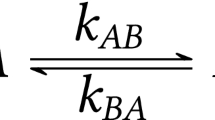Abstract
We have studied the recombination kinetics of carboxymyoglobin (after photodissociation of the CO ligand) by Mössbauer spectroscopy for temperatures in the range 4.2 – 60 K. The observed kinetics display non-exponential behaviour which was monitored over periods of a few days. It is shown that the time dependence of the kinetics can be reduced to a single universal function of the temperature-dependent variable (t/τ 1/2(T))β(T). The half-decay time τ 1/2(T) and the scaling parameter β(T) are analysed for the presence of tunneling effects. The non-Arrhenius temperature dependence of the half-decay time below 60 K is interpreted as activated tunneling in models with an Eckart barrier or a fluctuating barrier.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 19 November 1996 / Accepted: 17 March 1997
Rights and permissions
About this article
Cite this article
Ober, C., Burkardt, M., Winkler, H. et al. Low temperature study of myoglobin-ligand rebinding kinetics with Mössbauer spectroscopy. Eur Biophys J 26, 227–237 (1997). https://doi.org/10.1007/s002490050075
Issue Date:
DOI: https://doi.org/10.1007/s002490050075