency coronary artery bypass graft was performed in either of the groups (Table 2).
Eleven patients had radial artery angiography and were diagnosed with RAP when resistance was encountered during guiding catheter advancement (Supplementary Figure 2). RAP induced by advancing STGC was observed in two patients in the STGC group and nine patients during catheter exchange in the control group. The RAP rate was significantly higher in the control group than in the STGC group (3.2% vs. 0.7%, p = 0.033). The balloon-assisted tracking technique allowed the guiding catheter to pass through the site of perforation and PCI in 10 patients (two in the STGC group and eight in the control group), and radial artery angiography revealed that all the RAPs were sealed. Another patient in the control group switched to femoral access after guiding catheter-induced RAP, which was treated with external compression.
Treatment time
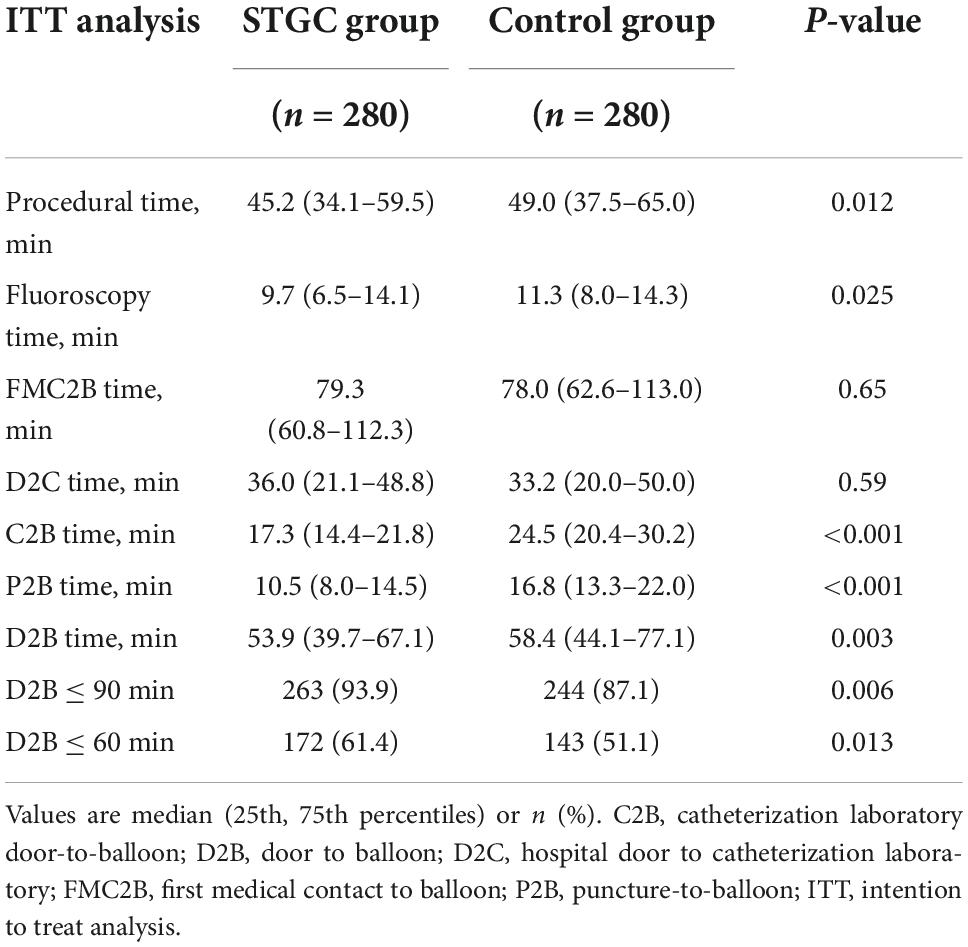
The median D2B (53.9 vs. 58.4 min, p = 0.003), C2B (17.3 vs. 24.5 min, p < 0.001), procedural (45.2 vs. 49.0 min, p = 0.012), and fluoroscopy times (9.7 vs. 11.3 min, p = 0.025) were significantly shorter in the STGC group compared with the control group (Figure 2 and Table 3). Per-protocol analyses come to essentially the same conclusions (Supplementary Table 1). The proportion of patients achieving D2B time within 90 and 60 min increased significantly in the STGC group (93.9% vs. 87.1%, p = 0.006; 61.4% vs. 51.1%, p = 0.013, respectively; Figure 3). There was no difference in the D2B time between the four operators (Figure 4). The proportion of C2B time in the symptom-to-balloon time was significantly higher for patients with symptom-to-balloon time ≤3, 3–6, and ≥6 h (15.6% vs. 9.0% vs. 4.6%, p < 0.001, respectively) and subgroup patients (Figure 5).
