Abstract
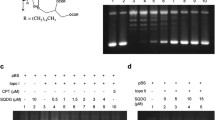
We investigated the antitumor effect of oral administration of etoposide and arabinofuranosylcytosine-5′-stearylphosphate (C18PCA) against P388 ascites tumors in B6D2F1 mice. Etoposide (25 mg/kg) and C18PCA (5 mg/kg) were given orally on days 1–5 after tumor inoculation. The median life span of the mice treated with etoposide or C18PCA alone was 19.5 and 18 days, respectively. The combination of both drugs significantly extended the median life span to 33 days. To clarify this enhancement of the increase in median life span, we examined intracellular deoxyribonucleoside triphosphate (dNTP) pools, cell-cycle distribution, DNA fragmentation, and the time course of the plasma drug concentration. Etoposide had no effect on intracellular dNTP pools in this experimental system, whereas treatment of cells with C18PCA or with the combination of both drugs resulted in a significant increase in dTTP pools to values ranging from 1.8-to 2.0-fold higher than the control levels. There was a significant increase in cells in the S+G2/M phase when cells had been treated with both etoposide and C18PCA. Agarose-gel electrophoresis of the extracted DNA revealed that C18PCA enhanced the fragmentation of DNA, with a length of about 180 bp being induced by etoposide. The plasma peak levels of etoposide (100 nM) and ara-C (50 nM) were observed at 20 and 30 min after the simultaneous administration of both drugs, respectively. The plasma etoposide level gradually decreased to 10% of the peak level at 240 min after administration. On the other hand, the plasma concentration of ara-C was maintained at above 20 nM at 240 min. These observations suggest that C18PCA and etoposide act on P388 murine leukemic cells by accumulating cells in the S+G2/M phase. Even if the plasma concentration of ara-C is low, the repair of DNA damage by etoposide may be hindered in the presence of ara-C following an increase in DNA fragmentation.
Similar content being viewed by others
References
Ellison RR, Holland JF, Weil M, Jacquillat C, Boiron M, Bernard J, Sawitsky A, Rosner F, Gussoff B, Silver RT, Karanas A, Cuttner J, Spurr CL, Hayes DM, Blom J, Leone LA, Haurani F, Kyle R, Hutchinson JL, Forcier RJ, Moon JH (1968) Arabinosyl cytosine: a useful agent in the treatment of acute leukemia in adults. Blood 32507–523
Furth JJ, Cohen SS (1968) Inhibition of mammalian DNA polymerase by the 5′-triphosphate of 1-β-D-arabinofuranosylcytosine and the 5′-triphosphate of 9-β-D-arabinofuranosyladenosine. Cancer Res 28:2061–2067
Garrett C, Santi DV (1979) A rapid and sensitive high pressure liquid chromatography assay for deoxyribonucleoside triphosphates in cell extracts. Anal Biochem 99:268–273
Hainsworth JD, Johnson DH, Frazier SR, Greco FA (1990) Chronic daily administration of oral etoposide in refractory lymphoma. Eur J Cancer 26:818–821
Higasnigawa M, Hori H, Ohkubo T, Kawasaki H, Yoshizumi T, Sakurai M (1990) Deoxyribonucleoside triphosphate pools and ara-CTP levels in P388 murine leukemic cells treated with 1-β-D-arabinofuranosylcytosine-5′-stearlyphosphate, which is a newly synthesized derivative of 1-β-D-arabinofuranosylcytosine. Med Oncol Tumor Pharmacother 7:223–226
