Paralytic Toxin Producing Dinoflagellates in Latin America: Ecology and Physiology
- 1Instituto Politécnico Nacional-Centro Interdisciplinario de Ciencias Marinas, La Paz, Mexico
- 2CONACYT, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, Ciudad de México, Mexico
- 3Centro de Investigaciones Biológicas del Noroeste, La Paz, Mexico
- 4CONACYT-Instituto Politécnico Nacional, Centro Interdisciplinario de Ciencias Marinas, La Paz, Mexico
In this review we summarize the current state of knowledge regarding taxonomy, bloom dynamics, toxicity, autoecology, and trophic interactions, of saxitoxin producing dinoflagellates in this region. The dinoflagellates Gymnodinium catenatum, Pyrodinium bahamense and several species of Alexandrium are saxitoxin producers, and have been responsible of paralytic shellfish poisoning in different regions of Latin America, causing intoxications and important fisheries losses. The species distribution differ; most harmful algal blooms of G. catenatum are from the northern region, however this species has also been reported in central and southern regions. Blooms of P. bahamense are mostly reported in North and Central America, while blooms of Alexandrium species are more common in South America, however this genus is widely spread in Latin America. Species and regional differences are contrasted, with the aim to contribute to future guidelines for an international scientific approach for research and monitoring activities that are needed to increase our understanding of paralytic toxin producing dinoflagellates in this region.
Introduction
Neurotoxic paralytic shellfish toxins (PSTs) are produced in the marine environment mainly by dinoflagellates of three genera associated with harmful algal blooms (HABs). These include about a dozen species of Alexandrium, a single species of Gymnodinium (G. catenatum) and a single species of Pyrodinium (P. bahamense).
PSTs molecules comprise saxitoxin (STX) and over 57 analogs have been described (Wiese et al., 2010) that vary in toxicity, being the carbamoyl [STX, neosaxitoxin (NEO), gonyautoxins (GTX)] the most potent, followed by decarbamoyl (dcSTX, dcNEO, dcGTX) and the deoxydecarbamoyl analogs (doSTX, doGTX2, doGTX3). The least toxic analogs are the N-sulfocarbamoyl (B and C toxins). Only G. catenatum produces benzoyl analogs (GC), and out of 18 theoretical toxins (Negri et al., 2003a, 2007; Vale, 2008, 2010), 15 benzoyl analogs have been confirmed (Durán-Riveroll et al., 2017) (Figure 1).
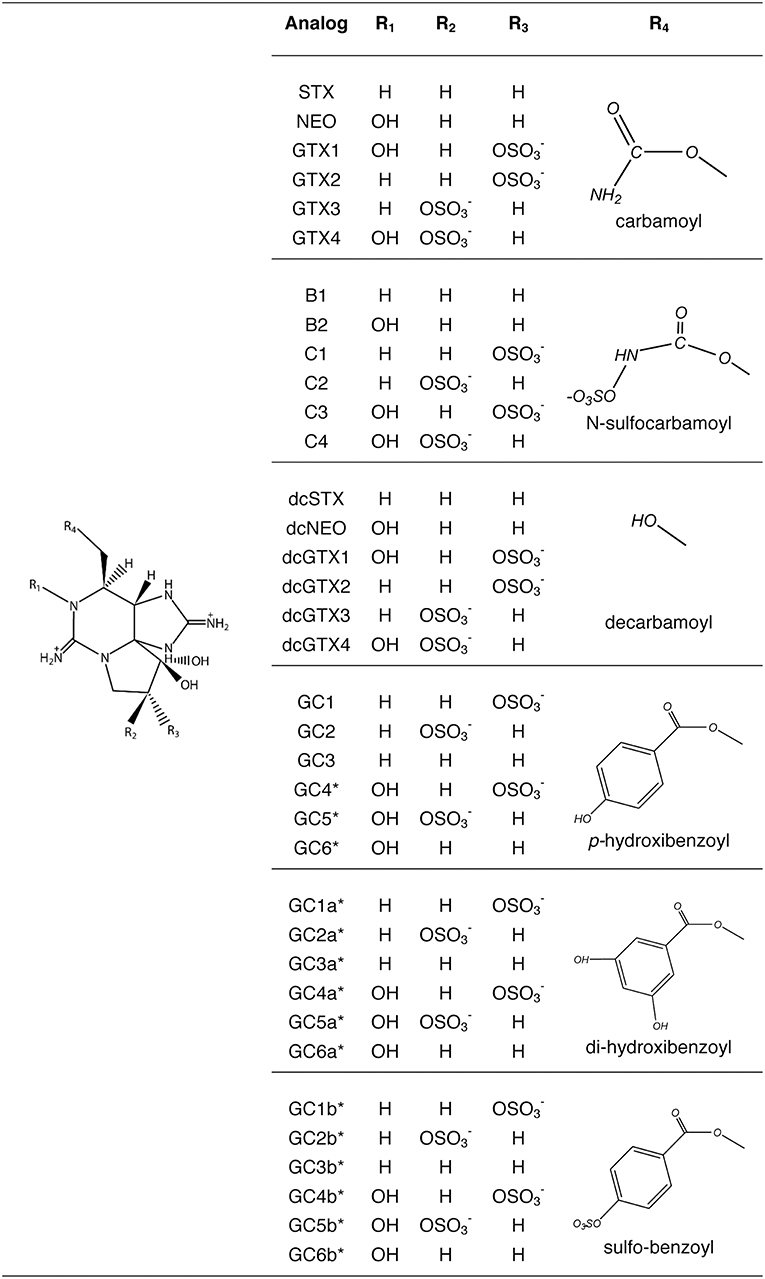
Figure 1. Paralytic shellfish toxins produced by dinoflagellates. Benzoyl toxins are only produced by Gymnodinium catenatum. (Modified from Durán-Riveroll and Cembella, 2017).