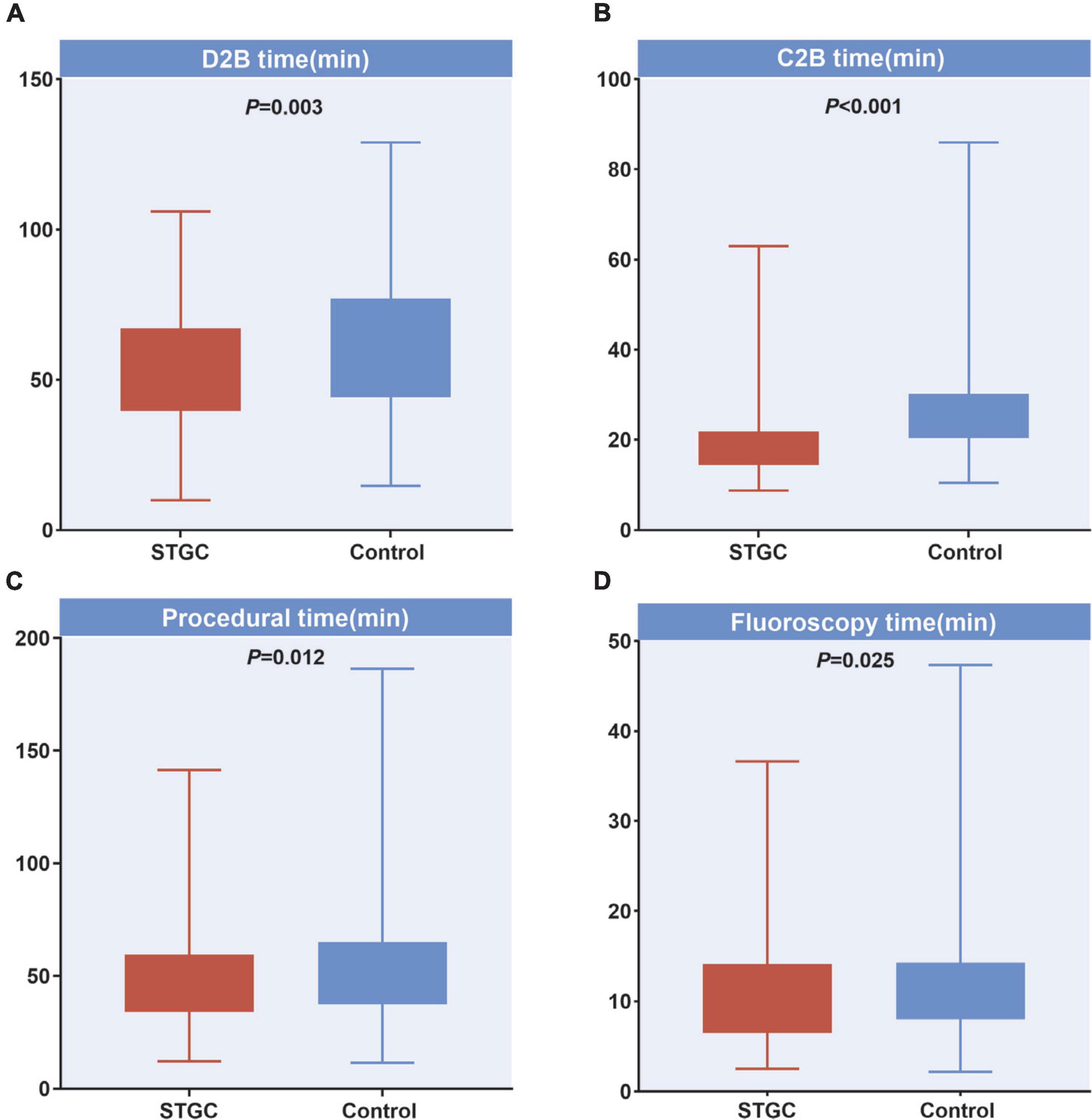
Figure 2. Difference of treatment time between two groups. (A) D2B time, (B) C2B time, (C) procedural time, and (D) fluoroscopy time. C2B, catheterization laboratory door-to-balloon; D2B, door to balloon; STGC, single transradial guiding catheter.
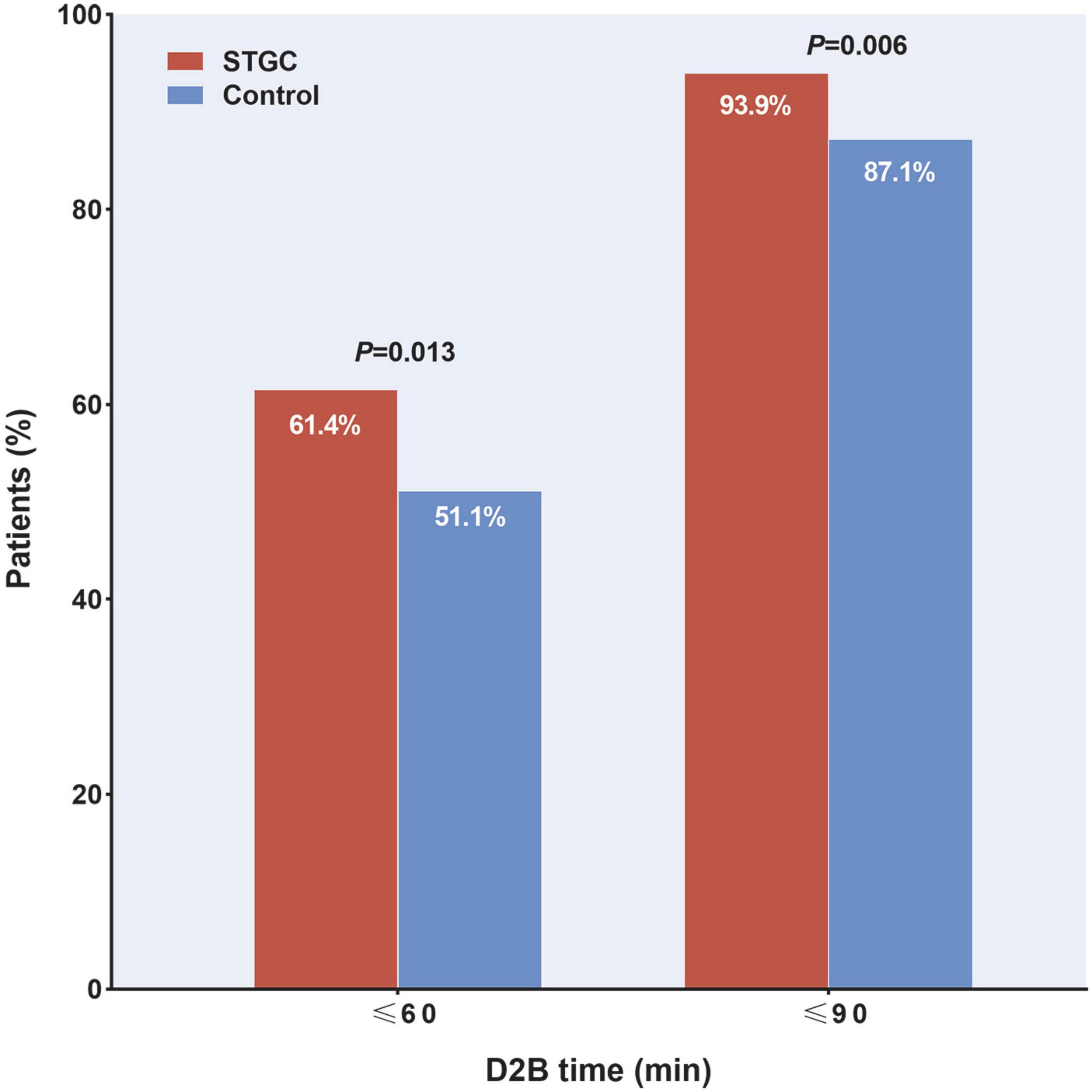
Figure 3. Proportion of patients achieving door-to-balloon (D2B) target. Proportion of patients with D2B time within 90 and 60 min were significantly higher in the STGC group than in control group (p = 0.006 and p = 0.013, respectively). D2B, door-to-balloon; STGC, single transradial guiding catheter.
