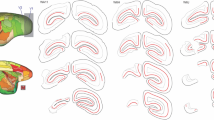
Summary
Unilateral lesions of the right superior colliculus (SC) were made in hamsters on the day after birth. In order to quantify the extent of abnormal innervation by left eye fibers in the diencephalon and midbrain, the left eye was removed on postnatal day 12 or 36, and after an appropriate survival time, the brains were stained for degenerating axons and axon terminals with the Fink-Heimer method. In additional cases, anterograde transport of 3H proline-leucine or horseradish peroxidase was used to assess left eye connectivity. In agreement with previous reports we found abnormal projections in the ventral nucleus of the lateral geniculate body (LGv), in the lateral posterior nucleus (LP) of the thalamus, and in the left SC (the ‘recrossing’ pathway). We also noted areas of abnormally heavy terminal fields arranged in patches in coronal sections in the dorsal nucleus of the lateral geniculate body (LGd). These patches arise from columns of dense innervation that are oriented along a rostral-to-caudal axis. If the right SC lesion was made large enough to diminish the recrossing pathway, retinofugal axons establish a significantly smaller distal terminal field in the left SC. In these cases, a corresponding increase in the size of terminal fields in all major proximal structures (LGd, LGv, LP, DTN) was observed. The sum of abnormal proximal growth (“compensatory sprouting”) was found to truly compensate for the distal loss of terminals. The evaluation of hamsters in which left eye connectivity was assessed at the age of 12 days revealed that lesion-induced patches of abnormal growth have already reached their full size by that time. These findings provide evidence for the ‘pruning -effect’ and demonstrate that retinofugal axons support a fixed number of terminal arborizations (the principle of ‘conservation of total axonal arborizations’).
Similar content being viewed by others
References
Baisinger J, Lund RD, Miller B (1977) Aberrant retinothalamic projections resulting from unilateral tectal lesions made in fetal and neonatal rats. Exp Neurol 54: 369–382
Bryman BS, Goldberger ME (1982) Anatomical plasticity and sparing of function after spinal cord damage in neonatal cats. Science 217: 553–555
Cotman CW (ed) (1978) Neuronal plasticity. Raven Press, New York
Cotman CW, Matthews DA, Taylor DA, Lynch G (1973) Synaptic rearrangement in the dentate gyrus: histochemical evidence of adjustments after lesions in immature and adult rats. Proc Natl Acad Sci USA 70: 3473–3477
Crain BJ, Hall WC (1980) The organization of afferents to the lateral posterior nucleus in the golden hamster after different combinations of neonatal lesions. J Comp Neurol 193: 403–412
Devor M (1976) Neuroplasticity in the rearrangement of olfactory tract fibers after neonatal transection in hamsters. J Comp Neurol 166: 49–72
Devor M, Schneider GE (1975) Neuroanatomical plasticity: the principle of conservation of total axonal arborization. Inserm 43: 191–200
Finger S, Stein DG (1982) Brain damage and recovery. Academic Press, New York
Fink RP, Heimer L (1967) Two methods for selective silver impregnation of degenerating axons and their synaptic endings in the central nervous system. Brain Res 4: 369–374
Finlay BL, Wilson KG, Schneider GE (1979) Anomalous ipsilateral retinotectal projections in Syrian hamsters with early lesions: topography and functional capacity. J Comp Neurol 183: 721–740
Frost DO (1982) Anomalous visual connections to somatosensory and auditory systems following brain lesions in early life. Dev Brain Res 3: 627–635
Frost DO (1984) Axonal growth and target selection during development: retinal projections to the ventrobasal complex and other “nonvisual” structures in neonatal Syrian hamsters. J Comp Neurol 230: 576–592
Frost DO, So K-F, Schneider GE (1979) Postnatal development of retinal projections in Syrian hamsters: a study using autoradiographic and anterograde degeneration techniques. Neuroscience 4: 16–49
Gall C, McWilliams R, Lynch G (1979) The effect of collateral sprouting on the density of innervation of normal target sites: implications for theories on the regulation of the size of developing synaptic domains. Brain Res 157: 37–47