Higashigawa M, Ido M, Nagao Y, Kuwabara H, Hori H, Ohkubo T, Kawasaki H, Sakurai M (1991) Decreased DNA polymerase sensitivity to 1-β-D-arabinofuranosylcytosine-5′-triphosphate in P388 murine leukemic cells resistant to vincristine. Leuk Res 15:675–681
Higashigawa M, Ido M, Ohkubo T, Kawasaki H, Kamiya H, Sakurai M, Taniguchi K, Hamazaki M, Hamazaki M (1989) Increased sensitivity to 1-β-D-arabinofuranosylcytosine in P388 murine leukemic cells resistant to etoposide. Leuk Res 13:39–42
Higashigawa M, Ochiai H, Ohkubo T, Kawasaki H, Nobori T, Kamiya H, Sakurai M (1988) Incorporation ofN 4-behenoyl-1-β-D-arabinofuranosylcytosine into DNA as 1-β-D-arabinofuranosycytosine. Med Oncol Tumor Pharmacother 5:265–271
Ho DHW, Frei E III (1971) Clinical pharmacology of 1-β-D-arabinofuranosylcytosine. Clin Pharamcol Ther 12:944–954
Idzu G, Yazawa Y, Tachibana M, Terada T, Hashimoto Y, Shimazu T (1989) Determination of etoposide in plasma by high performance liquid chromatography with fluorescence detector. Clin Rep 23:4809–4813
Jensen MK, Stentoft J (1986) Low-dose cytosine arabinoside in the treatment of relapsed and refractory acute non-lymphocytic leukemia. Acta Haematol (Basel) 76:127–129
Johnson DH, Greco A, Strupp J, Hande KR, Hainsworth JD (1990) Prolonged administration of oral etoposide in patients with relapsed or refractory small-cell lung cancer: a phase II trial. J Clin Oncol 8:1613–1617
Kaufmann SH (1989) Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemia cells by etoposide, camptothecin, and other cytotoxic anticancer drugs: a cautionary note. Cancer Res 49:5870–5878
Kawasaki H, Ochiai H, Ohkubo T, Nobori T, Kamiya H, Sakurai M (1986) Analysis of intracellular deoxyribonucleic acid pool using HPLC equipped with DEAE-2SW column. Igakunoayumi 137:1019–1020
Khym JX (1975) An analytical system for rapid separation of tissue nucleotides at low pressures of conventional anion exchangers. Clin Chem 21:1245–1252
Kiya K, Uozumi T, Ogasawara H, Sugiyama K, Hotta T, Mikami T, Kurisu K (1992) Penetration of etoposide into human malignant brain tumors after intravenous and oral administration. Cancer Chemother Pharmacol 29:339–342
Kodama K, Morozum M, Saitoh K, Kuninaka A, Yoshino H, Saneyoshi M (1989) Antitumor activity and pharmacology of 1-β-d-arabinofuranosylcytosine-5′-stearylphosphate: an orally active derivative of 1-β-d-arabinofuranosylcytosine. Jpn J Cancer Res 80:679–685
Koyama S, Itou S, Shibata A (1990) Low dose continuous infusion therapy with etoposide (VP-16) and cytosine arabinoside (ara-C) for a patient with refractory acute myelogenous leukemia. Jpn J Clin Hematol 31:1891–1892
Kufe DW, Major PP, Egan EM, Beardsley GP (1980) Correlation of cytotoxicity with incorporation of ara-C into DNA. J Biol Chem 255:8997–9000
Kusama K, Ekimoto H, Ishii T, Okamoto K, Takahashi K (1991) Antitumor activity by long-term administration of low-dose etoposide. Jpn J Chemother 18:959–963
Long BH, Musial ST, Brattain MG (1984) Comparison of cytotoxicity and DNA breakage activity of congeners of podophyllotoxin including VP16-213 and VM-26: a quantitative structure-activity relationship. Biochemistry 23:1183–1188
Mabel JA, Merker PC, Sturgeon ML, Woldinsky I, Geran RI (1978) Combination chemotherapy against B16 melanoma: bleomycin/vinblastine, bleomycin/cis-diamminedichloroplatinum, 5-fluorouracil/BCNU and 5-fluorouracil/methyl-CCNU. Cancer 42:1711–1719