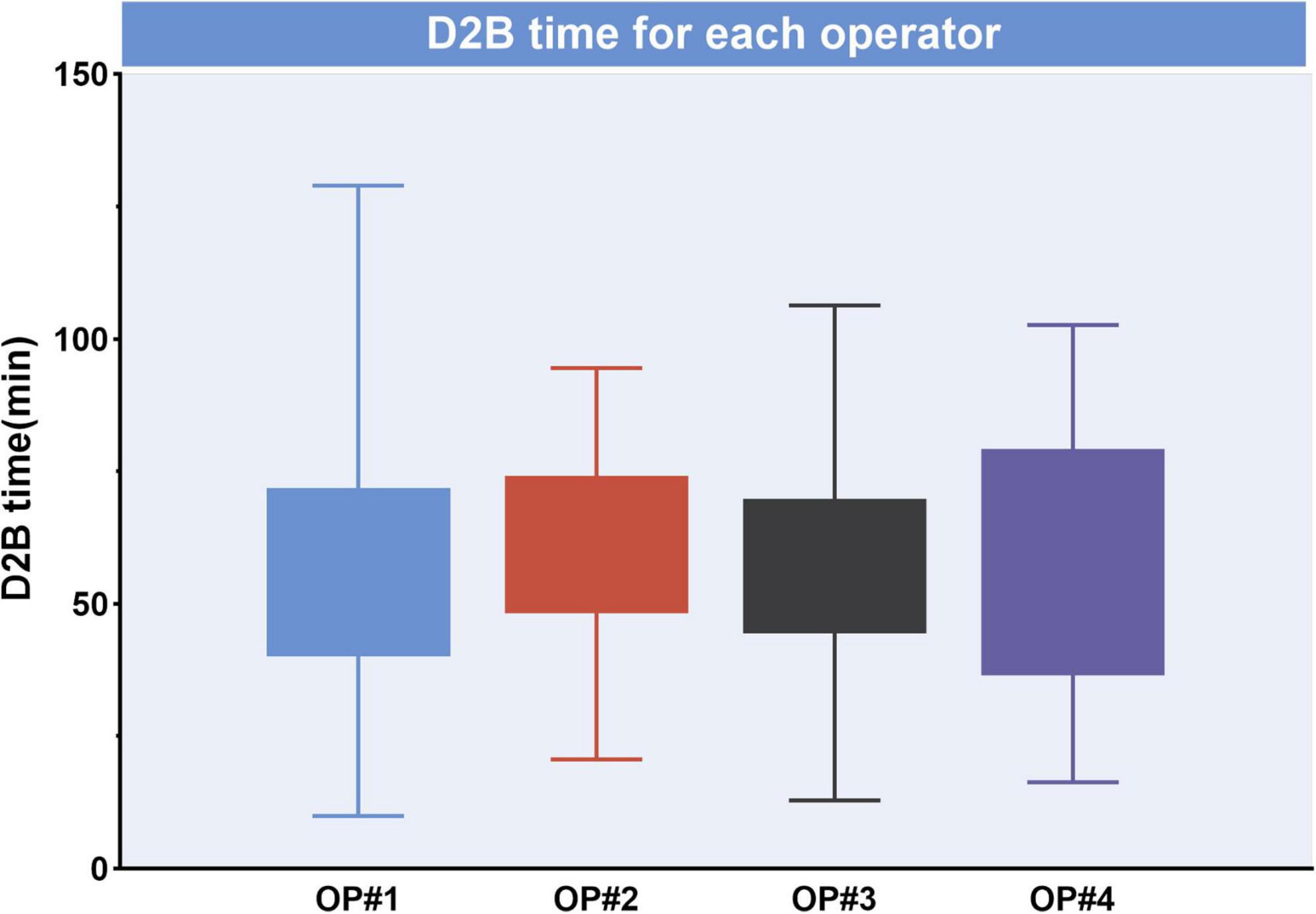
Figure 4. Median door-to-balloon (D2B) time for each operator. D2B was not significantly different between operators (p > 0.05), D2B, door-to-balloon.
