Abstract
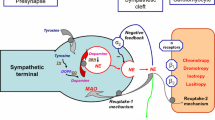
Iodine-123 metaiodobenzylguanidine (MIBG) is a noradrenaline analogue which can be used as a tracer to investigate the cardiac sympathetic nervous system. Regional ischaemia leads to noradrenaline depletion with functional denervation which can be demonstrated by reduced MIBG uptake. In order to evaluate the reversibility of ischaemia-associated damage to the sympathetic nervous system, neuronal scintigraphy with 123I-MIBG and myocardial rest and stress perfusion scintigraphy with technetium-99m sestamibi was performed in 16 patients with coronary artery disease before and 3–4 months after percutaneous transluminal coronary angioplasty (PTCA). Partial re-innervation ocurred in five patients, the degree of stenosis of remaining lesions being estimated by repeat angiography to be below 40%. Unchanged MIBG defects cold be confirmed in four patients with residual lesions of between 40% and 50%. Increased MIBG defects were shown in three patients with significant restenoses of more than 70%. In all patients the neuronal defects exceeded the ischaemia-induced or scar-associated perfusion defects. Three patients dropped out of this study: one for technical reasons, one due to emergency aortocoronary bypass surgery and one due to diabetic polyneuropathy. This investigation shows that the sympathetic nervous system is highly sensitive to ischaemia. Further studies need to be done to assess the conditions allowing re-innervation after PTCA.
Similar content being viewed by others
References
Manger WM. Adrenergic involvement in cardiac pathophysiology. In: Manger WM, ed. Catecholamines in normal and abnormal cardiac function. New York: Karger Press; 1982.
Sisson JC, Wieland DM, Sherman P, et al. Metaiodobenzylguanidine as an index of the adrenergic nervous system integrity and function. J Nucl Med 1987;28:1620–1624.
Jaques S Jr, Tobes MC, Sisson JC, et al. Comparison of sodium dependency of uptake of meta-iodo-benzylguanidine and norepinephrine into cultured bovine adrenomedullary cells. Mol Pharmacol 1984;26:539–546.
Wellman HN, Zipes DP. Cardiac sympathetic imaging with radioiodinated metaoiodobenzylguanidine (MIBG). Prog Cardiol 1990;3:161–174.
Wieland DM, Brown LE, Tobes MC, et al. Imaging the primate adrenal medullae with 123-I and 131-I metaiodobenzylguanidine: concise communication. J Nucl Med 1981;22:358–364.
Gasnier B, Rosin MP, Scherman D, et al. Uptake of metaiodobenzylguanidine by bovine chromaffin granule membranes. Mol Pharmacol 1986;29:275–280.
Sisson JC, Shapiro B, Meyers L, et al. Metaiodobenzylguanidine to map scintigraphically the adrenergic nervous system in man. J Nucl Med 1987;28:1625–1636.
Dae MW, O'Connell JW, Botvinick EH, et al.. Scintigraphic assessment of regional cardiac adrenergic innervation. Circulation 1989;79:634–644.
Stenton MS, Tuli M, Radke ML, et al. Regional sympathetic denervation after myocardial infarction in humans detected noninvasively using I-123 metaiodobenzylguanidine. J Am Coll Cardiol 1989;14:1519–1526.
Fagret D, Wolf JE, Comet M. Myocardial uptake of meta-123 I-iodobenzylguanidine (123I-MIBG) in patients with myocardial infarct. Eur J Nucl Med 1989;15:624–628.
Nishimura T, Oka H, Sago M, et al. Serial assessment of denervated but viable myocardium following acute myocardial infarction in dogs using iodine-123 metaiodobenzylguanidine and thallium-201 chloride myocardial single photon emission tomography. Eur J Nucl Med 1992;19:25–29.
Schwaiger M, Guibourg H, Rosenspire K, et al. Effect of regional myocardial ischemia on sympathetic nervous system was assessed by fluorine- l8-metaraminol. J Nucl Med 1990; 31:1352–1357.
Shapiro B, Sisson JC, Eyre P, et al. 131-I-MIBG, a new agent in diagnosis and treatment of pheochromocytoma. Cardiology 1985;72:137–142.
Henderson EB, Kahn JK, Corbett LR, et al. Abnormal I-123-metaiodobenzylguanidine myocardial washout and distribution may reflect myocardial adrenergic derangement in patients with congestive cardiomyopathy. Circulation 1988;78:1192–1199.
Schofer J, Spielmann R, Schuchert A, et al. Meta-(123) iodo benzylguanidine in idiopathic dilated cardiomyopathy: a non-invasive method to assess myocardial catecholamine depletion? Circulation 1987;76 Suppl IV:IV-1225.
Glowniak JV, Turner FE, Gray LL, et al. I-123 metaiodobenzylguanidine (MIBG) cardiac imaging in idiopathic congestive cardiomyopathy (ICC). J Nucl Med 1987;28:667.
Merlet P, Bourguignon MH, Valette H, et al. I-123 metaiodobenzylguanidine (MIBG) myocardial uptake in patients with primary hypertrophic cardiomyopathy (PHC). J Nucl Med 1989;30:810.
Nagami K, Iwanaga S, Gotoh S, et al. Regional abnormality of myocardial sympathetic nervous activity in patients with hypertrophic cardiomyopathy. J Nucl Med 1990;31:772.
