Abstract
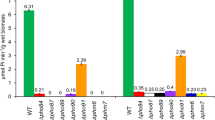
Transcription of a regulatory gene,PHO81, in the phosphatase regulon ofSaccharomyces cerevisiae is repressed by inorganic phosphate (Pi) in the medium via that same regulatory system. The activity of Pho81p, the product ofPHO81, is also inhibited by a high concentration of Pi in the medium. Increased dosage ofPHO86, a gene encoding a putative membrane protein associated with a Pi transporter complex, activates the Pi-inhibited Pho81p produced under the control of theGAL1 promoter. A new gene,PHO88/YBR106w, has now been identified as a multicopy suppressor of the rAPase− phenotype of the cells caused by theP i inhibition of Pho81p. Thepho86 disruptant expressed rAPase activity in high-Pi medium, while thepho88 disruptant did not. The Δpho86 Δpho88 double disruption resulted in enhanced synthesis of rAPase under the high-Pi condition and conferred arsenate resistance on the cells than those in single disruptants of these genes. Its hydropathy profile and the results of an analysis of its cellular localization suggested that Pho88p is a membrane protein similar to Pho86p. Both disruption and high dosage ofPHO88 orPHO86 resulted in reduced Pi uptake. These findings suggest that Pho88p is also involved in Pi transport and modulates Pho81p function together with Pho86p.
Similar content being viewed by others
References
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1987) Current protocols in molecular biology. John Wiley and Sons, New York
Bennett RL, Malamy MH (1970) Arsenate resistant mutants ofEscherichia coli and phosphate transport. Biochem Biophys Res Commun 40:496–503
Bun-ya M, Nishimura M, Harashima S, Oshima Y (1991) ThePHO84 gene ofSaccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol Cell Biol 11:3229–3238
Bun-ya M, Harashima S, Oshima Y (1992) Putative GTP-binding protein, Gtrl, associated with the function of the Pho84p inorganic phosphate transporter inSaccharomyces cerevisiae. Mol Cell Biol 12:2958–2966
Bun-ya M, Shikata K, Nakade S, Yompakdee C, Harashima S, Oshima Y (1996) Two new genes,PHO86 andPHO87, involved in inorganic phosphate uptake inSaccharomyces cerevisiae. Curr Genet 29:344–351
Clark-Adams CD, Norris D, Osley MA, Fassler JS, Winston F (1988) Changes in histone gene dosage alter transcription in yeast. Genes Dev 2:150–159
DeFranco AL, Koshland DE Jr (1981) Molecular cloning of chemotaxis genes and overproduction of gene products in the bacterial sensing system. J Bacteriol 147:390–400
Eisenberg D (1984) Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem 53:595–623
Feldmann H, Aigle M, Aljinovic G, André B, Baclet MC, Barthe C, Baur A, Bécam A-M, Biteau N, Boles E, Brandt T, Brendel M, Brückner M, Bussereau F, Christiansen C, Contreras R, Crouzet M, Cziepluch C, Démolis N, Delaveau Th, Doignon F, Domdey H, Düsterhus S, Dubois E, Dujon B, Bakkoury MEI, Entain K-D, Feuerman M, Fiers W, Fobo GM, Fritz C, Gassenhuber H, Glansdorff N, Goffeau A, Grivell LA, de Hann M, Hein C, Herbert CJ, Hollenberg CP, Holmstrøm K, Jacq C, Jacquet M, Jauniaux JC, Jonniaux JL, Kallesøe T, Kiesau P, Kirchrath L, Kötter P, Korol S, Liebl S, Logghe M, Lohan AJE, Louis EJ, Li ZY, Maat MJ, Mallet L, Mannhaupt G, Messenguy F, Miosga T, Molemans F, Müller S, Nasr F, Obermaier B, Perea J, Piérard A, Piravandi E, Pohl FM, Pohl TM, Potier S, Proft M, Purnelle B, Ramezani Rad M, Rieger M, Rose M, Schaaff-Gerstenschläger I, Scherens B, Schwarzlose C, Skala J, Slonimski PP, Smits PHM, Souciet JL, Steensma HY, Stucka R, Urrestarazu A, van der Aart QJM, van Dyck L, Vassarotti A, Vetter I, Vierendeels F, Vissers S, Wagner G, de Wergifosse P, Wolfe KH, Zagulski M, Zimmermann FK, Mewes HW, Kleine K (1994) Complete DNA sequence of yeast chromosome II. EMBO J 13:5795–5809
Floor E (1970) Interaction of morphogenetic genes of bacteriophage T4. J Mol Biol 47:293–306
Gallwitz D, Sures I (1980) Structure of a split yeast gene: complete nucleotide sequence of the actin gene inSaccharomyces cerevisiae. Proc Natl Acad Sci USA 77:2546–2550
Gietz RD, Sugino A (1988) New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74:527–534
Johnson DL (1971) Simultaneous determination of arsenate and phosphate in natural waters. Environ Sci Technol 5:411–414
Johnston M, Carlson M (1992) Regulation of carbon and phosphate utilization. In: Jones EW, Pringle JR, Broach JR (eds) The molecular and cellular biology of the yeastSaccharomyces: gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, pp 193–281
Kaffman A, Herskowitz I, Tjian R, O'Shea EK (1994) Phosphorylation of the transcription factor PHO4 by a cyclin-CDK complex, PHO80-PHO85. Science 263:1153–1156
Kubo Y, Baldwin TJ, Jan YN, Jan LY (1993) Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature 362:127–133
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132
Mortimer RK, Contopoulou CR, King JS (1992) Genetic and physical maps ofSaccharomyces cerevisiae, edition 11, Yeast 8:817–902