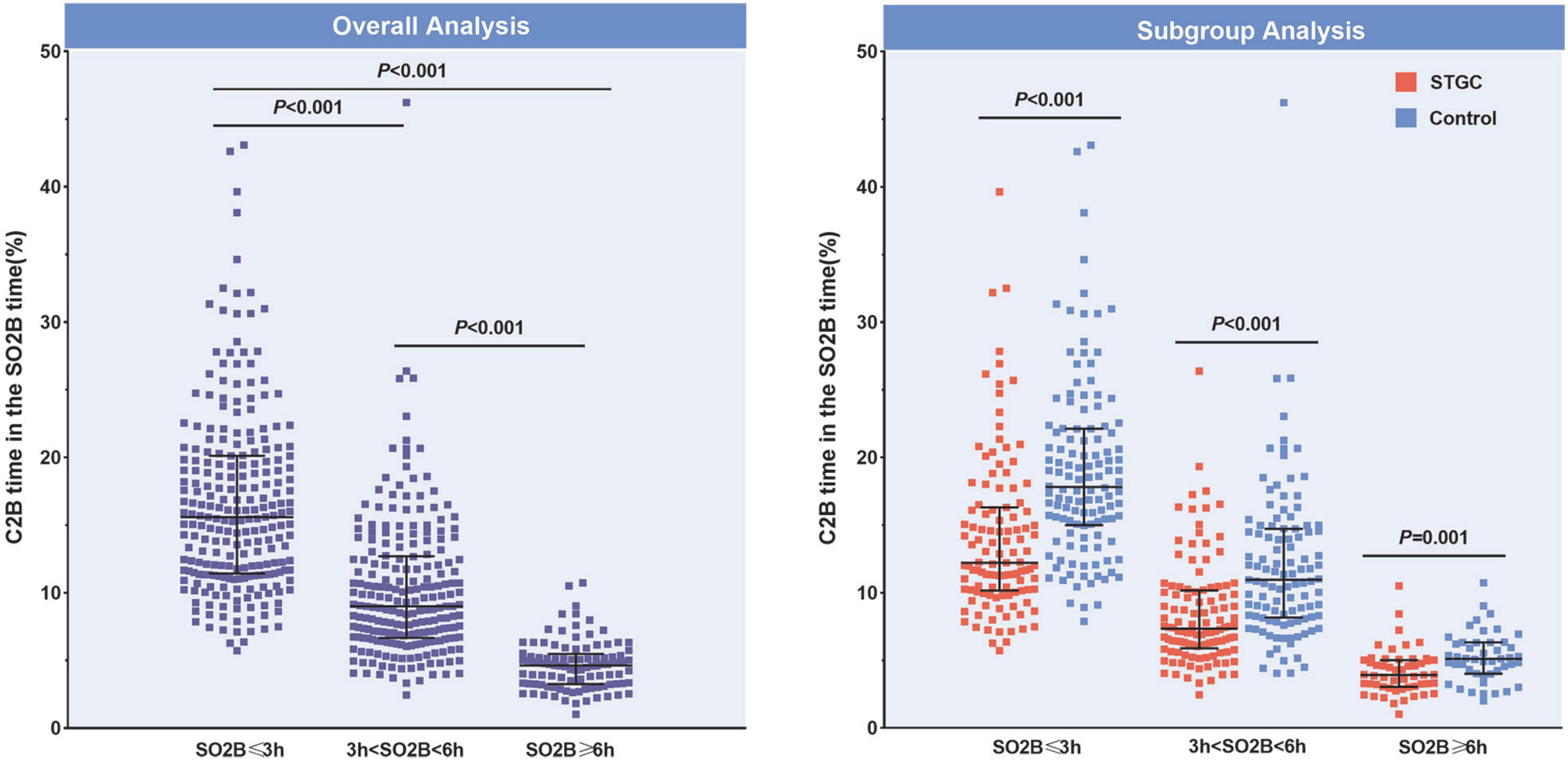
Figure 5. Proportions of C2B time in the SO2B time. The proportion of C2B time in the SO2B time was significantly different for patients with SO2B time ≤3, 3–6, and ≥6 h (15.6% vs. 9.0% vs. 4.6%, p < 0.001, respectively). In subgroup analysis, the proportion was also significant different between the STGC group and the control group (p < 0.001, respectively). C2B, catheterization laboratory door-to-balloon; SO2B, symptom to balloon; STGC, single transradial guiding catheter.
Clinical outcomes
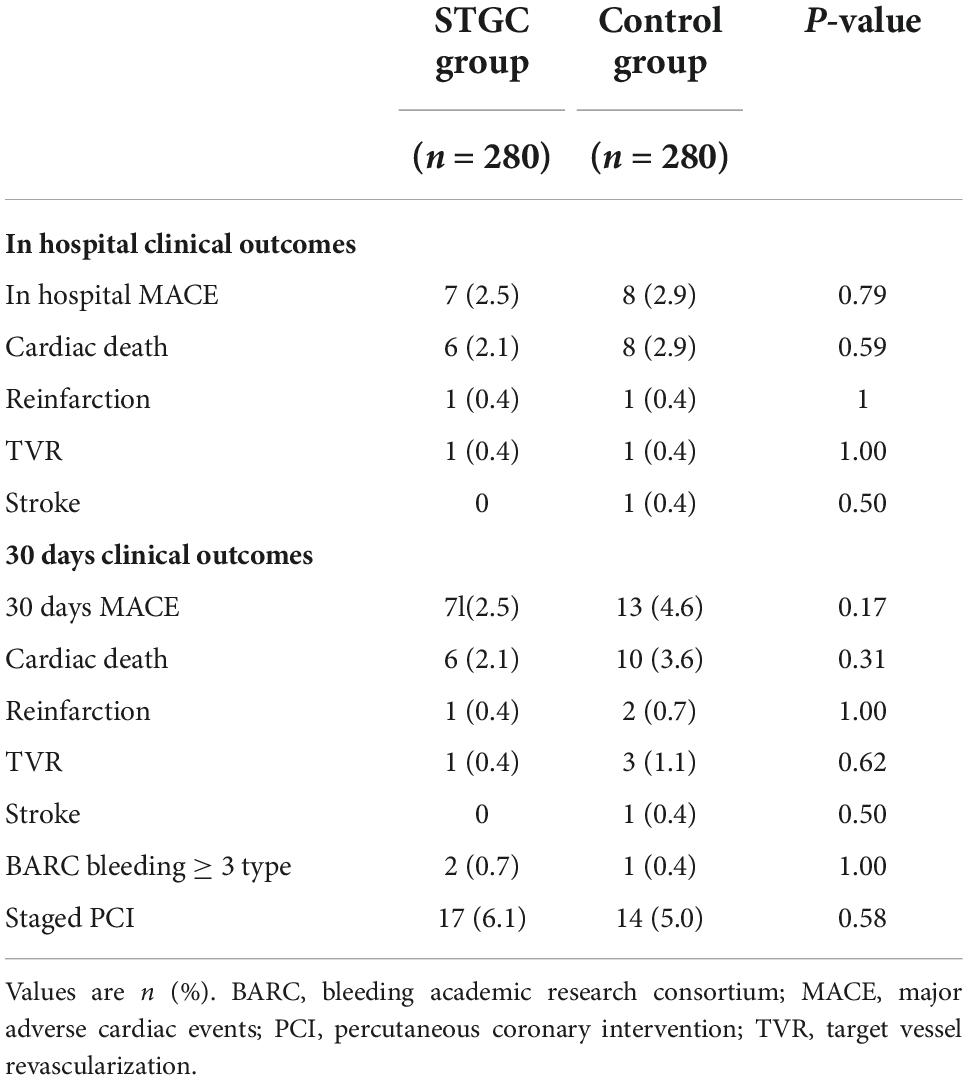
No catheter-associated coronary complications were observed in either group. There was no significant difference in MACE at 30 days (2.5% vs. 4.6%, p = 0.17) between the two groups (Table 4). After discharge, 17 patients in the STGC group and 14 in the control group underwent staged PCI.