Maeda T, Ueda M, Yamada T, Kubo H, Okamura S, Sano T (1986) Sensitivity to etoposide of cultured cells from cervical squamous cell carcinoma. Acta Obstet Gynaecol Jpn 38:2050–2056
O'Dwyer PJ, Leyland-Jones B, Alonso MT, Marsoni S, Wittes RE (1985) Etoposide (VP-16-213): current status of an anticancer drug. N Engl J Med 312:692–700
Ohkubo T, Higashigawa M, Kawasaki H, Kamiya H, Sakurai M, Kagawa Y, Kakito E, Sumida K, Ooi K (1988) Sequence-dependent antitumor effect of VP-16 and 1-β-D-arabinofuranosylcytosine in L1210 ascites tumor. Eur J Cancer Clin Oncol 24:1823–1828
Okamoto K, Nishikawa K, Seki T, Shibasaki C, Uchida T, Takahashi K (1985) The antitumor activity of intraperitoneally or orally administered etoposide in animals and its administration schedule dependency. Jpn J Cancer Chemother 12:2331–2337
Ooi K, Ohkubo T, Kuwabara H, Higashigawa M, Kawasaki H, Kakitoh H, Kagawa Y, Inagaki S, Sumida K, Sakurai M (1993) Enhanced incorporation of 1-β-D-arabinofuranosylcytosine by pretreatment with etoposide. Cancer Invest (in press)
Rivera G, Avery T, Roberts D (1975) Response of L1210 to combinations of cytosine arabinoside and VM-26 or VP16-213. Eur J Cancer 11:639–647
Saneyoshi M, Morozumi M, Kodama K, Machida H, Kuninaka A, Yoshino H (1980) Synthetic nucleosides and nucleotides. XVI. Synthesis and biological evaluations of a series of 1-β-D-arabinofuranosylcytosine 5′-alkyl or aryl phosphates. Chem Pharm Bull (Tokyo) 28:2915–2923
Sato T, Morozumi M, Kodama K, Kuninaka A, Yoshino H (1984) Sensitive radioimmunoassay for cytarabine and uracil arabinoside in plasma. Cancer Treat Res 68:1357–1366
Sauter C, Fehr J, Frick P, Gmuer J, Honegger H, Martz G (1982) Acute myelogenous leukemia: successful treatment of relapse with cytosine arabinoside, VP 16-213, vincristine and vinblastine (A-triple-V). Eur J Cancer Clin Oncol 18:733–737
Shackney SE, Ford SS, Occhipinti SJ, Ritch PS, Riccardi RR, Erickson BW Jr (1982) Schedule optimization of hydroxyurea and 1-β-D-arabinofuranosylcytosine in sarcoma 180 in vitro. Cancer Res 42:4339–4347
Shimizu T, Kubota M, Tanizawa A, Sano H, Kasai Y, Hashimoto H, Akiyama Y, Mikawa H (1990) Inhibition of both etoposide-induced DNA fragmentation and activation of poly (ADP-ribose) synthesis by zinc ion. Biochem Biophys Res Commun 169:1172–1177
Takeda S, Takada S, Kojima T, Kinoshita K, Sakamoto S (1990) Oral etoposide therapy in stage III–IV ovarian carcinoma. J Jpn Soc Cancer Ther 25:2562–2566
Wozniak AJ, Ross WE (1983) DNA damage as a basis for 4′-demethylepipodophyllotixin-9-(4,6-O-ethylidene-β-d-glucopyranoside) (etoposide) cytotoxicity). Cancer Res 43:120–124
Yuki K, Kodama Y, Emoto K, Yukawa O, Onda J, Uozumi T (1989) A case report of advanced malignant mixed germ cell tumor of parasellar origin indicating marked efficacy of a salvage combined chemotherapy of CDDP and etoposide and subsequent chemotherapy using oral etoposide. Jpn J Cancer Chemother 16:2651–2654
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Higashigawa, M., Cao, DC., Matsui, K. et al. Combined oral administration of etoposide and arabinofuranosylcytosine-5′-stearylphosphate enhances the antitumor effect against P388 ascites tumors. Cancer Chemother. Pharmacol. 33, 281–285 (1994). https://doi.org/10.1007/BF00685900
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00685900