From Macroscopy to Ultrastructure: An Integrative Approach to Pulmonary Pathology
- 1Antwerp Surgical Training, Anatomy and Research Centre (ASTARC), Antwerp University, Antwerp, Belgium
- 2Division of Pneumology, University Hospital Antwerp, Edegem, Belgium
- 3Department of Thoracic and Vascular Surgery, University Hospital Antwerp, Edegem, Belgium
- 4Member of the German Center for Lung Research (DZL), Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), Hannover, Germany
- 5Institute for Pathology, Hannover Medical School, Hannover, Germany
- 6Division of Radiology, University Hospital Antwerp and University of Antwerp, Edegem, Belgium
- 7Division of Pneumology, Hannover Medical School, Hannover, Germany
- 8Division of Thoracic Surgery, Hannover Medical School, Hannover, Germany
- 9Department of Radiology, Hannover Medical School, Hannover, Germany
- 10Division of Pathology, University Hospital Antwerp, Edegem, Belgium
- 11Laboratory of Experimental Medicine and Pediatrics (LEMP), Antwerp University, Antwerp, Belgium
- 12Institute of Pathology and Department of Molecular Pathology, Helios University Clinic Wuppertal, University of Witten-Herdecke, Witten, Germany
- 13Institute of Functional and Clinical Anatomy, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany
Pathology and radiology are complimentary tools, and their joint application is often crucial in obtaining an accurate diagnosis in non-neoplastic pulmonary diseases. However, both come with significant limitations of their own: Computed Tomography (CT) can only visualize larger structures due to its inherent–relatively–poor resolution, while (histo) pathology is often limited due to small sample size and sampling error and only allows for a 2D investigation. An innovative approach of inflating whole lung specimens and subjecting these subsequently to CT and whole lung microCT allows for an accurate matching of CT-imaging and histopathology data of exactly the same areas. Systematic application of this approach allows for a more targeted assessment of localized disease extent and more specifically can be used to investigate early mechanisms of lung diseases on a morphological and molecular level. Therefore, this technique is suitable to selectively investigate changes in the large and small airways, as well as the pulmonary arteries, veins and capillaries in relation to the disease extent in the same lung specimen. In this perspective we provide an overview of the different strategies that are currently being used, as well as how this growing field could further evolve.
Introduction
Computed tomography (CT), positron emission tomography (PET) and histopathology are major components in the differential diagnosis and follow-up of patients with acute and chronic non-neoplastic respiratory diseases. Given the diversity in CT and histologic presentation and the inherent morphologic differences in strategies of diagnosis between neoplastic and non-neoplastic disease, we will specifically focus on non-neoplastic diseases. Especially as these tend to result in significant day-to-day challenges in the differential diagnosis.
Particularly in the field of interstitial lung diseases (ILD), there is an important diagnostic and prognostic role for CT and histopathology, with a growing emphasis on the relative weight of chest imaging as a diagnostic tool (1). Thus, the need for an invasive video-assisted or robot- assisted thoracic surgery biopsy to confirm differential diagnosis has drastically reduced. Consequently, lung biopsies are currently only recommended for those challenging cases where major discrepancies are observed between CT and clinical findings and a clear diagnosis cannot be rendered. Given this important role of imaging in the management of ILD, it is primordial to integrate the existing clinical, imaging and histopathologic data as much as possible.
Initially, assessment of remodeling and fibrosis patterns by experienced radiologists was the only way to leverage the available imaging data, but gradually and partly due to the large inter-observer variability, automated image analysis tools for characterization and quantification of CT signs and patterns have been developed of which some are on the verge of making their appearance in routine clinical care. CT can provide 3D insight into the (gross) morphological changes in the lung. However, CT patterns are often nonspecific and may change during the evolution of a disease. Importantly, CT changes may also have more than one (histo-) pathological correlate. For example, a study demonstrated that more than 90% of patients presenting with CT findings that were inconsistent with usual interstitial pneumonia demonstrated histological evidence of usual interstitial pneumonia (2). Additionally, in vivo resolution is rather limited with a slice thickness of 1 mm most commonly being used for routine patient CT scans due to radiation concerns. This is sufficient to investigate airways and vessels >1 mm in diameter and gross changes in the secondary pulmonary lobule and the lung parenchyma. However, the small airways, the cellular composition and specific smaller sized structures are impossible to address, although morphologic secondary effects of primary changes such as hyperinflation and air trapping may be observed.