Discussion
Our study showed that D2B time and C2B time in the STGC group were significantly reduced by 4.4 and 7.2 min, respectively. This reduction translates to a higher proportion of cases with a D2B time of less than 90 min (93.9%). Procedural and fluoroscopy times were also significantly shorter in the STGC group. RAP occurred less frequently in the STGC group vs control group (3.2% vs. 0.7%, p = 0.03). There were no significant differences in MACE at 30 days (2.5% vs. 4.6%, p = 0.17) between the two groups.
Decreasing door-to-balloon time
The survival benefit of primary PCI depends on the timely opening of the culprit vessel within the recommended 90 min or less D2B time and radial access first according to the current guidelines (1, 14). A shorter D2B time for individual patients is associated with lower mortality in hospitals (2, 15, 16), at 30 days (2, 16), at 6 months (17), or 1 year (2, 3). There has been extensive research on strategies to improve D2B time, including shortening the door to ECG and ECG to activation time (18), prehospital activation and direct to the cath lab (19, 20), and data feedback with each member in the system of care (20). However, little attention has been paid to the effect of the sequence of the catheter on D2B time in transradial access.
Choice of the catheter on door-to-balloon time
One way to shorten D2B time is to perform transradial coronary angiography using a STGC. Several randomized trials have investigated the performance of either standard Judkins-shaped or dedicated catheters (Tiger II, Kimny, and DxTerity) for transradial coronary angiography (21–23). The potential objective of introducing the STGC method of transradial intervention is to avoid catheter exchange and achieve shorter procedural times. A recent meta-analysis showed comparable results for either dual- or single catheter strategies for transradial coronary angiography (23). It is unclear whether an STGC strategy for diagnostic coronary angiography is a time-saving measure in the setting of acute coronary syndrome.
Another way to shorten the D2B time is to use a STGC for both non-culprit and culprit vessel angiography and intervention. Several guiding catheters (Kimny, Ikari Left, RM, and MAC) have been studied during primary PCI (9–11, 24–27). In this strategy, an STGC is used to perform contralateral coronary angiography, followed by complete culprit vessel angiography and intervention. In a randomized study by Guo et al. (9), compared with conventional methods (universal diagnostic Tiger catheter followed by guiding catheter), using a single MAC3.5 guiding catheter significantly reduced the median procedure time by 3.4 min, and the D2B time was similar between the two groups. In another multicenter retrospective study, D2B time was significantly reduced by 6.0 min in the STGC group compared with the conventional group (10).
Culprit vessel percutaneous coronary intervention first on door-to-balloon time
Data obtained in previous retrospective studies (12, 28) using an ECG-guided culprit-vessel first strategy before contralateral or complete diagnostic angiography indicated that a lower D2B time was achieved by skipping several steps compared with the conventional procedure. According to Couture et al. (12), the median vascular access-to-balloon time was 4–6 min shorter with a culprit-vessel PCI strategy. Plourde et al. (28) reported that the median D2B time in the immediate PCI group was 8 min less than that in the control group (32 vs. 40 min, p < 0.0001). In the present randomized study, ECG-guided culprit vessel-first PCI enabled the improvement of D2B and C2B by 4.4 and 7.2 min, respectively, which was similar to the above reports (12, 28). In addition, in our study, the same guiding catheter was also applied to perform the contralateral angiography after culprit vessel-PCI, which resulted in a shorter fluoroscopy time by 1.6 min and procedure time by 4.0 min compared to a standard approach. These results are also consistent with our previous study (9). Furthermore, no guiding catheter-induced coronary complications were observed in either group.
Potential benefits for radial access
Radial artery spasm is the most frequent complication of transradial cardiac catheterization. Previous studies (23, 29) have shown that the number of catheters used is associated with radial artery spasm. RAP is extremely rare, occurring in <1% of cases, and may occur as a consequence of guidewire advancement and avulsion due to catheter advancement (29). In the present study, we did not calculate the variable of radial artery spasm, but we found that the rate of RAP was significantly lower in the STGC group than in the control group (0.7% vs. 3.2%, p = 0.03). All the RAPs occur at the time of guiding catheter advancement, in the nine cases of the control group, spasmolytic agents were not administered intra-arterially before catheter exchanges to avoid spasm, which may the reason of RAPs (22). Therefore, spasmolytic agents should be used during catheter exchange.
Important questions that need to be focused on
In contrast to traditional approach, our new strategy proposed here allows issues such as failure to identify the culprit artery by ECG in inferior STEMI, emergency coronary artery bypass graft, and time-saving clinical implications to be considered.
Identification of the culprit artery in inferior STEMI plays an important role in the culprit vessel first strategy; misdiagnosis of culprit vessels may delay C2B time. In our study, the diagnostic accuracy was 95.4%, which is consistent with results obtained in previous studies (30, 31). Owing to the use of a STGC strategy, the STGC can be easily manipulated to engage the contralateral artery without a time delay.
