Summary
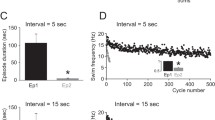
1. Neurons from the isolated pedal ganglia of the marine mollusc Clione limacina were recorded from intracellularly during generation of the locomotory rhythm. Polarization of single type 7 or type 8 interneurons (which discharge in the D-and V-phases of a swim cycle, respectively) strongly affected activity of the rhythm generator. Injection of depolarizing and hyperpolarizing current usually resulted in shortening and lengthening of a swim cycle, respectively. A short pulse of hyperpolarizing current shifted the phase of the rhythmic generator. The same effect could be evoked by polarization of efferent neurons of types 2, 3 and 4 which are electrically coupled to interneurons. On the contrary, polarization of types 1, 6 and 10 efferent neurons, having no electrical connections with interneurons, did not affect the locomotory rhythm. 2. A number of observations indicate that type 7 and 8 interneurons constitute the main source of postsynaptic potentials that were observed in all the “rhythmic” neurons of the pedal ganglia. Type 7 interneurons excited the D-phase neurons and inhibited the V-phase neurons; type 8 interneurons produced opposite effects. 3. Tetrodotoxin eliminated spike generation in all efferent neurons of the pedal ganglia, while in interneurons spike generation persisted. After blocking the spike discharges in all the efferent neurons, type 7 and 8 interneurons were capable of generating alternating activity. One may conclude that these interneurons determine the main features of the swim pattern, i.e., the rhythmic alternating activity of two (D and V) populations of neurons. 4. Both type 7 and type 8 interneurons were capable of endogenous rhythmic discharges with a period like that in normal swimming. This was demonstrated in experiments in which one of the two populations of “rhythmic” neurons (D or V) was inhibited by means of strong electrical hyperpolarization, as well as in experiments in which interaction between the two populations, mediated by chemical synapses, was blocked by Co2+ ions. 5. Type 7 and 8 interneurons were capable of “rebound”, i.e. they had a tendency to discharge after termination of inhibition. 6. V-phase neurons exerted not only inhibitory but also excitatory action upon D-phase neurons, the excitatory action being longer than the inhibitory one. 7. The main experimental findings correspond well to the model of rhythm generator consisting of two half centres possessing endogenous rhythmic activity. The half-centres exert strong, short duration inhibitory and weak long duration excitatory actions upon one another. The behaviour of such a model is considered and compared with that of the locomotor generator of Clione.
Similar content being viewed by others
References
Arshavsky YuI, Beloozerova IN, Orlovsky GN, Panchin YuV, Pavlova GA (1985a) Control of locomotion in marine mollusc Clione limacina. I. Efferent activity during actual and fictitious swimming. Exp Brain Res 58: 255–262.
Arshavsky YuI, Beloozerova IN, Orlovsky GN, Panchin YuV, Pavlova GA (1985b) Control of locomotion in marine mollusc Clione limacina. II. Rhythmic neurons of pedal ganglia. Exp Brain Res 58: 263–272.
Arshavsky YuI, Beloozerova IN, Orlovsky GN, Panchin YuV, Pavlova GA (1985c) Control of locomotion in marine mollusc Clione limacina. IV. Role of type 12 interneurons. Exp Brain Res 58: 285–293.
Arshavsky YuI, Beloozerova IN, Orlovsky GN, Pavlova GA, Panchin YuV (1984a) Activity of interneurons from pedal ganglia of pteropodial mollusc during generation of locomotory rhythm. Nejrofiziologia 16: 272–275 (in Russian).
Arshavsky YuI, Beloozerova IN, Orlovsky GN, Pavlova GA, Panchin YuV (1984b) Neurons of pedal ganglia of pteropodial mollusc controlling activity of the locomotor generator. Nejrofiziologia 16: 543–546 (in Russian).
Brown TG (1911) The intrinsic factors in the act of progression in the mammal. Proc R Soc Ser B 84: 309–319.
Brown TG (1914) On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression and a theory of the evolution of function in the nervous system. J Physiol (Lond) 48: 18–46.