Histopathology on the other hand provides the advantage of a detailed analysis of the morphologic and cellular changes within the lung, whereby structures in the micrometer range can be readily resolved. However, (histo-) pathology is limited due to relatively small tissue samples and only allows for a 2D investigation. This can lead to discordance between initial diagnosis made on transbronchial cryobiopsy or surgical lung biopsy and the final diagnosis made in explant lung specimens at the time of transplantation (15–22 and 12.4% respectively) (3, 4). In addition, the lung is considered quite fragile, making it vulnerable for deformation and distortion during the preparation resulting in histologic artifacts potentially hampering microscopic examination.
As, especially non-neoplastic pulmonary diseases, show a great spatial heterogeneity in disease activity and progression, targeted sampling is essential. It is reasonable to assume that often important patterns/changes in the lung can be overlooked due to sampling bias or sectioning artifacts upon histologic examination.
Proof of concept data of the huge potential of microCT, a tool designed to combine the 3D aspect of CT with almost full histological resolution, to investigate human lung (disease) was provided in 2005. However, at the time, the authors were still struggling to obtain sufficient contrast between air and tissue (5). A subsequent porcine study by Litzlbauer et al. (6) extensively validated the use of microCT with histology, showing great promise in measuring morphological changes such as alveolar surface density and mean linear intercept. Therefore, we and others have further optimized these protocols for ex-vivo scanning of human lung tissue to bridge the gap between radiology and histopathology, and thus between macroscopy and near-cellular resolution. In this article, we describe a unique approach of air-inflating whole human lung/lobe explants and subjecting these to a wide spectrum of investigations. We propose to use this macro to micro approach leveraging CT and microCT to bridge the gap between radiologic data and histopathology with illustrative examples. Further, we propose to use this approach to selectively investigate changes in the large and small airways, the interstitial compartment as well as the pulmonary arteries, veins and capillaries in relation to the disease extent in the same lung specimen. In addition, other promising tools are discussed in more detail including their potential applications. We acknowledge that a plethora of techniques has also been investigated in animal studies, which can readily be leveraged to combine imaging data with mechanistical insights (7); however, we deliberately focus on studies in humans that can be implemented more easily into routine clinical practice.
Methodology of Lung Processing
Obtaining sufficient contrast between tissue and air is of major importance in the imaging of lung explants. Therefore, immediately following surgical resection, the lung is collected and cannulated via the main stem bronchus. Applying water-controlled air pressure, inflating the lung with 30 cm of water pressure and subsequently lowering the pressure to 10 cm of water pressure allows for an even and uniform recruitment of the lung specimen. The lungs are then mounted in a styrofoam box and frozen in liquid nitrogen vapors. At this moment, the lungs can be safely stored at−80°C until further use. Ex vivo CT scanning can be used to correlate with the last available in vivo CT, which already allows a higher spatial resolution due to higher radiation doses that can be used. MicroCT scanning of entire lung specimens is more complicated and a dedicated device is needed, where large samples can be mounted while keeping the lung frozen solid inside a styrofoam box. Depending on the size of the specimen, a resolution of 90–150 micron is feasible, thereby increasing the resolution 6–8 fold compared to conventional in vivo CT imaging.
After CT and microCT imaging, which can be used to identify structures of interest, the lung is cut in even slices using a band saw and smaller sized samples with a diameter typically between 12 and 22 mm can be extracted with a core bore or power drill depending on the degree of fibrosis within the samples. These samples can subsequently be re-scanned with high-resolution microCT preferably while keeping the sample frozen (8), where a further increase in resolution can be achieved given the smaller size of the respective specimen, typically up to 5–15 microns.
Subsequently, the lung can be further processed for histopathologic and molecular assessment, e.g., by formalin fixation and dehydration of samples. Our workflow is further illustrated in Figure 1. The quality of standard histology is not severely impaired by the prior process of freezing and scanning the lung. Although minor freezing artifacts can be observed, the quality of the histology is much better compared to frozen sections, explained by the fact that the tissue is never in direct contact with ice-cold solutions such as glutaraldehyde. It is very important to emphasize that this process of freezing the lung in toto and using microCT also has limited negative impact on further downstream molecular analysis illustrated by e.g., similar gene expression signatures in scanned vs. non-scanned specimens (8). Also bulk RNA-sequencing (9, 10), immunohistochemistry (11), and microbiome sequencing (Einarsson et al. accepted for publication) have already been performed, making ours a valid tool for more molecular-based research.