Consumption of PSTs-contaminated seafood results in a variety of gastro-intestinal and neurologic symptoms known as paralytic shellfish poisoning (PSP) that depend on the toxin concentration and can lead, in extreme cases, to human death. PSTs are usually transferred to humans by the consumption of mollusks such as clams, oysters, and mussels; other toxin vectors that have been reported are gastropods, crustaceans and fish (Llewellyn et al., 2006; Deeds et al., 2008; McLeod et al., 2017). These toxins act at the nervous system level by blocking the voltage-gated sodium channels (NaV) in mammals (Llewellyn, 2006). They can also bind to voltage-gated calcium (CaV) (Su et al., 2004) and potassium (KV) channels (Wang et al., 2003). In the case of GC analogs, their binding to NaV channels has only been demonstrated by in silico analyses (Durán-Riveroll et al., 2016). PSTs have been related to the death and intoxication of diverse organisms such as shrimp, fish, sea birds, turtles, and whales; however, the knowledge of toxin action in marine organisms is still scarce (Pérez-Linares et al., 2008; Núñez-Vázquez et al., 2011; Costa et al., 2012).
In Latin America (LAm), ~1,410 people have been intoxicated (94 fatalities) by PSP from 1970 to 2016 (Table 1). Pyrodinium bahamense has caused the highest number of intoxications (819 cases), followed by Alexandrium spp. (350 cases), and G. catenatum (241 cases). In the genus Alexandrium the highest mortalities have been caused by A. catenella (Lagos, 2003), causing 10 M USD losses in salmon industry (Mardones et al., 2015). In spite that intoxications and economic losses due to PSP are becoming an important public concern, few Latin American countries have established and/or maintained monitoring programs.
In this review, we summarize the current state of knowledge regarding PSTs producing dinoflagellates in LAm, contrasting the regional differences with the aim to contribute to future guidelines for an international scientific approach for research and monitoring activities that are needed to increase our understanding of PSP events in this region.
Gymnodinium catenatum
Gymnodinium catenatum is the only gymnodinioid known to produce PSTs. The first description of the species was in the northern region of the Gulf of California (GOLCA) in 1939 (Graham, 1943). Forty years later, in 1979, the first PSP related to this species in LAm was reported along the coasts of Sinaloa, Mexico, during an upwelling event, causing an extensive fish kill (~200 km), 19 human intoxications and 3 fatalities (de la Garza-Aguilar, 1983; Mee et al., 1986).