Calabrese RL (1979) The roles of endogenous membrane properties and synaptic interaction in generating the heartbeat rhythm of the leech, Hirudo medicinialis. J Exp Biol 82: 163–176.
Friesen WO, Stent G (1978) Neuronal circuit for generating rhythmic movements. Ann Rev Biophys Bioeng 7: 37–61.
Friesen WO, Wyman RJ (1979) Model circuit for the control of motor neuron activity pattern in Drosophila light. Soc Neurosci Abst 5: 494.
Getting PA (1981) Mechanisms of pattern generation underlying swimming in Tritonia. I. Neuronal network formed by monosynaptic connections. J Neurophysiol 46: 65–79.
Getting PA (1983a) Mechanisms of pattern generation underlying swimming in Tritonia. II. Network reconstruction. J Neurophysiol 49: 1017–1035.
Getting PA (1983b) Mechanisms of pattern generation underlying swimming in Tritonia. III. Intrinsic and synaptic mechanisms for delayed excitation. J Neurophysiol 49: 1036–1050.
Grillner S (1981) Control of locomotion in bipeds, tetrapods, and fish. In: Brooks V (ed) Handbook of physiology. The nervous system, Vol II. Waverly Press, Baltimore, pp 1179–1236.
Harmon LD (1964) Neuromimes: action of a reciprocally inhibitory pair. Science 146: 1323–1325.
Harmon LD, Lewis ER (1966) Neural modeling. Physiol Revs 46: 513–591.
Kahn JA, Roberts A (1982) Experiments on the central pattern generator for swimming in amphibian embryos. Philos Trans R Soc B 296: 229–243.
Kawato M, Suzuki R (1980) Two coupled neural oscillators as a model of the circadian pacemaker. J Theor Biol 86: 547–575.
Lundberg A (1980) Half-centres revised. In: Symp XXVIII Internat Congr Physiol Sci. Académia Kiado, Budapest, pp 155–167.
Maynard DM, Selverston AI (1975) Organization of the stomatogastric ganglion of the spiny lobster. IV. The pyloric system. J Comp Physiol 100: 161–182.
Merickel M, Gray R (1980) Investigation of burst generation by the electrically coupled cyberchron network in the snail Helisoma using a single-electrode voltage clamp. J Neurobiol 11: 73–102.
Miller JP, Selverston AI (1982a) Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. II. Oscillatory properties of pyloric neurons. J Neurophysiol 48: 1378–1391.
Miller JP, Selverston AI (1982b) Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. IV. Network properties of pyloric system. J Neurophysiol 48: 1416–1432.
Perkel DH, Mulloney B (1974) Motor pattern production in reciprocally inhibitory neurons exhibiting postinhibitory rebound. Science 185: 181–183.
Reiss RF (1962) A theory and simulation of rhythmic behavior due to reciprocal inhibition in small nerve nets. Am Fed Inf Process Soc Proc Spring Computer Conf 21: 171–194.
Roberts A, Kahn JA (1982) Intracellular recordings from spinal neurons during “swimming” in paralysed amphibian embryos. Philos Trans Soc B 296: 213–228.
Rose RM, Benjamin PR (1981) Interneuronal control of feeding in the pond snail Lymnaea stagnalis. II. The interneuronal mechanism generating feeding cycles. J Exp Biol 92: 203–228.
Selverston AI, Miller JP (1980) Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. I. Pyloric system. J Neurophysiol 44: 1102–1121.
Stent GS, Thompson WJ, Calabrese RL (1979) Neural control of heartbeat in the leech and in some other invertebrates. Physiol Rev 59: 101–136.
Wilson DM, Waldron I (1968) Models for the generation of the motor output pattern in flying locust. Proc IEEE 56: 1058–1064.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arshavsky, Y.I., Beloozerova, I.N., Orlovsky, G.N. et al. Control of locomotion in marine mollusc Clione limacina III. On the origin of locomotory rhythm. Exp Brain Res 58, 273–284 (1985). https://doi.org/10.1007/BF00235309
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00235309