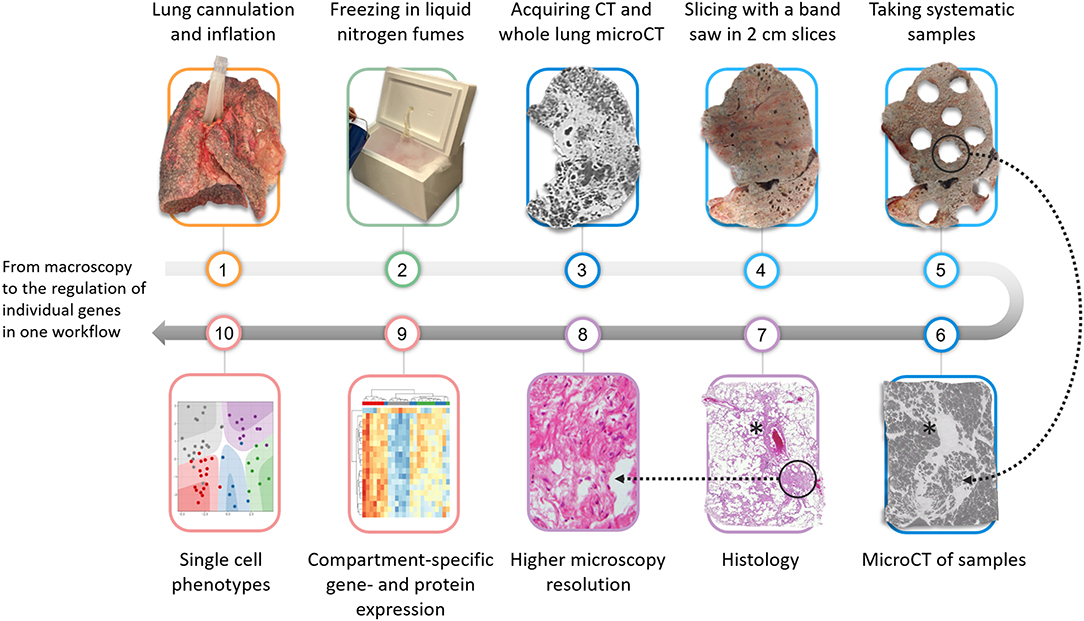
Figure 1. Overview of the proposed protocol that can be used to investigate the lung from macroscopy to microscopical ultrastructure. The lung is firstly cannulated via the main stem bronchus and inflated using a compressed air source and subsequently fixed in liquid nitrogen fumes. Following an ex-vivo CT and microCT, the lung is sliced in 2 cm slices and samples are systematically extracted using a core bore or power drill. Pre-selected or randomly selected samples can be scanned with microCT to further improve the spatial resolution. Further validation can be performed with histopathology. More downstream possible molecular analysis includes compartment-specific gene expression analysis or single nuclear analysis which can be used to elucidate specific pathways in pathological areas. *blood vessel.
CT and microCT of Explant Lungs
Leveraging CT and whole lung microCT, an easier separation of the lung into morphologically inconspicuous, moderate and severely diseased areas can be made, that can be specifically sampled using the targeted sampling protocol described above. The healthy appearing areas as evident from the imaging can be used as a proxy of early disease. This is valuable given the temporal heterogeneity in non-neoplastic lung diseases, since genuine specimens with early disease features for research purposes are difficult to obtain. There is certainly some ground truth in such a hypothesis as distinct morphological and molecular processes have been demonstrated in minimally affected vs. severely affected regions (9, 10).
Next to pattern and structure analysis, it is also possible to visually identify and segment the entire bronchial tree from the main stem bronchus until the last branch of conducting airways (i.e., terminal bronchioles). This approach can therefore be used to investigate airway abnormalities like airway collapse, airway obstruction or bronchiectasis on a whole lung scale. In addition quantitative data such as the numbers of airways per generation, airway diameters and segment lengths can be generated as has been described already in COPD (12), graft-vs.-host-disease post-allogenic stem cell transplantation (13), lymphangioleiomyomatosis (11) and physiologic aging (14). The use of a contrast agent, for example osmium staining, also allows for the investigation of (micro-)vascular structures, making the estimation of vascular volumes relative to the total tissue volume possible (15). Specifically in rare pulmonary diseases, whole lung microCT can bridge the gap between research and clinical routine as exemplified in Figure 2, where a representative ex-vivo CT scan (Figure 2A), whole lung microCT scan (Figure 2B) and the segmented airway tree based on whole lung microCT (Figure 2C) are shown from a patient who underwent pneumonectomy for unilateral congenital emphysema (Figures 2D,E). The airway segmentation shows an aberrant airway bifurcation pattern with a marked decrease in the number of visible airways. Remarkably, there are large airway segments without notable airway branching; especially airway segments in severely emphysematous areas show a lack of airway bifurcations (Figure 2C).
