Summary
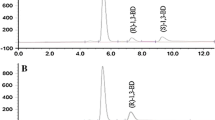
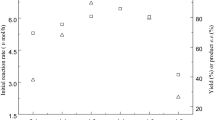
The sequential two-step conversion of 4-oxoisophorone (OIP) to 4-hydroxy-2,2,6-trimethylcyclohexanone (4-HTMCH) via diyhdrooxoisophorone (DOIP) was achieved using two kinds of thermophiles, Thermomonospora curvata and Bacillus stearothermophilus. In the first step, 83% OIP was converted to DOIP by T. curvata during 12 h incubation at 50° C. The resulting reaction mixture containing thermophile cells, DOIP (2.5 mg/ml), and OIP (0.5 mg/ml) was used directly in the second step after adjusting to pH 7 and adding glycerol. In the second step, DOIP in the reaction mixture was converted to 4-HTMCH by B. stearothermophilus. The final concentration of 4-HTMCH and DOIP after 24 h of incubation was 2.5 mg/ml and 0.5 mg/ml respectively; OIP was not detected. The total conversion yield of 4-HTMCH from OIP was 83% through the two-step conversion. The two-step conversion by a sequential culture system using T. curvata and B. stearothermophilus was found to be suitable for 4-HTMCH production.
Similar content being viewed by others
References
Fukumura T (1977) Conversion of d- and dl-α-amino-ɛ-caprolactam into l-lysine using both yeast cells and bacterial cells. Agric Biol Chem 41:1327–1330
Hori N, Hieda T, Mikami Y (1984) Microbial conversion of 4-oxoisophorone by thermophile Thermomonospora curvata. Agric Biol Chem 48:123–129
Lee BK, Brown WE, Ryu DY, Thoma RW (1971) Sequential 11α-hydroxylation and 1-dehydrogenation of 16α-hydroxycortexolone. Biotechnol Bioeng 13:503–515
Leuenberger HGW, Boguth W, Widmer E, Zell R (1976) Synthesis of optically active natural carotenoids and structurally related compounds. Helv Chim Acta 59:1832–1849
Mazumder TK, Sonomoto K, Tanaka A, Fukui S (1985) Sequential conversion of cortexolone to prednisolone by immobilized mycelia of Curvularia lunata and immobilized cells of Arthrobacter simplex. Appl Microbiol Biotechnol 21:154–161
Mori K (1974) Synthesis of optically active grasshopper ketone and dehydrovomifoliol as a synthetic support for the revised absolute configuration of (+)-abscisic acid. Tetrahedron 30:1065–1072
Nishii K, Sode K, Karube I (1989) Microbial conversion of dihydrooxoisophorone (DOIP) to 4-hydroxy-2,2,6-trimethyl-cyclohexanone (4-HTMCH) by thermophilic bacteria. J Biotechnol 9:117–128
Shimizu S, Hattori S, Hata H, Yamada H (1987) Stereoselective enzymatic oxidation and reduction system for the production of d(−)-pantoyllactone from a racemic mixture of pantoyllactone. Enzyme Microb Technol 9:411–416
Sode K, Kajiwara K, Tamiya E, Karube I (1987) Continuous asymmetric reduction of 4-oxoisophorone by thermophilic bacteria using hollow-fiber reactor. Biocatalysis 1:77–86
Sonoyama T, Tani H, Matsuda K, Kageyama B, Tanimoto M, Kobayashi K, Yagi S, Kyotani H, Mitsushima K (1982) Production of 2-keto-l-gulonic acid from d-glucose by two-stage fermentation. Appl Environ Microbiol 43:1064–1069
Takamatsu S, Umemura I, Yamamoto K, Sato T, Tosa T, Chibata I (1982) production of l-alanine from ammonium fumarate using two immobilized microorganisms. Eur J Appl Microbiol Biotechnol 15:147–152
Author information
Authors and Affiliations
Additional information
Offprint requests to: I. Karube
Rights and permissions
About this article
Cite this article
Nishii, K., Sode, K. & Karube, I. Sequential two-step conversion of 4-oxoisophorone to 4-hydroxy-2,2,6-trimethylcyclohexanone by thermophilic bacteria. Appl Microbiol Biotechnol 33, 245–250 (1990). https://doi.org/10.1007/BF00164515
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00164515