Although it was rarely observed after being described, from the decade of 1970 on, blooms have been reported and associated with human poisonings and fatalities in several countries (de la Garza-Aguilar, 1983; Hallegraeff et al., 2012; Cembella and Band-Schmidt, 2018). An apparent increase in frequency, duration, and distribution of blooms are probably related to anthropogenic activities (Hallegraeff, 1993, 1995; Hallegraeff et al., 2012). To date, it has been reported in nine countries of LAm (Figure 2). In Mexican coasts there have been numerous reports since 1990 (Band-Schmidt et al., 2010; Gárate-Lizárraga et al., 2016; Medina-Elizalde et al., 2018), associated with human intoxications and death of marine organisms (de la Garza-Aguilar, 1983; Mee et al., 1986; Núñez-Vázquez et al., 2011, 2016). HABs of this species have resulted in 241 cases of PSP (14 fatalities) in Mexico and Venezuela (Table 1), and epizootic diseases with mass mortalities of fish, seabirds, and marine mammals. Losses in shrimp cultures have also been reported. Also, prolonged sanitary closures by contamination with PSTs in shellfish, have affected commercialization, causing un-estimated but significant economic losses due to the closure of important shellfish fisheries in the upper GOLCA (García-Mendoza et al., 2016).
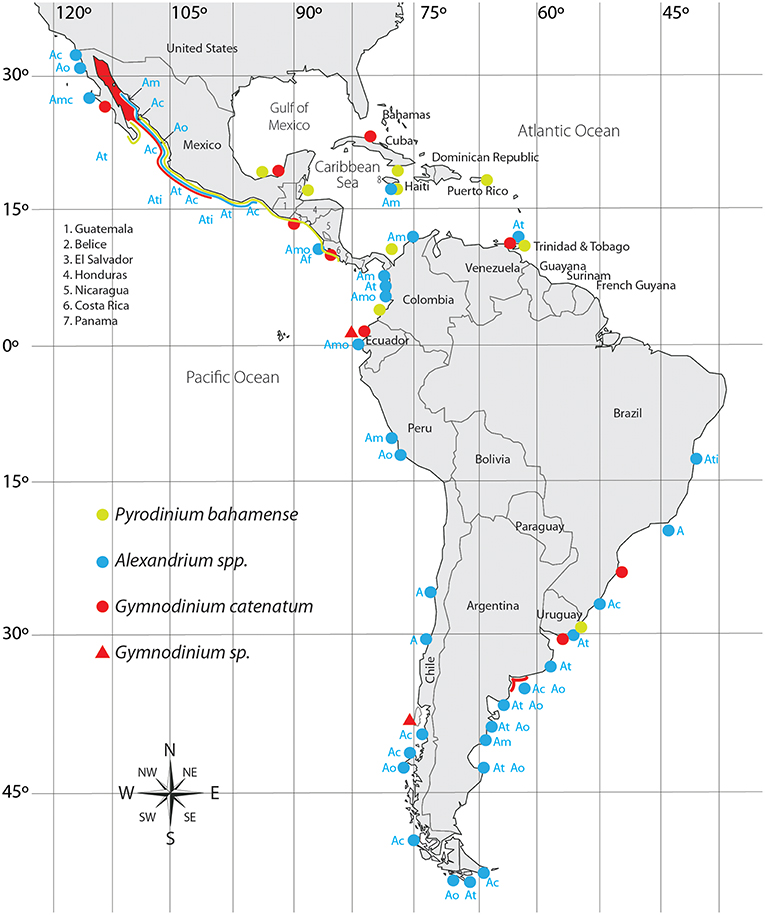
Figure 2. Distribution of PST producing dinoflagellates in Latin America. Ac, A. catenella; Am, A. minutum; Amo, A. monilatum; Ao, A. ostenfeldii; At, A. tamarense; Ati, A. tamiyavanichi; A, Alexandrium sp.
Gymnodinium catenatum Bloom Dynamics
Mexico
One of the most recent and lengthy HABs of this species occurred in the northern part of the GOLCA with maximum abundances of 152 × 103 cell L−1 (Medina-Elizalde et al., 2018). This bloom lasted from January to March in 2015, affecting a large number of seabirds, mammals and people (García-Mendoza et al., 2016). In Bahía Concepción (BACO), G. catenatum is often present without forming blooms and is more abundant during hydrographic transitional periods during spring and early fall (Morquecho and Lechuga-Devéze, 2004). BACO is one of the few bays where studies on cyst dynamics have been realized, cyst yields are low, but seem to be a constant inoculum that sustains the population for long periods (Morquecho and Lechuga-Devéze, 2004).