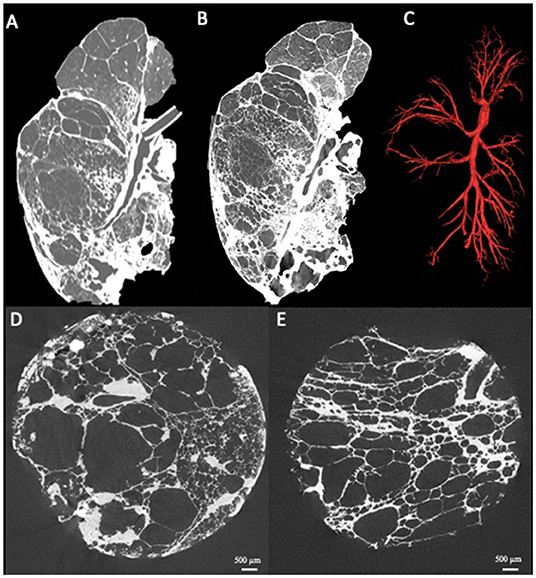
Figure 2. Presentation of a case where clinical routine meets research. Patient with unilateral congenital emphysema that underwent pneumonectomy. The ex-vivo clinical CT scan shows severe emphysematous destruction (A) with some fibrosis in the lower lung lobe. The whole lung microCT shows greatly improved details providing near alveolar resolution (B). Airway segmentation of the whole lung by microCT demonstrating the simplification of the airway tree characterized by a low number of visible airways and remarkable long airway segments without airway branching (C). MicroCT imaging of extracted lung specimens showing the severe emphysematous destruction in this lung (D,E).
Correlation with Histopathology
Numerous insights can be generated by high-resolution imaging, however the correlation between imaging and histopathology is often difficult, given the inherent differences in spatial resolution. While high-resolution imaging data allows for the investigation of morphological changes, it only provides black and white images and changes at the cellular level or minimal density differences cannot be resolved, making routine pathology still an indispensable tool for both research and routine clinical purposes. Some attempts have, however, been made to correlate imaging with histopathology data, especially with the recent introduction of synchrotron imaging, an extremely powerful source of X-rays, of paraffin embedded samples where sub-stacks of images with (sub)micrometer resolution can be created. Hierarchical phase contrast imaging is another application of synchrotron imaging, where specialized sample preparation and equipment enable the scanning of entire lungs, but also other organs, with a resolution of 25 micron. Furthermore, this technology allows the acquisition of sub-scans with a resolution of 2.5 micron due to the high X-ray photon flux and coherence achieved at modern fourth-generation synchrotron sources (16). This technique has been employed by Ackermann and colleagues to reveal microvascular changes (i.e., broncho-pulmonary shunting) in the bronchial circulation of COVID-19 patients (17). Synchrotron imaging of paraffin embedded samples on the other hand has been used by another group to study morphological changes in the vascularity in pulmonary vascular diseases. Westroo and colleagues leveraged synchrotron imaging to demonstrate a previously unappreciated plexiform lesion heterogeneity, the pathological hallmark of (idiopathic) pulmonary hypertension (18). The same group also used synchrotron-based phase-contrast microCT in combination with vascular dye injection to investigate the vascular morphology in alveolar capillary dysplasia with misaligned pulmonary veins, with vascular dye injection being of significant help in the analysis and further segmentation of the vascular structures (19). A 3D approach for virtual histology and histopathology based on multi-scale phase contrast x-ray tomography has also been applied to punches of tissue samples with a maximum cross section of 8 mm derived from COVID-19 victims, which allows the segmentation of individual cells with a minimal resolution of 167 nm (20). Lastly, a 36 M-pixel synchrotron radiation microCT has been successfully used to study the secondary pulmonary lobule from a large human lung specimen (21).