Ogawa N, Oshima Y (1990) Functional domains of a positive regulatory protein, PHO4, for transcriptional control of the phosphatase regulon inSaccharomyces cerevisiae. Mol Cell Biol 10:2224–2236
Ogawa N, Noguchi K, Yamashita Y, Yasuhara T, Hayashi N, Yoshida K, Oshima Y (1993) Promoter analysis of thePHO81 gene encoding a 134 kDa protein bearing ankyrin repeats in the phosphatase regulon ofSaccharomyces cerevisiae. Mol Gen Genet 238:444–454
Ogawa N, Hayashi N, Saito H, Noguchi K, Yamashita Y, Oshima Y (1994) Regulatory circuit for phosphatase genes inSaccharomyces cerevisiae: specificcis-acting sites inPHO promoters for binding the positive regulator Pho4p. In: Torriani-Gorini A, Yagil E, Silver S (eds) Phosphate in microorganisms: cellular and molecular biology. American Society for Microbiology, Washington DC, pp 56–62
Ogawa N, Noguchi K, Sawai H, Yamashita Y, Yompakdee C, Oshima Y (1995) Functional domains of Pho81p, an inhibitor of Pho85p protein kinase, in the transduction pathway of Pj signals inSaccharomyces cerevisiae. Mol Cell Biol 15:997–1004
Parent SA, Fenimore CM, Bostian K (1985) Vector systems for the expression, analysis and cloning of DNA sequences inS. cerevisiae. Yeast 1:83–138
Philippsen P, Thomas M, Kramer RA, Davis RW (1978) Unique arrangement of coding sequences for 5S, 5.8S, 18S and 25S ribosomal RNA inSaccharomyces cerevisiae as determined by R-loop and hybridization analysis. J Mol Biol 123:387–404
Rose MD, Winston F, Hieter P (1990) Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Rothstein RJ (1983) One-step gene disruption in yeast. Methods Enzymol 101:202–211
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual (2nd edn). Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Schneider KR, Smith RL, O'Shea EK (1994) Phosphate-regulated inactivation of the kinase PHO80-PHO85 by the CDK inhibitor PHO81. Science 266:122–126
Steed PM, Wanner BL (1993) Use of therep technique for allele replacement to construct pstSCAB-phoU operon mutants: evidence for a new role for the PhoU protein in the PHO regulon. J Bacteriol 175:6797–6809
Sternberg N (1976) A genetic analysis of bacteriophage head assembly. Virology 71:568–582
Stotz A, Linder P (1990) TheADE2 gene fromSaccharomyces cerevisiae: sequence and new vectors. Gene 95:91–98
Tamai Y, Toh-e A, Oshima Y (1985) Regulation of inorganic phosphate transport systems inSaccharomyces cerevisiae. J Bacteriol 164:964–968
Toh-e A, Oshima Y (1974) Characterization of a dominant, constitutive mutation,PHOO, for the repressible acid phosphatase synthesis inSaccharomyces cerevisiae. J Bacteriol 120:608–617
Torriani-Gorini A (1994) Introduction: the Pho regulon ofEscherichia coli. In: Torriani-Gorini A, Yagil E, Silver S (eds) Phosphates in microorganisms: cellular and molecular biology. American Society for Microbiology, Washington DC, pp 1–4
Ueda Y, Oshima Y (1975) A constitutive mutation,phoT, of the repressible acid phosphatase synthesis with inability to transport inorganic phosphate inSaccharomyces cerevisiae. Mol Gen Genet 136:255–259
Wanner BL (1994) Multiple controls of theEscherichia coli Pho regulon by the Pi sensor PhoR, the catabolite regulatory sensor CreC, and Acetyl Phosphate. In: Torriani-Gorini A, Yagil E, Silver S (eds) Phosphates in microorganisms: cellular and molecular biology. American Society for Microbiology, Washington DC, pp 13–21
Webb DC, Cox GB (1994) Proposed mechanism for phosphate translocation by the phosphate-specific transport (Pst) system and role of the Pst system in phosphate regulation. In: Torriani-Gorini A, Yagil E, Silver S (eds) Phosphates in microorganisms: cellular and molecular biology. American Society for Microbiology, Washington DC, pp 37–42
Willsky GR, Leung JO, Offermann PV Jr, Plotnick EK, Dosch SF (1985) Isolation and characterization of vanadate-resistant mutants ofSaccharomyces cerevisiae. J Bacteriol 164:611–617
Yompakdee C, Bun-ya M, Shikata K, Ogawa N, Harashima S, Oshima Y (1996) A putative new membrane protein, Pho86p, in the inorganic phosphate uptake system ofSaccharomyces cerevisiae. Gene 171:41–47
Yoshida K, Kuromitsu Z, Ogawa N, Oshima Y (1989a) Mode of expression of the positive regulatory genesPHO2 andPHO4 of the phosphatase regulon inSaccharomyces cerevisiae. Mol Gen Genet 217:31–39
Yoshida K, Ogawa N, Oshima Y (1989b) Function ofPHO regulatory genes for repressible acid phosphatase synthesis inSaccharomyces cerevisiae. Mol Gen Genet 217:40–46
Author information
Authors and Affiliations
Additional information
Communicated by C. P. Hollenberg
Rights and permissions
About this article
Cite this article
Yompakdee, C., Ogawa, N., Harashima, S. et al. A putative membrane protein, Pho88p, involved in inorganic phosphate transport inSaccharomyces cerevisiae . Molec. Gen. Genet. 251, 580–590 (1996). https://doi.org/10.1007/BF02173648
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02173648