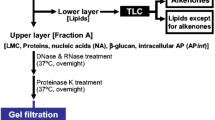
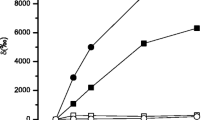
Abstract
Distribution of photoassimilated carbon into major metabolite classes differed between two Antarctic diatom species, Nitzschia curta and a small unicellular Chaetoceros sp.. Time course uptake studies (over 54 h) revealed that 14C allocation appeared to be equilibrated after approximately 8 h at light saturated photosynthesis. During short term dark periods (6 h), polysaccharides as well as low-molecular-weight compounds were catabolised to sustain protein synthesis in the dark, whilst lipid reserves were not mobilised for this process. Experiments with these two species were conducted at 0 and -1.5°C, although no difference in the distribution of radiolabel was measured between the two temperatures. It is hypothesised that under near-optimal conditions fast growing species are characterised by a high carbon turnover associated with a rapid flow of newly assimilated carbon into polymeric compound classes. On the other hand, slower growing species (such as N. curta) may store a significant amount of surplus carbon in the low-molecular-weight metabolite fraction. Species specific preferences were observed when comparing the accumulation of radiolabel into the lipid pools.
Similar content being viewed by others
References
Aletsee L, Jahnke J (1992) Growth and productivity of the psycrophilic marine diatoms Thalassiosira antarctica Comber and Nitzschia frigida Grunow in batch cultures at temperatures below the freezing point of sea water. Polar Biol 11:643–647
Almgren T, Dyrssen D, Fonselius S (1983) Determination of alkalinity and total carbonate. In: Grasshoff K, Ehrhardt M, Kremling K (eds) Methods of Seawater Analysis. 2nd rev ext ed, Verlag Chemie, Weinheim, pp 99–123
Barlow RG (1982) Phytoplankton ecology in the southern Benguela Current. II. Carbon assimilation patterns. J Exp Mar Biol Ecol 63:229–237
Barlow RG (1984) Time-series uptake of carbon into photosynthetic products of Benguela phytoplankton populations. J Plank Res 6:435–442
Barlow RG, Henry JL (1982) Patterns of carbon assimilation in phytoplankton from the Southern Ocean. Fish Bull S Afr 16:25–29
Bartsch A (1989) Die Eisalgenflora des Weddellmeeres (Antarktis): Artenzusammensetzung und Biomasse sowie Ökophysiologie ausgewählter Arten (German with English abstract). Ber Polarforsch 63, pp 110
Ben-Amotz A, Tornabene TG, Thomas WH (1985) Chemical profile of selected species of microalgae with emphasis on lipids. J Phycol 21:72–81
Cuhel RL, Ortner PB, Lean DRS (1984) Night synthesis of protein by algae. Limnol Oceanogr 29:731–744
Garrison DL (1991) Antarctic sea ice biota. Am Zool 31:17–33
Garrison DL, Sullivan CW, Ackley SF (1986) Sea ice microbial communities in Antarctica. BioScience 36:243–250
Geider RJ (1987) Light and temperature dependence of the carbon to chlorophyll a ratio in microalgae and cyanobacteria: Implications for physiology and growth of phytoplankton. New Phytol 106:1–34
Gleitz M, Kirst GO (1991) Photosynthesis-irradiance relationships and carbon metabolism of different ice algal assemblages collected from Weddell Sea pack ice during austral spring (EPOS 1). Polar Biol 11:385–392
Gleitz M, Thomas DN (1992) Physiological responses of a small Antarctic diatom (Chaetoceros sp.) to simulated environmental constraints associated with sea-ice formation. Mar Ecol Prog Ser 88:271–278
Gosselin M, Legendre L, Therriault JC, Demers S (1990) Light and nutrient limitation of sea-ice microalgae (Hudson Bay, Canadian Arctic). J Phycol 26:220–232
Hitchcock GL (1983) Photosynthate partitioning in cultured marine phytoplankton. I. Dinoflagellates. J Exp Mar Biol Ecol 69:21–36
Hitchcock GL, Goldman JC, Dennett MR (1986) Photosynthate partitioning in cultured marine phytoplankton: Metabolic patterns in a marine diatom under constant and variable light intensities. Mar Ecol Prog Ser 30:77–84
Kirst GO (1990) Salinity tolerance in eukaryotic marine algae. Annu Rev Plant Physiol Plant Mol Biol 41:21–53
Lancelot C, Mathot S (1985) Biochemical fractionation of primary production by phytoplankton in Belgian coastal waters during shortand long-term incubations with 14C-bicarbonate. I. Mixed diatom population. Mar Biol 86:219–226
Li WKW, Harrison WG (1982) Carbon flow into the end-products of photosynthesis in short and long incubations of a natural phytoplankton population. Mar Biol 72:175–182
Li WKW, Platt T (1982) Distribution of carbon among photosynthetic end-products in phytoplankton of the eastern Canadian Arctic. J Phycol 18:466–471
Li WKW, Glover HE, Morris I (1980) Physiology of carbon photoassimilation by Oscillatoria thiebautii in the Caribbean Sea. Limnol Oceanogr 25:447–456