However, this approach is not readily available due to the specific nature of the required equipment for both acquisition and analysis, the need for extensive experience with the interpretation of the images and is therefore not (yet) ready for high-throughput screening.
microCT Imaging as a Scouting Tool
Histopathologic analysis remains the only reliable option for identifying cellular and morphologic patterns keeping the limitation of small tissue samples and the lack of 3D insight in mind. Serial sectioning of paraffin blocks has been used to extensively demonstrate the correlation between microCT and histology (14), however the process of aligning, matching and cutting the samples is tedious and time-consuming. As an alternative, microCT scanning of paraffin blocks or microCT as a scouting tool have been proposed (22). This can especially be interesting for retrospective studies, in situations where no whole organ is available or when detailed and fast histopathologic assessment is essential for routine patient care such as surgical lung biopsies in the context of a possible interstitial lung disease or suspicion of neoplasms in a resected lung specimen. Interesting and relevant regions can be selected for further sectioning based on the microCT selection which enables time-efficient preparation of conventional histological sections (22). In that aspect, it is also of interest that a novel X-ray microtomosynthesis of unstained pathology tissue samples has also been proposed. Its unique design maximizes the photon flux density through the sample as no rotation is required and therefore samples can be scanned closer to the source resulting in higher resolution (23).
In addition to its potential as a scouting tool, X-ray imaging can also assist in generating a 3D overview of morphological changes in the lung structure. Jones et al. leveraged microCT of paraffin embedded samples to investigate the 3D morphology of fibroblast foci, a key histologic feature of active fibroproliferation in the context of interstitial lung disease. In contrast to the general belief at that time, the fibroblast foci in the lungs of IPF patients were not interconnected and displayed a wide plasticity (24). Wells et al. also used microCT, histopathology, and immunohistochemistry to investigate necrotic granuloma, a characteristic feature of tuberculosis and showed that necrotic granulomas exhibit more complex shapes than anticipated, including cylindrical, branched morphologies that are connected to the airways and shaped by the bronchi (25).
The 3D overview provided by microCT is not only helpful to investigate morphologic changes but can also be used to investigate structural changes in the airways. Mcdonough et al. were the first to employ microCT to quantify the size and number of terminal bronchioles, the last generation of conducting airways before the respiratory bronchioles and alveoli, in tissue samples removed from explanted lungs from COPD patients. Terminal bronchioles can be visually identified on microCT imaging by the loss of the airway wall and appearance of alveolar buds and are of considerable importance in chronic non-neoplastic lung diseases. A significant decrease in the number and size of terminal bronchioles in end-stage COPD lungs was found compared to controls. Given that repeated sampling of the same lung was possible and given that the tissue volume of the sample could be determined, it not only allowed the assessment of the number of terminal bronchioles in the sample, but by carefully measuring the lung volume, these numbers could be extrapolated to the entire lung. This approach provided quantitative data of the actual small airway involvement in COPD for the first time, something that was always assumed but never conclusively demonstrated in a quantitative way (26). Later on, this approach has also been successfully leveraged to demonstrate small airway involvement in post lung transplantation rejection, cystic fibrosis and idiopathic pulmonary fibrosis (27–30). Over the last years, this approach has been further refined to allow scanning of the samples in frozen condition, making destructive dehydration which was applied at first, unnecessary. In addition, an improvement in the analysis tools has allowed the researchers to extract additional quantitative parameters such as the number of alveolar attachments, the thickness of the airway wall, the circularity of the lumen, but also size and diameter of the preterminal bronchioles (31).
Discussion
Given the above evidence, it is clear that microCT can serve as a complementary, and in the future perhaps even an indispensable tool for routine pathology (32). Although significant technical advances have been made, there is still some work to be done. Indeed, specific microCT devices have already been optimized for 3D imaging of non-stained soft tissue at a resolution of 5–10 micron. These scans could be implemented in routine care and could assist in histology-guided identification of a range of tissue structures and diagnostically relevant histologic criteria and moreover may allow easy quantification of relevant measures such as for example tissue thickness (33). It is our belief that similar advances could also assist in improving the differential diagnosis, particularly in the field of non-neoplastic diseases. However, these approaches could also be leveraged to better understand neoplastic lung diseases such as micro-metastasis, the role of the tumor micro-environment and the role of the vasculature in neoplastic lung disease. Our approach also allows for structurally targeted molecular investigation with both fresh frozen–or formalin fixed tissue being available after non-destructive imaging, allowing panel-based gene expression analysis or broad next-generation sequencing approaches (34). Additionally, microorganisms such as bacteria from biofilms can be recultivated from native flash frozen tissue and used in in-vitro assays.