It is important to consider that diagnostic angiography before PCI might provide valuable anatomical information that could change the reperfusion strategy, such as an emergency coronary artery bypass graft. However, coronary artery bypass graft was performed as reperfusion therapy in only 0.8% of patients (32), and patients requiring urgent or emergency coronary artery bypass graft within 24 h of STEMI have mortality rates of 8.2∼15.8% (33, 34). In fact, for patients with STEMI and multivessel disease, immediate or staged complete revascularization with PCI significantly improves hard clinical outcomes (35). In our study, immediate complete revascularization was performed in 7.5% of the patients (contralateral 4.3%, ipsilateral 3.2%) in the STGC group and 6.8% in the control group (contralateral 4.6%, ipsilateral 2.2%), and there was no coronary artery bypass graft in the acute period and 30 days after PCI.
Previous studies have demonstrated that every minute of delay in primary PCI affects 1 year mortality (36). Recently, Park et al. (3) showed that reduction in D2B time by 30 min was associated with a continuous reduction in 1 year mortality. However, population-level studies revealed that reducing D2B time in primary PCI did not improve mortality (17, 37). These results raise questions regarding the value of shortening D2B time. In our study, a reduction of 4.4 min D2B time failed to translate into clinical outcomes, which is in agreement with previous studies (12, 38). The following possible reasons should be considered: (1) the primary endpoint is D2B time other than symptom to balloon time in our study, and clinical outcome differences cannot be identified due to the small sample size. (2), the C2B time represents only a small fraction of the symptom to balloon time. In our study, the C2B time encompassed 15.6% of the symptom to balloon time in patients with symptom to balloon time ≤3 h. Improvement in C2B time might have a significant effect on the reduction of symptom to balloon time, especially in patients presenting within 3 h of symptom onset. (3) “Time is muscle” remains indisputable. Time may also be critical in certain clinical situations, for example, in a patient with cardiogenic shock (39). (4) The contribution of radial access to better outcomes may cause bias in the interpretation of the relationship between our time-saving strategy and the outcomes.
To our knowledge, this is the first randomized study to investigate the effectiveness of two-strategy combination of using a STGC and culprit vessel-PCI first. Our results provide compelling evidence for this new strategy with shorter D2B, C2B, fluoroscopy, and procedure times.
Study limitations
Our study has several limitations. First, this was an open-label trial, which may have caused potential bias in the results. Second, this study was conducted at a high-volume tertiary center for primary PCI. Therefore, the results may not be generalizable to low-volume primary PCI centers. Third, the right radial artery is the default access. MAC3.5 or JL3.5 manipulation technique may not apply to the left radial access. Fourth, the primary endpoint was D2B time, but not clinical endpoint. Fifth, although prasugrel is not available in China, the use of ticagrelor remains low, only 52.9% of patients were under ticagrelor treatment in the present study, highlighting a therapeutic inertia with considerable gap between guidelines and clinical practice. Sixth, there was a higher proportion (>80%) of male in the current study, may not be representative of acute coronary syndrome in usual clinical practice. Seventh, aspiration thrombectomy were performed in a high rate of patients in current study. However, there was no significant difference between the two groups, which would not be expected to influence the primary endpoint. Eighth, low incidence of AF patients were detected. Finally, the follow-up duration in this study was relatively short. Additional longer follow-up should be performed to evaluate clinical outcomes.
Conclusion
ECG-guided immediate intervention on the culprit vessel followed by contralateral angiography with a STGC in STEMI patients is safe and is associated with a 4.4 min decrease in D2B time, 7.2 min in C2B time, reduced procedural time, fluoroscopy time, and RAP complications. Further studies are required to evaluate the effects of this strategy on the clinical outcomes.
Data availability statement
The datasets used and/or analyzed during this current study are available from the corresponding authors on reasonable request. Requests to access these datasets should be directed to JCG, guojcmd@126.com.
Ethics statement
The studies involving human participants were reviewed and approved by the Review Board of Beijing Luhe Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JCG and QH: conception, design, final approval of the manuscript, and overall responsibility. ZJL, YJW, SHW, YTW, YXW, HTW, YPW, and LBZ: data collection. JCG, GZW, and ZXL: analysis, interpretation, and manuscript writing. QH: critical revision. JCG: obtain funding. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Grant Nos. 2018-2-7082 and 2022-2-7086 from the Capital’s Funds for Health Improvement and Research (CFH) to JCG.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1022488/full#supplementary-material
Abbreviations
C2B, catheterization laboratory door-to-balloon; D2B, door-to-balloon; LAD, left anterior descending artery; LCX, left circumflex artery; MACE, major adverse cardiac events; PCI, percutaneous coronary intervention; RAP, radial artery perforation; RCA, right coronary artery; STGC, single transradial guiding catheter; STEMI, ST-elevation myocardial infarction.