Nakajiama K, Bunko H, Taki J, et al. Analysis of I-123 MIBG yptake and clearance in hypertrophic cardiomyopathy. J Nucl Med 1990;31:772.
Tanaka T, Aizawa T, Kato K, et al. Estimation of regional myocardial sympathetic neuronal function with I-123 metaiodobenzylguanidine (MIBG) myocardial images in patients with cardiomyopathy. J Nucl Med 1989;26:257–261.
Glowniak JV, Turner FE, Gray LL, et al. Iodine-123 metaiodobenzylguanidine imaging of the heart in idiopathic congestive cardiomyopathy and in cardiac transplantation. J Nucl Med 1989;30:1182–1191.
Hiroe M, Otha Y, Kusakabe K, et al. Cardiac tomographic assessment of regional sympathetic denervation in transient myocardial ischemia of coronary artery disease. J Nucl Med 1990;31:793.
Holmgren S, Abrahamsson T, Almgren O, et al. Effect of ischemia on the adrenergic neuron of the rat heart: a fluorescence histochemical and biochemical study. Cardiovasc Res 1981;15:680–689.
Goldstein DS, Brush JE Jr, Eisenhofer G, et al. In vivo measurement of neuronal uptake of norepinephrine in the human heart. Circulation 1988;78:41–48.
Dart AM, Dietz R, Kübler W, et al. Effects of cocaine and desimipramine on the neuronally evoked overflow of endogenous noradrenaline from the rat heart. Br J Pharmacol 1983;79:71–74.
Zaza A, Schwartz PJ. Role of autonomic nervous system in the genesis of early ischemic arrhythmias. J Cardiovasc Pharmacol 1985;7 Suppl 5:5–12.
Kaltenbach M. The long wire technique — a new technique for steerable balloon catheter dilatation of coronary artery stenoses. Eur Heart J 1984;5:1004–1009.
Klepzig H Jr, Kober G, Satter P. Analysis of 100 emergency bypass operations after percutaneous transluminal coronary angioplasty: which patients are at risk for large infections? Eur Heart J 1991;12:946–951.
Tobes MC, Sandford J Jr, Wieland DM. Effect of uptake inhibitors on the uptake of norepinephrine and metaiodobenzylguanidine. J Nucl Med 1985;26:897–907.
Wakasugi S, Wada A, Hasegawa Y. Detection of abnormal cardiac adrenergic neuron activity in adriamycin-induced cardiomyopathy with iodine- 125-metaiodobenzylguanidine. J Nucl Med 1992;33:208–214.
Guertner C, Hoer G. Neuroadrenerge Funktionsszintigraphie des Herzens (“Neurotransmitter Mapping”) — derzeitiger Stand. Nucl Med 1991;30:11–114.
Reimer KA, Jennings RB. The “wavefront phenomenon” of myocardial ischemic cell death. Lab Invest 1979;40:633–644.
Dae MW, Chin MC, O'Connel W. Effects of reperfusion on myocardial sympathetic nerves. Circulation 1989;80:11–415.
Richalet JP, Bourguignon M, Le-Trong JL. MIBG scintigraphic assessment of cardiac adrenergic activity in response to altitude hypoxia. J Nucl Med 1990;31:34–37.
Kober G, Vallbacht C, Lang H. Transluminale koronare Angioplastik 1977–1985 — Erfahrungen bei 1000 Eingriffen. Radiologe 1985;25:346–353.
Kaltenbach M, Kober G, Scherer D. Recurrence rate after successful coronary angioplasty. Eur Heart J 1985;6:276–281.
Eisen HJ, Nader Gr, Reily J. Assessment of regional myocardial adrenergic activity following myocardial infarction using I-123-metaiodobenzylguanidine. Circulation 1989;80:11–514.
Dae M, Herre J, Botvinick E. Scintigraphic detection of denervated myocardium after infarction. J Nucl Med 1986;27:949.
Inoue H, Zipes DP. Results of sympathetic denervation on the canine heart: supersensitivity that may be arrhythmogenic. Circulation 1987;75:877.
Tuli M, Minardo J, Mock B. SPECT with high purity I-123-MIBG after transmural myocardial infarction (TMI), demonstrating sympathetic denervation followed by reinnervation in a dog model. J Nucl Med 1987;28:669.
Dolezel S, Gerova M, Hartmannova B. Cardiac adrenergic innervation after instrumentation of the coronary artery in dog. Am J Physiol 1984;246:459–465.
Kaye MP, Tyce GM. Norepinephrine uptake as an indicator of cardiac reinnervation in dogs. Am J Physiol 1978;253:H289-H294.
Pfeifer MA, Weinberg CR, Cook DL. Autonomic neural dysfunction in recently diagnosed diabetic subjects. Diabetes Care 1984;7:447–453.
Masaoka S, Lev-Ran A, Hill LR. Heart rate variability in diabetes: relationship to age and duration of the disease. Diabetes Care 1985;8:64–68.
Author information
Authors and Affiliations
Additional information
This work is dedicated to Prof. Kaltenbach on the occasion of his sixty-fifth birthday
Rights and permissions
About this article
Cite this article
Guertner, C., Klepzig, H., Maul, F.D. et al. Noradrenaline depletion in patients with coronary artery disease before and after percutaneous transluminal coronary angioplasty with iodine-123 metaiodobenzylguanidine and single-photon emission tomography. Eur J Nucl Med 20, 776–782 (1993). https://doi.org/10.1007/BF00180908
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00180908