Abstract
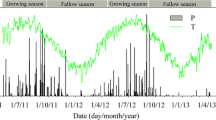
Methane emission rates from an Italian rice paddy field showed diel and seasonal variations. The seasonal variations were not closely related to soil temperatures. However, the dieL changes of CH4 fluxes were significantly correlated with the diel changes of the temperature in a particular soil depth. The soil depths with the best correlations between CH4 flux and temperature were shallow (1–5cm) in May and June, deep (10–15cm) in June and July, and again shallow (1–5 cm) in August. Apparent activation energies (Ea) calculated from these correlations using the Arrhenius model were relatively low (50–150 kJ mol−1) in May and June, but increased to higher values (80–450 kJ mol−1) in August. In the laboratory, CH4 emission from two rice cultures incubated at temperatures between 20 and 38°C showed E α. values of 41 and 53 kJ mol−1) Methane production in anoxic paddy soil suspensions incubated between 7 and 43°C showed E α values between 53 and 132 kJ mol−1 with an average value of 85 kJ mol−1) and in pure cultures of hydrogenotrophic methanogenic bacteria E a values between 77 and 173 (average 126) kJ mol−1. It is suggested that diel changes of soil properties other than temperature affect CH4 emission rates, e.g. diel changes in root exudation or in efficiency of CH4 oxidation in the rhizosphere.
Similar content being viewed by others
References
Cicerone RJ, Shetter JD & Delwiche CC (1983) Seasonal variation of methane flux from a California rice paddy. Journal of Geophysical Research 88: 11022–11024
Conrad R (1989) Control of methane production in terrestrial ecosystems. In: Andreae MO & Schimel DS (Eds) Exchange of Trace Gases Between Terrestrial Ecosystems and the Atmosphere. Dahlem Konferenzen (pp 39–58). Wiley, Chichester
Conrad R, Schütz H & Babbel M (1987) Temperature limitation of hydrogen turnover and methanogenesis in anoxic paddy soil. FEMS Microbiology Ecology 45: 281–289
Conrad R, Bak F, Seitz HJ, Thebrath B, Mayer HP & Schütz H (1989a) Hydrogen turnover by psychrotrophic homoacetogenic and mesophilic methanogenic bacteria in anoxic paddy soil and lake sediment. FEMS Microbiology Ecology 62: 285–294
Conrad R, Mayer HP & Wüst M (1989b) Temporal change of gas metabolism by hydrogen-synthrophic methanogenic bacterial associations in anoxic paddy soil. FEMS Microbiology Ecology 62: 265–274
DeBont JEM, Lee KK & Bouldin DF (1978) Bacterial oxidation of methane in a rice paddy. Ecological Bulletin (Stockholm) 26: 91–96
Gujer W & Zehnder AJB (1983) Conversion processes in anaerobic digestion. Water Science Technology 15: 127–167
Hasebe A, Kanazawa S & Takai Y. (1984) Microbial biomass in paddy soil. I. Microbial biomass calculated from direct count using fluorescence microscope. Soil Science and Plant Nutrition 30: 175–187
Holzapfel-Pschorn A, Conrad R & Seiler W (1985) Production, oxidation, and emission of methane in rice paddies. FEMS Microbiology Ecology 31: 343–351
Holzapfel-Pschorn A, Conrad R & Seiler W (1986) Effects of vegetation on the emission of methane by submerged paddy soil. Plant and Soil 92: 223–233
Holzapfel-Pschorn A & Seiler W (1986) Methane emission during a cultivation period from an Italian rice paddy. Journal of Geophysical Research 91: 11803–11814
Kelly CA & Chynoweth DP (1981) The contribution of temperature and the input of organic matter in controlling rates of sediment methanogenesis. Limnology and Oceanography 26: 891–897
Koyama T (1963) Gaseous metabolism in lake sediments and paddy soils and the production of atmospheric methane and hydrogen. Journal of Geophysical Research 68: 3971–3973
Mayer HP & Conrad R (1990) Factors influencing the population of methanogenic bacteria and the initiation of methane production upon flooding of paddy soil. FEMS Microbiology Ecology 73: 103–112
Moriarty DJW (1986) Measurement of bacterial growth rates in aquatic systems from rates of nucleic acid synthesis. Advances in Microbial Ecology 245–292
Oremland RS & Taylor BF (1977) Diurnal fluctuations of O2, N2, and CH4 in the rhizosphere of Thalassia testudinum. Limnology and Oceanography 22: 566–570
Sand-Jensen K, Prahl C & Stokholm H (1982) Oxygen release from roots of submerged aquatic macrophytes. Oikos 38: 349–354
Schütz H, Holzapfel-Pschorn A, Conrad R, Rennenberg H & Seiler W (1989a) A three years continuous record on the influence of daytime, season, and fertilizer treatment on methane emission rates from an Italian rice paddy field. Journal of Geophysical Research 94: 16,405–16,416
Schütz H, Seiler W & Conrad R (1989b) Processes involved in formation and emission of methane in rice paddies. Biogeochemistry 7: 33–53
Seiler W, Holzapfel-Pschorn A, Conrad R & Scharffe D (1984) Methane emission from rice paddies. Journal of Atmospheric Chemistry 1: 241–268
Sculthorpe CD (1967) The Biology of Aquatic Vascular Plants. Edward Arnold, London
Sieburth JM (1967) Seasonal selection of estuarine bacteria by water temperature. Journal of Experimental Marine Biology and Ecology 1: 98–121
Svensson BH (1984) Different temperature optima for methane formation, when enrichments from acid peat are supplemented with acetate or hydrogen. Applied and Environmental Microbiology 48: 389–394
Svensson BH & Rosswall T (1984) In situ methane production from acid peat in plant communities with different moisture regimes in a subarctic mire. Oikos 43: 342–350
Thorn PM & Ventullo RM (1988) Measurement of bacterial growth rates in subsurface sediments using the incorporation of tritiated thymidine into DNA. Microbial Ecology 16: 3–16
Wang MX, Dai A, Shen RX, Wu HB, Schütz H, Rennenberg H & Seiler W (1990) CH4 emission from a China rice paddy field. Journal of Science China (in press)
Westermann P & Ahring BK (1987) Dynamics of methane production, sulfate reduction, and denitrification in a permanently waterlogged alder swamp. Applied and Environmental Microbiology 53: 2554–2559
Westermann P, Ahring BK & Mah RA (1989) Temperature compensation in Methanosarcina barkeri by modulation of hydrogen and acetate affinity. Applied and Environmental Microbiology 55: 1262–1266
Westrich JT & Berner RA (1988) The effect of temperature on rates of sulfate reduction in marine sediments. Geomicrobiology Journal 6: 99–117
Widdel F & Pfennig N (1981) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen.nov.sp.nov. Archives of Microbiology 129: 395–400
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schütz, H., Seiler, W. & Conrad, R. Influence of soil temperature on methane emission from rice paddy fields. Biogeochemistry 11, 77–95 (1990). https://doi.org/10.1007/BF00002060
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00002060