References
1. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European society of cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
2. Foo CY, Bonsu KO, Nallamothu BK, Reid CM, Dhippayom T, Reidpath DD, et al. Coronary intervention door-to-balloon time and outcomes in ST-elevation myocardial infarction: a meta-analysis. Heart. (2018) 104:1362–9. doi: 10.1136/heartjnl-2017-312517
3. Park J, Choi KH, Lee JM, Kim HK, Hwang D, Rhee TM, et al. Prognostic implications of door-to-balloon time and onset-to-door time on mortality in patients with ST -segment-elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Heart Assoc. (2019) 8:e012188. doi: 10.1161/JAHA.119.012188
4. Pulia M, Salman T, O’Connell TF, Balasubramanian N, Gaines R, Shah F, et al. Impact of emergency medical services activation of the cardiac catheterization laboratory and a 24 h/Day in-hospital interventional cardiology team on treatment times (Door to Balloon and Medical Contact to Balloon) for ST-elevation myocardial infarction. Am J Cardiol. (2019) 124:39–43. doi: 10.1016/j.amjcard.2019.03.046
5. Di Santo P, Simard T, Wells GA, Jung RG, Ramirez FD, Boland P, et al. Transradial versus transfemoral access for percutaneous coronary intervention in ST-segment-elevation myocardial infarction: a systematic review and meta-analysis. Circ Cardiovasc Interv. (2021) 14:e009994. doi: 10.1161/CIRCINTERVENTIONS.120.009994
6. Shroff AR, Gulati R, Drachman DE, Feldman DN, Gilchrist IC, Kaul P, et al. SCAI expert consensus statement update on best practices for transradial angiography and intervention. Catheter Cardiovasc Interv. (2020) 95:245–52. doi: 10.1002/ccd.28672
7. Singh S, Singh M, Grewal N, Khosla S. The fluoroscopy time, door to balloon time, contrast volume use and prevalence of vascular access site failure with transradial versus transfemoral approach in ST segment elevation myocardial infarction: a systematic review and meta-analysis. Cardiovasc Revasc Med. (2015) 16:491–7. doi: 10.1016/j.carrev.2015.08.013
8. Le May M, Wells G, So D, Chong AY, Dick A, Froeschl M, et al. Safety and efficacy of femoral access vs radial access in ST-segment elevation myocardial infarction: the SAFARI-STEMI randomized clinical trial. JAMA Cardiol. (2020) 5:126–34. doi: 10.1001/jamacardio.2019.4852
9. Guo J, Chen W, Wang G, Liu ZJ, Hao MH, Xu M, et al. Safety and efficacy of using a single transradial MAC guiding catheter for coronary angiography and intervention in patients with ST elevation myocardial infarction. J Interv Cardiol. (2017) 30:33–42. doi: 10.1111/joic.12346
10. Lee KH, Torii S, Oguri M, Miyaji T, Kiyooka T, Ono Y, et al. Reduction of door-to-balloon time in patients with ST-elevation myocardial infarction by single-catheter primary percutaneous coronary intervention method. Catheter Cardiovasc Interv. (2022) 99:314–21. doi: 10.1002/ccd.29797
11. Chow J, Tan CH, Tin AS, Ong SH, Tan VH, Goh YS, et al. Feasibility of transradial coronary angiography and intervention using a single Ikari left guiding catheter for ST elevation myocardial infarction. J Interv Cardiol. (2012) 25:235–44. doi: 10.1111/j.1540-8183.2011.00710.x
12. Couture EL, Berube S, Dalery K, Gervais A, Harvey R, Nguyen M, et al. Culprit vessel revascularization prior to diagnostic angiography as a strategy to reduce delays in primary percutaneous coronary intervention: a propensity-matched analysis. Circ Cardiovasc Interv. (2016) 9:e003510. doi: 10.1161/CIRCINTERVENTIONS.115.003510
13. Han Y, Guo J, Zheng Y, Zang HY, Su X, Wang Y, et al. Bivalirudin vs heparin with or without tirofiban during primary percutaneous coronary intervention in acute myocardial infarction: the BRIGHT randomized clinical trial. JAMA. (2015) 313:1336–46. doi: 10.1001/jama2015.2323
14. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedettoet U. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165. doi: 10.1093/eurheartj/ehy394
15. Huded CP, Kumar A, Johnson M, Abdallah M, Ballout JA, Kravitz K, et al. Incremental prognostic value of guideline-directed medical therapy, transradial access, and door-to-balloon time on outcomes in ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. (2019) 12:e007101. doi: 10.1161/CIRCINTERVENTIONS.118.007101
16. Foo CY, Andrianopoulos N, Brennan A, Roquelaure Y, Descathaet A. Re-examining the effect of door-to-balloon delay on STEMI outcomes in the context of unmeasured confounders: a retrospective cohort study. Sci Rep. (2019) 9:19978. doi: 10.1038/s41598-019-56353-7
17. Nallamothu BK, Normand SL, Wang Y, Hofer TP, Brush JE Jr., Messenger JC, et al. Relation between door-to-balloon times and mortality after primary percutaneous coronary intervention over time: a retrospective study. Lancet. (2015) 385:1114–22. doi: 10.1016/S0140-6736(14)61932-2
18. McLaren JTT, Kapoor M, Yi SL, Chartier LB. Using ECG-To-activation time to assess emergency physicians’ diagnostic time for acute coronary occlusion. J Emerg Med. (2021) 60:25–34. doi: 10.1016/j.jemermed.2020.09.028
19. Kontos MC, Gunderson MR, Zegre-Hemsey JK, Lange DC, French WJ, Henryet TD. Prehospital activation of hospital resources (PreAct) ST-segment-elevation myocardial infarction (STEMI): a standardized approach to prehospital activation and direct to the catheterization laboratory for STEMI recommendations from the american heart association’s mission: lifeline program. J Am Heart Assoc. (2020) 9:e011963. doi: 10.1161/JAHA.119.011963
20. Jacobs AK, Ali MJ, Best PJ, Bieniarz MC, Bufalino VJ, French WJ, et al. Systems of care for ST-segment-elevation myocardial infarction: a policy statement from the American Heart Association. Circulation. (2021) 144:e310–27. doi: 10.1161/CIR.0000000000001025
21. Chyrchel M, Bartus S, Dziewierz A, Legutko J, Kleczyński P, Januszek R, et al. Safety and efficacy of four different diagnostic catheter curves dedicated to one-catheter technique of transradial coronaro-angiography-prospective, Randomized Pilot study. TRACT 1: trans RAdial coronary angiography trial 1. J Clin Med. (2021) 10:4722. doi: 10.3390/jcm10204722