Applying microCT or synchrotron imaging directly to living patients remains an elusive dream, yet a recent study applied propagation-based phase-contrast CT on a human-scale chest phantom prepped with an inflated fresh porcine lung. The authors demonstrated that a resolution of 100 micron could be obtained in a limited local area of interest with significant less radiation than used in conventional CT scanning (35), indicating the possibilities of in vivo high resolution scanning. This indicates that we could dream big and hope to apply similar high-resolution imaging in vivo, making it perhaps even possible to avoid invasive biopsies. Caution is however needed as rigorous interpretation of the, often immense, imaging data is needed, which could cause a new wave for artificial intelligence based analysis of microCT scans, similar to the current wave of research in the use of artificial intelligence analysis of conventional CT images (36).
Therefore, the imaging-based approaches to close the gap from macroscopy to ultrastructure seems a safe and trustworthy option, which can be relatively fast implemented in the routine care of patients suffering from acute or chronic lung disease and which can facilitate the differential diagnosis.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by University Hospital of Antwerp Ethical committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SV, PB, CW, MA, JH, TL, JK-V-E, and DJ: responsible for conception and design of the study. EP, MK, AS, HE, TW, AH, FL, SD, PP, VV, and PV: wrote sections of the manuscript. All authors contribute to sample preparation, processing, contributed to manuscript revision, read, and approved the submitted version.
Funding
The grants of the European Research Council (ERC); European Consolidator Grant, XHale to DJ (Ref. No. 771883).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of idiopathic pulmonary fibrosis. an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. (2018) 198:e44–68. doi: 10.1164/rccm.201807-1255ST
2. Yagihashi K, Huckleberry J, Colby TV, Tazelaar HD, Zach J, Sundaram B, et al. Radiologic–pathologic discordance in biopsy-proven usual interstitial pneumonia. Eur Respir J. (2016) 47:1189–97. doi: 10.1183/13993003.01680-2015
3. Unterman A, Wand O, Fridel L, Edelstein E, Pertzov B, Kramer MR. High diagnostic accuracy of transbronchial cryobiopsy in fibrotic interstitial lung diseases compared to final explant diagnosis. Respiration. (2019) 98:421–7. doi: 10.1159/000502893
4. Panchabhai TS, Arrossi AV, Highland KB, Bandyopadhyay D, Culver DA, Budev MM, et al. A single-institution study of concordance of pathological diagnoses for interstitial lung diseases between pre-transplantation surgical lung biopsies and lung explants. BMC Pulm Med. (2019) 19:20. doi: 10.1186/s12890-019-0778-x
5. Watz H, Breithecker A, Rau WS, Kriete A. Micro-CT of the human lung: imaging of alveoli and virtual endoscopy of an alveolar duct in a normal lung and in a lung with centrilobular emphysema—initial observations. Radiology. (2005) 236:1053–8. doi: 10.1148/radiol.2363041142
6. Litzlbauer HD, Neuhaeuser C, Moell A, Greschus S, Breithecker A, Franke FE, et al. Three-dimensional imaging and morphometric analysis of alveolar tissue from microfocal X-ray-computed tomography. Am J Physiol Lung Cell Mol Physiol. (2006) 291:L535–45. doi: 10.1152/ajplung.00088.2005
7. Tielemans B, Dekoster K, Verleden SE, Sawall S, Leszczyński B, Laperre K, et al. From mouse to man and back: closing the correlation gap between imaging and histopathology for lung diseases. Diagnostics. (2020) 10:636. doi: 10.3390/diagnostics10090636
8. Vasilescu DM, Phillion AB, Tanabe N, Kinose D, Paige DF, Kantrowitz JJ, et al. Nondestructive cryomicro-CT imaging enables structural and molecular analysis of human lung tissue. J Appl Physiol (1985). (2017) 122:161–9. doi: 10.1152/japplphysiol.00838.2016
