Abstract
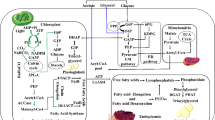
The effects of cold hypoxia were examined during a time-course at 2 °C on levels of glycolytic metabolites: glycogen, glucose, glucose-1-phosphate, glucose-6-phosphate, fructose-6-phosphate, fructose-1,6-bisphosphate, phosphoenolpyruvate, pyruvate, lactate and energetics (ATP, ADP, AMP) of livers from rats and columbian ground squirrels. Responses of adenylate pools reflected the energy imbalance created during cold hypoxia in both rat and ground squirrel liver within minutes of organ isolation. In rat, ATP levels and energy charge values for freshly isolated livers were 2.54 μmol·g-1 and 0.70, respectively. Within 5 min of cold hypoxia, ATP levels had dropped well below control values and by 8 h storage, ATP, AMP, and energy charge values were 0.21 μmol·g-1, 2.01 μmol·g-1, and 0.17, respectively. In columbian ground squirrels the patterns of rapid ATP depletion and AMP accumulation were similar to those found in rat. In rat liver, enzymatic regulatory control of glycolysis appeared to be extremely sensitive to the decline in cellular energy levels. After 8 h cold hypoxia levels of fructose-6-phosphate decreased and fructose-1,6-bisphosphate increased, thus reflecting an activation of glycolysis at the regulatory step catalysed by phospho-fructokinase fructose-1,6-bisphosphatase. Despite an initial increase in flux through glycolysis over the first 2 min (lactate levels increased 3.7 μmol·g-1), further flux through the pathway was not permitted even though glycolysis was activated at the phosphofructokinase/fructose-1,6-bisphosphatase locus at 8 h, since supplies of phosphorylated substrate glucose-1-phosphate or glucose-6-phosphate remained low throughout the duration of the 24-h period. Conversely, livers of Columbian ground squirrels exhibited no activation or inactivation of two key glycolytic regulatory loci, phosphofructokinase/fructose-1,6-bisphosphatase and pyruvate kinase/phosphoenolpyruvate carboxykinase and pyruvate carboxylase. Although previous studies have shown similar allosteric sensitivities to adenylates to rat liver phospho-fructokinase, there was no evidence of an activation of the pathway as a result of decreasing high energy adenylate, ATP or increasing AMP levels. The lack of any apparent regulatory control of glycosis during cold hypoxia may be related to hibernator-specific metabolic adaptations that are key to the survival of hypothermia during natural bouts of hibernation.
Similar content being viewed by others
Abbreviations
- DHAP:
-
dihydroxyacetonephosphate
- EC:
-
energy charge
- F1,6P2 :
-
fructose-1,6-bisphosphate
- F2,6P2 :
-
fructose-2,6-bisphosphate
- F6P:
-
fructose-6-phosphate
- FBP:
-
fructose-1,6-bisphosphatase
- G1P:
-
glucose-1-phosphate
- G6P:
-
glucose-6-phosphate
- GAP:
-
glyceraldehyde-3-phosphate
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- L/R:
-
lactobionate/raffinose-based solution
- MR:
-
metabolic rate
- PDH:
-
pyruvate dehydrogenase
- PEP:
-
phosphoenolpyruvate
- PEPCK & PC:
-
phosphoenolpyruvate carboxykinase and pyruvate carboxylase
- PFK:
-
phosphofructokinase; PK, pyruvate kinase
- Q 10 :
-
the effect of a 10 °C drop in temperature on reaction rates (generally, Q 10=2–3)
- TA:
-
total adenylates
- UW solution:
-
University of Wisconsin solution (L/R-based)
References
Adam R, Astarcioglu I, Gigou M, Isaac J, Bismuth H (1992) The influence of the glycogen content of the donor liver on subsequent graft function and survival in rat liver transplantation. Transplantation 54:753–756
Atkinson DE (1968) The energy charge of the adenylate pool as a regulatory parameter. Biochemistry 7:4030
Attenburrow VD, Fuller BJ, Hobbs KEF (1981) Effects of temperature and method of hypothermic preservation on hepatic energy metabolism. Cryo-Lett 2:15–20
Belzer FO, Southard JH (1988) Principles of solid-organ preservation by cold storage. Transplantation 45:673–676
Brooks SPJ, Storey KB (1992) Mechanisms of glycolytic control during hibernation in the ground squirrel Spermophilus lateralis. J Comp Physiol B 162:23–28
Busza AL, Fuller BJ, Proctor E, Gadian DG (1988) The time course of changes in liver phosphorus metabolites during hypothermic preservation measured by 31Phosphorus nuclear magnetic resonance. Cryo-Lett 9:200–209
Cohen P (1980) Recently discovered systems of enzyme regulation by reversible phosphoryation. Elsevier/North Holland Biochemical Press, Amsterdam