22. Mason PJ, Shah B, Tamis-Holland JE, Bittl JA, Cohen MG, Safirstein J, et al. An update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome: a scientific statement from the American Heart Association. Circ Cardiovasc Interv. (2018) 11:e000035. doi: 10.1161/HCV0000000000000035
23. Alushi B, Lauten A, Ndrepepa G, Leistner DM, Kufner S, Xhepa E, et al. Procedural and clinical performance of dual- versus single-catheter strategy for transradial coronary angiography: a meta-analysis of randomized trials. Catheter Cardiovasc Interv. (2020) 96:276–82. doi: 10.1002/ccd.28458
24. Torii S, Fujii T, Murakami T, Nakazawa G, Ijichi T, Nakano M, et al. Impact of a single universal guiding catheter on door-to-balloon time in primary transradial coronary intervention for ST segment elevation myocardial infarction. Cardiovasc Interv Ther. (2017) 32:114–9. doi: 10.1007/s12928-016-0395-z
25. Roberts EB, Wood A. Use of a single Q guide catheter for complete assessment and treatment of both coronary arteries via radial access during acute ST elevation myocardial infarction: a review of 40 consecutive cases. J Interv Cardiol. (2011) 24:389–96. doi: 10.1111/j.1540-8183.2011.00655.x
26. Moon KW, Kim JH, Kim JY, Jung MH, Kim GH, Yoo KD, et al. Reducing needle-to-balloon time by using a single guiding catheter during transradial primary coronary intervention. J Interv Cardiol. (2012) 25:330–6. doi: 10.1111/j.1540-8183.2011.00717.x
27. Malaiapan Y, Leung M, Ahmar W, Hutchison AW, Prasad S, Katticaran T, et al. Guideline recommended door-to-balloon time can be achieved in transradial primary PCI–the usefulness of a dedicated radial guide catheter. Cardiovasc Revasc Med. (2013) 14:27–31. doi: 10.1016/j.carrev.2012.10.012
28. Plourde G, Abdelaal E, Bataille Y, MacHaalany J, Déry JP, Déry U, et al. Effect on door-to-balloon time of immediate transradial percutaneous coronary intervention on culprit lesion in ST-elevation myocardial infarction compared to diagnostic angiography followed by primary percutaneous coronary intervention. Am J Cardiol. (2013) 111:836–40. doi: 10.1016/j.amjcard.2012.11.059
29. Sandoval Y, Bell MR, Gulati R. Transradial artery access complications. Circ Cardiovasc Interv. (2019) 12:e007386. 119.007386 doi: 10.1161/CIRCINTERVENTIONS
30. Vives-Borras M, Maestro A, Garcia-Hernando V, Jorgensen D, Ferrero-Gregori A, Moustafa AH, et al. Electrocardiographic distinction of left circumflexand right coronary artery occlusion in patients with inferior acute myocardial infarction. Am J Cardiol. (2019) 123:1019–25. doi: 10.1016/j.amjcard.2018.12.026
31. Huang X, Ramdhany SK, Zhang Y, Yuan Z, Mintz GS, Guo N. New ST-segment algorithms to determine culprit artery location in acute inferior myocardial infarction. Am J Emerg Med. (2016) 34:1772–8. doi: 10.1016/j.ajem.2016.06.005
32. Zeymer U, Ludman P, Danchin N, Kala P, Laroche C, Sadeghi M, et al. Reperfusion therapies and in-hospital outcomes for ST-elevation myocardial infarction in Europe: the ACVC-EAPCI EORP STEMI Registry of the European Society of Cardiology. Eur Heart J. (2021) 42:4536–49. doi: 10.1093/eurheartj/ehab342
33. Lemaire A, Vagaonescu T, Ikegami H, Volk L, Verghis N, Lee LY. Delay in coronary artery bypass grafting for STEMI patients improves hospital morbidity and mortality. J Cardiothorac Surg. (2020) 15:86. doi: 10.1186/s13019-020-01134-x
34. Thielmann M, Wendt D, Slottosch I, Welp H, Schiller W, Tsagakis K, et al. Coronary artery bypass graft surgery in patients with acute coronary syndromes after primary percutaneous coronary intervention: a current report from the north-rhine westphalia surgical myocardial infarction registry. J Am Heart Assoc. (2021) 10:e021182. doi: 10.1161/JAHA.121.021182
35. Ahmad Y, Howard JP, Arnold A, Prasad M, Seligman H, Cook CM, et al. Complete revascularization by percutaneous coronary intervention for patients with ST-segment-elevation myocardial infarction and multivessel coronary artery disease: an updated meta-analysis of randomized trials.. J Am Heart Assoc. (2020) 9:e015263. doi: 10.1161/JAHA.119.015263
36. De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation. (2004) 109:1223–5. doi: 10.1161/01CIR.0000121424.76486.20
37. Zandecki L, Sadowski M, Janion M, Kurzawski J, Gierlotka M, Poloński L, et al. Survival benefit from recent changes in management of men and women with ST-segment elevation myocardial infarction treated with percutaneous coronary interventions. Cardiol J. (2019) 26:459–68. doi: 10.5603/CJ.a2018.0057
38. Meisel SR, Kleiner-Shochat M, Abu-Fanne R, Frimerman A, Danon A, Minha S, et al. Direct admission of patients with ST-segment-elevation myocardial infarction to the catheterization laboratory shortens pain-to-balloon and door-to-balloon time intervals but only the pain-to-balloon interval impacts short- and long-term mortality. J Am Heart Assoc. (2021) 10:e018343. doi: 10.1161/JAHA.120.018343
Keywords: percutaneous coronary intervention, myocardial infarction, door-to-balloon time, culprit vessel, ST-elevation myocardial infarction
Citation: Guo J, Wang G, Li Z, Liu Z, Wang Y, Wang S, Wang Y, Wu Y, Wang H, Wang Y, Zhang L and Hua Q (2022) Culprit vessel revascularization first with primary use of a dedicated transradial guiding catheter to reduce door to balloon time in primary percutaneous coronary intervention. Front. Cardiovasc. Med. 9:1022488. doi: 10.3389/fcvm.2022.1022488
Received: 18 August 2022; Accepted: 10 October 2022;
Published: 28 October 2022.
Edited by:
Turgay Celik, VM Medical Park Ankara (Keçiören), TurkeyReviewed by:
Martin Geyer, Johannes Gutenberg University Mainz, GermanyImad Sheiban, Casa di Cura Pederzoli, Italy
Copyright © 2022 Guo, Wang, Li, Liu, Wang, Wang, Wang, Wu, Wang, Wang, Zhang and Hua. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jincheng Guo, guojcmd@126.com; Qi Hua, huaqi5371@medmail.com.cn
†These authors have contributed equally to this work and share first authorship
 Jincheng Guo
Jincheng Guo Guozhong Wang
Guozhong Wang Zixuan Li
Zixuan Li Zijing Liu1
Zijing Liu1  Yujie Wang
Yujie Wang