Guillery RW (1972) Experiments to determine whether retinogeniculate axons can form translaminar collateral sprouts in the dorsal lateral geniculate nucleus of the cat. J Comp Neurol 46: 407–420
Heimer L, Peters A (1968) An electron microscope study of a silver stain for degenerating boutons. Brain Res 8: 337–346
Hefti F, Weiner WJ (1988) Adaptive changes in central dopaminergic neurons after injury: effects of drugs. In: Stein DG, Sabel BA (eds) Pharmacological approaches to the treatment of brain and spinal cord injury. Plenum Press, New York, pp 103–119
Hsiao CF, Sachs GM, Schneider GE (1984) A minute fraction of Syrian golden hamster retinal ganglion cells project bilaterally. J Neurosci 4: 359–367
Huerta MF, Harting JK (1984) The mammalian superior colliculus: studies of its morphology and connections. In: Vanegas H (ed) Comparative neurology of the optic tectum. Plenum Press, New York, pp 687–773
Innocenti GM (1981) Transitory structures as substrates for developmental plasticity of the brain. Dev Neurosci 13: 305–333
Jen LS, Lund RD (1981) Experimentally induced enlargement of the uncrossed retinotectal pathway in rats. Brain Res 211: 37–57
Jen LS, Woo HH, So K-F (1980) The uncrossed retinotectal pathways in golden hamsters — an orthograde HRP study. Soc Neurosci Abstr 6: 207
Jen LS, So K-F, Woo HH (1984) An anterograde HRP study of the retinocollicular pathways in normal hamsters and hamsters with one eye enucleated at birth. Brain Res 294: 169–173
Jhaveri S, Northmore DPM, Schneider GE (1985) Morphology of tectal efferents to diencephalon visualized with the anterograde transport of PHA-L. Soc Neurosci Abstr 69.15
Jonsson G, Pollare T, Hallman H, Sachs C (1978) Developmental plasticity of central serotonin neurons after 5,7-dihydroxytryptamine treatment. Ann N Y Acad Sci 305: 328–345
Kalil RE (1972) Formation of new retino-geniculate connections in kittens after removal of one eye. Anat Rec 172: 339–340
Kalil RE (1973) Formation of new retino-geniculate connections in kittens: effects of age and visual experience. Anat Rec 175: 353
Kalil RE, Schneider GE (1975) Abnormal synaptic connections of the optic tract in the thalamus after midbrain lesions in newborn hamsters. Brain Res 100: 690–698
Laurberg S, Zimmer J (1980) Lesion-induced rerouting of hippocampal mossy fibers in developing but not in adult rats. J Comp Neurol 190: 627–650
Leong SK, Lund RD (1973) Anomalous bilateral corticofugal pathway in albino rats after neonatal lesions. Brain Res 62: 218–221
Linden R, Perry VH (1983) Massive retinotectal projection in rats. Brain Res 272: 145–149
Lund RD, Cunningham TJ, Lund JS (1973) Modified optic projections after unilateral eye removal in young rats. Brain Behav Evol 8: 51–72
Lund JS, Remington FL, Lund RD (1976) Differential central distribution of optic nerve components in the rat. Brain Res 116: 83–100
Lund RD (1978) Development and plasticity of the brain, Oxford University Press, New York
Mason R, Groos GA (1981) Cortico-recipient and tecto-recipient visual zones in the rat's lateral posterior (pulvinar) nucleus: an anatomical study. Neurosci Lett 25: 107–112
Mesulam MM (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction-product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26: 106–117
Nakamura S, Shirokawa T, Sakaguchi T (1984) Increased projection from the locus coeruleus to the lateral geniculate nucleus and visual cortex in young adult rats following unilateral enucleation. Neurosci Lett 49: 77–80
Nauta WJH (1950) Über die sogenannte terminale Degeneration im Zentralnervensystem und ihre Darstellung durch Silberimpregnation. Arch Neurol Psychiatr 66: 353–376
Pasquier DA, Villar MJ (1982) Subcortical projections to the lateral geniculate body in the rat. Exp Brain Res 48: 409–419
Perry VH (1980) A tectocortical visual pathway in the rat. Neuroscience 5: 915–927
Perry VH, Cowey A (1979) Changes in the retino-fugal pathways following cortical and tectal lesions in neonatal and adult rats. Exp Brain Res 35: 97–108