9. McDonough JE, Ahangari F, Li Q, Jain S, Verleden SE, Herazo-Maya J, et al. Transcriptional regulatory model of fibrosis progression in the human lung. JCI Insight. (2019) 4:e131597. doi: 10.1172/jci.insight.131597
10. De Sadeleer LJ, McDonough JE, Schupp JC, Yan X, Vanstapel A, Van Herck A, et al. Lung microenvironments and disease progression in fibrotic hypersensitivity pneumonitis. Am J Respir Crit Care Med. (2021) 205:60–74. doi: 10.1164/rccm.202103-0569OC
11. Verleden SE, Vanstapel A, De Sadeleer L, Weynand B, Boone M, Verbeken E, et al. Quantitative analysis of airway obstruction in lymphangioleiomyomatosis. Eur Respir J. (2020) 56:1901965. doi: 10.1183/13993003.01965-2019
12. Everaerts S, McDonough JE, Verleden SE, Josipovic I, Boone M, Dubbeldam A, et al. Airway morphometry in COPD with bronchiectasis: a view on all airway generations. Eur Respir J. (2019) 54:1802166. doi: 10.1183/13993003.02166-2018
13. Verleden SE, McDonough JE, Schoemans H, Knoop C, Verschakelen J, Dubbeldam A, et al. Phenotypical diversity of airway morphology in chronic lung graft vs. host disease after stem cell transplantation. Mod Pathol. (2019) 32:817–29. doi: 10.1038/s41379-019-0203-2
14. Verleden SE, Kirby M, Everaerts S, Vanstapel A, McDonough JE, Verbeken EK, et al. Small airway loss in the physiologically ageing lung: a cross-sectional study in unused donor lungs. Lancet Respir Med. (2020) 9:167–74. doi: 10.1016/s2213-2600(20)30324-6
15. Kampschulte M, Schneider C, Litzlbauer H, Tscholl D, Schneider C, Zeiner C, et al. Quantitative 3D micro-CT imaging of human lung tissue. Fortschr Röntgenstr. (2013) 185:869–76. doi: 10.1055/s-0033-1350105
16. Walsh CL, Tafforeau P, Wagner WL, Jafree DJ, Bellier A, Werlein C, et al. Imaging intact human organs with local resolution of cellular structures using hierarchical phase-contrast tomography. Nat Method. (2021) 18:1532–41. Available online at: https://www.nature.com/articles/s41592-021-01317-x
17. Ackermann M, Tafforeau P, Wagner WL, Walsh C, Werlein C, Kühnel MP, et al. The bronchial circulation in COVID-19 pneumonia. Am J Respir Crit Care Med. (2022) 205:121–5. doi: 10.1164/rccm.202103-0594im
18. Westöö C, Norvik C, Peruzzi N, van der Have O, Lovric G, Jeremiasen I, et al. Distinct types of plexiform lesions identified by synchrotron-based phase-contrast micro-CT. Am J Physiol Lung Cell Mol Physiol. (2021) 321:L17–28. doi: 10.1152/ajplung.00432.2020
19. Norvik C, Westöö CK, Peruzzi N, Lovric G, van der Have O, Mokso R, et al. Synchrotron-based phase-contrast micro-CT as a tool for understanding pulmonary vascular pathobiology and the 3-D microanatomy of alveolar capillary dysplasia. Am J Physiol Lung Cell Mol Physiol. (2020) 318:L65–75. doi: 10.1152/ajplung.00103.2019
20. Eckermann M, Frohn J, Reichardt M, Osterhoff M, Sprung M, Westermeier F, et al. 3D virtual pathohistology of lung tissue from Covid-19 patients based on phase contrast X-ray tomography. Elife. (2020) 9:e60408. doi: 10.7554/eLife.60408
21. Umetani K, Okamoto T, Saito K, Kawata Y, Niki N. 36M-pixel synchrotron radiation micro-CT for whole secondary pulmonary lobule visualization from a large human lung specimen. Eur J Radiol Open. (2020) 7:100262. doi: 10.1016/j.ejro.2020.100262
22. Scott AE, Vasilescu DM, Seal KAD, Keyes SD, Mavrogordato MN, Hogg JC, et al. Three dimensional imaging of paraffin embedded human lung tissue samples by micro-computed tomography. Warburton D, editor. PLoS ONE. (2015) 10:e0126230. doi: 10.1371/journal.pone.0126230
23. Nguyen DT, Larsen TC, Wang M, Knutsen RH, Yang Z, Bennett EE, et al. X-ray microtomosynthesis of unstained pathology tissue samples. J Microsc. (2021) 283:9–20. doi: 10.1111/jmi.13003
24. Jones MG, Fabre A, Schneider P, Cinetto F, Sgalla G, Mavrogordato M, et al. Three-dimensional characterization of fibroblast foci in idiopathic pulmonary fibrosis. JCI Insight. (2016) 1:e86375. doi: 10.1172/jci.insight.86375