Lizotte MP, Sullivan CW (1992) Biochemical composition and photosynthate distribution in sea ice microalgae of McMurdo Sound, Antarctica: evidence for nutrient stress during the spring bloom. Antarctic Sci 4:23–30
Lombardi AT, Wangersky PJ (1991) Influence of phosphorus and silicon on lipid class production by the marine diatom Chaetoceros gracilis grown in turbidostat cage cultures. Mar Ecol Prog Ser 77:39–47
McConville MJ (1985) Chemical composition and biochemistry of sea ice microalgae. In: Horner RA (ed) Sea Ice Biota. CRC Press Inc, pp 105–129
McConville MJ, Mitchell C, Wetherbee R (1985) Patterns of carbon assimilation in a microalgal community from annual sea ice, East Antarctica. Polar Biol 4:135–141
Morris I (1981) Photosynthetic products, physiological state and phytoplankton growth. In: Platt T (ed) Physiological bases of phytoplankton ecology, Can Bull Fish Aquat Sci 210:83–102
Morris I, Glover HE, Yentsch CS (1974) Products of photosynthesis by marine phytoplankton: The effect of environmental factors on the relative rates of protein synthesis. Mar Biol 27:1–9
Myklestad S, Djurhuus R, Mohus A (1982) Demonstration of exo(ß-1,3)-D-Glucanase activity in some planktonic diatoms. J Exp Mar Biol Ecol 56:205–211
Palmisano AC, Sullivan CW (1982) Physiology of sea ice diatoms. Response of three polar diatoms to a simulated summer-winter transition. J Phycol 18:489–498
Palmisano AC, Sullivan CW (1985a) Pathways of photosynthetic carbon assimilation in sea-ice microalgae from McMurdo Sound, Antarctica. Limnol Oceanogr 30:674–678
Palmisano AC, Sullivan CW (1985b) Growth metabolism and dark survival in sea ice microalgae. In: Horner RA (ed) Sea Ice Biota. CRC Press Inc, pp 131–146
Palmisano AC, Lizotte MP, Smith GA, Nichols PD, White DC, Sullivan CW (1988) Photosynthetic carbon assimilation in Antarctic sea-ice diatoms during spring bloom: Variation in synthesis of lipid classes. J Exp Mar Biol Ecol 116:1–13
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press New York, pp 173
ap Rees, T (1990) Carbon metabolism in mitochondria. In: Dennis DT, Turpin DH (eds) Plant Physiology, Biochemistry and Molecular Biology. Longman Scientific and Technical, pp 106–123
Rivkin RB (1985) Carbon-14 labelling patterns of individual marine phytoplankton from natural populations. Mar Biol 89:135–142
Rivkin RB (1989) Influence of irradiance and spectral quality on the carbon metabolism of phytoplankton. I. Photosynthesis, chemical composition and growth. Mar Ecol Prog Ser 55:291–304
Rivkin RB, Voytek MA (1987) Photoadaptations of photosynthesis and carbon metabolism by phytoplankton from McMurdo Sound, Antarctica. 1. Species-specific and community responses to reduced irradiances. Limnol Oceanogr 32:249–259
Shifrin NS, Chisholm SW (1981) Phytoplankton lipids: Interspecific differences and effects of nitrate, silicate and light-dark cycles. J Phycol 17:374–384
Smith AE, Morris I (1980a) Synthesis of lipid during photosynthesis by phytoplankton of the Southern Ocean. Science 207:197–199
Smith AE, Morris I (1980b). Pathways of carbon assimilation in phytoplankton from the Antarctic Ocean. Limnol Oceanogr 25:865–872
Smith REH, Clement P, Head E (1989) Biosynthesis and photosynthate allocation patterns of Arctic ice algae. Limnol Oceanogr 34:591–605
Smith REH, Clement P, Cota GF, Li WKW (1987) Intracellular photosynthate allocation and the control of Arctic marine ice algal production. J Phycol 23:124–132
Spies A (1987) Growth rates of Antarctic marine phytoplankton in the Weddell Sea. Mar Ecol Prog Ser 41:267–274
Stosch HA von, Drebes G (1964) Entwicklungsgeschichtliche Untersuchungen an zentrischen Diatomeen. IV. Die Planktondiatomee Stephanopyxis turris, ihre Behandlung und Entwicklungsgeschichte. Helgoländer wiss Meeresunters 11:209–257
Suen Y, Hubbard JS, Holzer G, Tornabene TG (1987) Total lipid production of the green alga Nannochloropsis SP. Q11 under different nitrogen regimes. J Phycol 23:289–296
Taguchi S, Hirata JA, Laws EA (1987) Silicate deficiency and lipid synthesis of marine diatoms. J Phycol 23:260–267
Thomas DN, Baumann MEM, Gleitz M (1992) Efficiency of carbon assimilation and photoacclimation in a small unicellular Chaetoceros species from the Weddell Sea (Antarctica): Influence of temperature and irradiance. J Exp Mar Biol Ecol 157:195–209
Tillmann U, Baumann MEM, Aletsee L (1989) Distribution of carbon among photosynthetic end products in the bloomforming arctic diatom Thalassiosira antarctica COMBER. Polar Biol 10:231–238
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thomas, D.N., Gleitz, M. Allocation of photoassimilated carbon into major algal metabolite fractions: Variation between two diatom species isolated from the Weddell Sea (Antarctica). Polar Biol 13, 281–286 (1993). https://doi.org/10.1007/BF00238764
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00238764