Perry VH, Cowey A (1982) A sensitive period for ganglion cell degeneration and the formation of aberrant retino-fugal connections following tectal lesions in rats. Neuroscience 7: 583–594
Reese BE (1984) The projection from the superior colliculus to the dorsal lateral geniculate nucleus in the rat. Brain Res 305: 162–168
Sabel BA, Schneider GE (1988) Enhanced sprouting of retinotectal fibers after early superior colliculus lesions in hamsters treated with gangliosides. Exp Brain Res 71: 365–376
Sachs C, Pycock Ch, Jonsson G (1974) Altered development of central noradrenaline neurons during ontogeny by 6-hydroxy-dopamine. Med Biol 52: 55–65
Sachs GM, Schneider GE (1984) The morphology of optic tract axons arborizing in the superior colliculus of the hamster. J Comp Neurol 230: 155–167
Sakaguchi T, Shirokawa T, Nakamura S (1984) Changes in projection from locus coeruleus to lateral geniculate nucleus following ablation of visual cortex in adult rats. Brain Res 321: 319–322
Schneider GE (1970) Mechanisms of functional recovery following lesions of visual cortex or superior colliculus in neonate and adult hamsters. Brain Behav 3: 295–323
Schneider GE (1971) Competition for terminal space in formation of abnormal retinotectal connections, and a functional consequence. Anat Rec 169: 420
Schneider GE (1973) Early lesions of superior colliculus: factors affecting the formation of abnormal retinal projections. Brain Behav Evol 8: 73–109
Schneider GE (1978) Postnatal development of retinal projections to the lateral geniculate body in Syrian hamsters. Brain Res 142: 343–352
Schneider GE (1979) Is is really better to have your brain lesion early? A revision of the “Kennard principle”. Neuropsychologia 17: 557–583
Schneider GE (1981) Early lesions and abnormal neuronal connections. Trends Neurosci 4: 187–192
Schneider GE, Jhaveri S, Edwards M, So KF (1985) Regeneration, re-routing, and redistribution of axons after early lesions: changes with age, and functional impact. In: Eccles JC, Dimitrijevic MR (eds) Recent achievements in restorative neurology, Vol 1. Upper motor function and dysfunction. Karger, Basel, pp 291–310
Schneider GE, Jhaveri S, Davis WF (1987) On the development of neuronal arbors. Pontif Acad Sci Scripta Varia 59: 31–64
Sefton AJ (1968) The innervation of the lateral geniculate nucleus and anterior colliculus in the rat. Vision Res 8: 867–881
Sefton AJ, Dreher B (1985) Visual system. In: Paxinos (ed) The rat nervous system, Vol 1. Academic Press, pp 169–221
Sengelaub DR, Finlay BL (1981) Early removal of one eye reduces normally occuring cell death in the remaining eye. Science 213: 573–574
So K-F (1979) Development of abnormal recrossing retinotectal projections after superior colliculus lesions in newborn Syrian hamsters. J Comp Neurol 186: 241–258
So K-F, Schneider GE (1978) Abnormal recrossing retinotectal projections after early lesions in Syrian hamsters: age-related effects. Brain Res 147: 277–295
So K-F, Woo HH, Jen LS (1984) The normal and abnormal postnatal development of retinogeniculate projections in golden hamsters: an anterograde horseradish peroxidase tracing study. Dev Brain Res 12: 191–205
Steward O (1984) Lesion-induced neuroplasticity and the sparing or recovery of function following early brain damage. In: Almli CR, Finger S (eds) Early brain damage, Vol 1. Academic Press, New York, pp 59–77
Takahashi T (1985) The organization of the lateral thalamus of the hooded rat. J Comp Neurol 231: 281–309
Tolbert DL (1987) Intrinsically directed pruning as a mechanism regulating the elimination of transient collateral pathways. Dev Brain Res 33: 11–21
Woo HH, Jen LS, So K-F (1985) The postnatal development of retinocollicular projections in normal hamstes and in hamsters following neonatal monocular enucleation: a horseradish peroxidase tracing study. Dev Brain Res 20: 1–13
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sabel, B.A., Schneider, G.E. The principle of “conservation of total axonal arborizations”: massive compensatory sprouting in the hamster subcortical visual system after early tectal lesions. Exp Brain Res 73, 505–518 (1988). https://doi.org/10.1007/BF00406608
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00406608