25. Wells G, Glasgow JN, Nargan K, Lumamba K, Madansein R, Maharaj K, et al. Micro–computed tomography analysis of the human tuberculous lung reveals remarkable heterogeneity in three-dimensional granuloma morphology. Am J Respir Crit Care Med. (2021) 204:583–95. doi: 10.1164/rccm.202101-0032OC
26. McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. (2011) 365:1567–75. doi: 10.1056/NEJMoa1106955
27. Verleden SE, Vasilescu DM, Willems S, Ruttens D, Vos R, Vandermeulen E, et al. The site and nature of airway obstruction after lung transplantation. Am J Respir Crit Care Med. (2014) 189:292–300. doi: 10.1164/rccm.201310-1894OC
28. Verleden SE, Vasilescu DM, McDonough JE, Ruttens D, Vos R, Vandermeulen E, et al. Linking clinical phenotypes of chronic lung allograft dysfunction to changes in lung structure. Eur Respir J. (2015) 46:1430–9. doi: 10.1183/09031936.00010615
29. Boon M, Verleden SE, Bosch B, Lammertyn EJ, McDonough JE, Mai C, et al. Morphometric analysis of explant lungs in cystic fibrosis. Am J Respir Crit Care Med. (2016) 193:516–26. doi: 10.1164/rccm.201507-1281OC
30. Verleden SE, Tanabe N, McDonough JE, Vasilescu DM, Xu F, Wuyts WA, et al. Small airways pathology in idiopathic pulmonary fibrosis: a retrospective cohort study. Lancet Respir Med. (2020) 8:573–84. doi: 10.1016/S2213-2600(19)30356-X
31. Tanabe N, Vasilescu DM, McDonough JE, Kinose D, Suzuki M, Cooper JD, et al. Micro–computed tomography comparison of preterminal bronchioles in centrilobular and panlobular emphysema. Am J Respir Crit Care Med. (2017) 195:630–8. doi: 10.1164/rccm.201602-0278OC
32. Bourdin A, Gamez AS, Vachier I, Crestani B. LAM is another small airway disease: lessons from microCT. Eur Respir J. (2020) 56:2002162. doi: 10.1183/13993003.02162-2020
33. Katsamenis OL, Olding M, Warner JA, Chatelet DS, Jones MG, Sgalla G, et al. X-ray micro-computed tomography for nondestructive three-dimensional (3D) X-ray histology. Am J Pathol. (2019) 189:1608–20. doi: 10.1016/j.ajpath.2019.05.004
34. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. (2020) 383:120–8. doi: 10.1056/NEJMoa2015432
35. Wagner WL, Wuennemann F, Pacilé S, Albers J, Arfelli F, Dreossi D, et al. Towards synchrotron phase-contrast lung imaging in patients – a proof-of-concept study on porcine lungs in a human-scale chest phantom. J Synchrotron Rad. (2018) 25:1827–32. doi: 10.1107/S1600577518013401
Keywords: microCT, lung, histology, lung disease, imaging
Citation: Verleden SE, Braubach P, Werlein C, Plucinski E, Kuhnel MP, Snoeckx A, El Addouli H, Welte T, Haverich A, Laenger FP, Dettmer S, Pauwels P, Verplancke V, Van Schil PE, Lapperre T, Kwakkel-Van-Erp JM, Ackermann M, Hendriks JMH and Jonigk D (2022) From Macroscopy to Ultrastructure: An Integrative Approach to Pulmonary Pathology. Front. Med. 9:859337. doi: 10.3389/fmed.2022.859337
Received: 21 January 2022; Accepted: 07 February 2022;
Published: 16 March 2022.
Edited by:
Luca Di Tommaso, Humanitas Research Hospital, ItalyReviewed by:
Torsten Goldmann, Research Center Borstel (LG), GermanyCopyright © 2022 Verleden, Braubach, Werlein, Plucinski, Kuhnel, Snoeckx, El Addouli, Welte, Haverich, Laenger, Dettmer, Pauwels, Verplancke, Van Schil, Lapperre, Kwakkel-Van-Erp, Ackermann, Hendriks and Jonigk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danny Jonigk, jonigk.danny@mh-hannover.de
†These authors have contributed equally to this work
 Stijn E. Verleden
Stijn E. Verleden Peter Braubach
Peter Braubach Christopher Werlein5
Christopher Werlein5  Mark P. Kuhnel
Mark P. Kuhnel Florian P. Laenger
Florian P. Laenger Patrick Pauwels
Patrick Pauwels Veronique Verplancke
Veronique Verplancke Maximilian Ackermann
Maximilian Ackermann Jeroen M. H. Hendriks
Jeroen M. H. Hendriks