Dunaway GA (1983) A review of animal phosphofructokinase isozymes with an emphasis on their physiological role. Mol Cell Biochem 52:75–91
Fuller BJ (1991) The effects of cooling on mammalian cells. In: Fuller BJ, Grout BWW (eds) Clinical applications of cryobiology CRC Press, London, pp 1–21
Fuller BJ, Marley SPE, Green CJ (1985) Gluconeogenesis in stored kidneys from normal and cold-acclimated rats. Cryo-Lett 6:91–98
Fuller BJ, Busza AL, Proctor E, Myles M, Gadian DG, Hobbs KEF (1988) Control of pH during hypothermic liver storage: role of the storage solution. Transplantation 45:239–241
Hannon JP, Vaughan DA (1961) Initial stages of intermediary glucose catabolism in the hibernator and nonhibernator. Am J Physiol 201:217–223
Herbert CV, Jackson DC (1985) Temperature effects on the responses to prolonged submergence in the turtle Chrysemys picta bellii II. Metabolic rate, blood acid-base and ionic changes, and cardiovascular function in aerated and anoxic water. Physiol Zool 58:670–681
Hochachka PW (1986) Defense strategies against hypoxia and hypothermia. Science 23:234–241
Hue L, Rider MR (1987) Role of fructose-2,6-bisphophate in the control of glycolysis in mammalian tissues. Biochem J 245:313–324
Jamieson NV (1991) Review article: improved preservation of the liver for transplantation. Aliment Pharmacol Therap 5:91–104
Keppler D, Decker K (1974) Glycogen: determination with amyloglucosidase. In: Bergmeyer HU (ed) Methods for enzymatic analysis. Academic Press, New York, USA, pp 1127–1131
Lehninger AL (1984) Principles of biochemistry, Worth, New York, USA
Lowry OH, Passonneau JV (1972) A flexible system of enzymatic analysis. Academic Press, New York, USA
Lutaya G, Sharma RJ, Griffiths JR (1983) Glycogenolysis in liver of phosphorylase kinase-deficient rats during liver perfusion and ischaemia. Biochem J 214:645–648
Marshall VC, Howden BO, Jablonski P, Scott DF, Thomas AC, Cham CW, Biguzas M, Walls K (1990) Analysis of UW solution in a rat liver transplant model. Transplant Proc 22:503–505
McArthur MD, Jourdan ML, Wang LCH (1992) Prolonged stable hypothermia: effect on blood gases and pH in rats and ground squirrels. Am J Physiol 262:R190-R197
Palombo JD, Pomposelli JJ, Hirschberg Y, Blackburn GL, Bistrian BR (1989) Glycolytic support of adenine nucleotides in rat liver flush-preserved with UW or Collins' II. Transplantation 48:901–905
Reinhart GD, Lardy HA (1980) Rat liver phosphofructokinase: kinetic activity under near-physiological condition. Biochemistry 19:1477–1484
Sakakibara R, Uyeda K (1983) Differences in the allosteric properties of pure low and high phosphate forms of phosphofructokinase from rat liver. J Biol Chem 258:8656–8662
Sankary HN, Foster P, Brown E, Hart M, Williams JW (1991) A comparison of Collins and UW solutions for cold ischemic preservation of the rat liver. J Surg Res 51:87–91
Storey KB (1987) Investigations of the mechanisms of glycolytic control during hibernation. Can J Zool 65:3079–83
Storey KB, Storey JM (1990) Metabolic rate depression and biochemical adaptation in anaerobiosis, hibernation and estivation. Q Rev Biol 65:145–174
Tashima LS, Adelstein SJ, Lyman CP (1970) Radioglucose utilization by active, hibernating, and arousing ground squirrels Am J Physiol 218:303–309
Wang LCH (1989) Ecological, physiological, and biochemical aspects of torpor in mammals and birds. In: Wang LCH (ed) Advances in comparative and environmental physiology, vol 4. Springer, Berlin
Wang LCH, Wolowyk MW (1988) Torpor in mammals and birds. Can J Zoll 66:133–137
Williamson JR (1970) General features of metabolic control as applied to the erythrocyte. Adv Biol Med 6:117–136
Willis JS (1987) Cold tolerance in mammalian cells chapter. In: Bowler K, Fuller BJ (eds) Temperature and animal cells. The Company of Biologists Ltd for the Society for Experimental Biology, Symposium XXXXI, pp 285–310
Willis JS (1982) Intermediary metabolism in hibernation. In: Lyman CP, Willis JS, Malan A, Wang LCH (eds) Hibernation and torpor in mammals and birds. Academic Press, London, UK
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Churchill, T.A., Cheetham, K.M., Simpkin, S. et al. Liver metabolism in cold hypoxia: a comparison of energy metabolism and glycolysis in cold-sensitive and cold-resistant mammals. J Comp Physiol B 164, 396–404 (1994). https://doi.org/10.1007/BF00302556
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00302556