In Bahía de La Paz (BAPAZ), in the south of the California Peninsula, SW GOLCA, there is only one report of a HAB in February/March 2007, with abundances from 6 × 105 to 2.4 × 106 cell L−1, an average sea surface temperature (SST) of 20.9 ± 0.7°C and low nitrate (1.0 ± 0.3 μM) and phosphate (0.8 ± 0.8 μM) concentrations (Hernández-Sandoval et al., 2009). In the same bay, in 2010, this dinoflagellate was reported in low abundances (21 × 103 cell L−1) (Muciño-Márquez, 2010).
On the NE GOLCA, in Bahía Bacochibampo, Sonora, a 24-year study was performed on HABs (1970–1994), recording 43 events, where one of the main species was G. catenatum (Manrique and Molina, 1997); this led to conclude that NW winds during colder months promoted blooms. They also agreed with Cortés-Altamirano (1987) that HABs had an inverse relationship with El Niño. Unfortunately, this is the only study for this region.
In April 1979 a HAB was reported in Bahía de Mazatlán (BAMAZ), Mexico, (SE GOLCA) with high abundances (6.6 × 106 cell L−1) (Mee et al., 1986). A thermal gradient up to 5°C was registered between 0 and 10 m, associating the bloom with an upwelling event. From 1979 to 1985, several HABs occurred in this bay, being this species one of the most frequently reported (Morey-Gaines, 1982; Cortés-Altamirano, 1987). In 1997, it was concluded that blooms in this region were inhibited due to the influence of El Niño, that attenuated the upwelling of deep nutrient rich waters (Cortés-Altamirano, 2002).
In the Mexican Pacific coast, in Nayarit, two HABs were reported in December 2005. The average abundances were 1010 × 103 cell L−1, at a SST of 25°C and high nutrient concentrations (Castillo-Barrera and García-Murillo, 2007). Further south, in Bahía Manzanillo (BMANZ), Colima, HABs have increased in frequency, duration, and coverage. During winter and spring of 1999, HABs were reported with abundances of 35 × 102 cell L−1 (Blanco-Blanco et al., 1999; Morales-Blake et al., 2000). The temperature during the initiation of the bloom was 23°C, and at the highest abundance it reached 25°C. In the same bay, in spring of 2007, blooms were reported, with abundances of 3532 × 106 cell L−1, SST ranged between 24.8 and 26.3°C, salinity from 31.8 to 32.6, and dissolved O2 from 5.1 to 6.1 mg L−1 (González-Chan et al., 2007). The authors suggested that HABs were caused by upwelling events documented by satellite images.
In May 2010, the species proliferated again in BMANZ, and Bahía de Santiago (Quijano-Scheggia et al., 2012). Water temperature was 21°C at the beginning of May, with salinities of 32.5–34.6. Again, during the HAB, temperature raised from 25.5 to 27.5°C, and when temperature decreased to 22.5°C, the highest abundances (3.7 × 106 cell L−1) were found. Also, high mean values of dissolved inorganic nitrogen (6.16–6.45 μM), orthophosphates (0.27–0.51 μM), and silicates (7.49–21.07 μM) were reported, coinciding with an upwelling event in the Central Mexican Pacific that lasted 2 weeks and was more intense than previous events. Morning winds flowed from the coast to the ocean, carrying the HAB off the coast; and in the early afternoon, the flow was in the opposite direction, causing cells to accumulated near the coast, suggesting that this dinoflagellate is present in the oceanic zone in low abundances, and that when the abundance increases, cells are transported to coastal areas during more favorable conditions, such as relaxation periods during upwellings (Quijano-Scheggia et al., 2012).
In the southern Mexican Pacific, in Bahía de Acapulco (BACA), the first record occurred in March 1999 with low abundances from 7 to 78 × 103 cell L−1 (Gárate-Lizárraga et al., 2009). Co-occurrence with M. polykrikoides was also reported during a HAB from December 2005 to February 2006, with an abundance of G. catenatum from 141 × 103 cell L−1 to 604 × 103 cell L−1. In December 2007 once again there was a HAB of both species at a SST of 26–27°C.
In the last decade, the species has been reported in the Gulf of Mexico, with the highest densities in October 2008, between 183 and 1797 cell L−1 (Zamudio et al., 2013). Gymnodinium cf. catenatum was reported in oyster beds in Campeche (Poot-Delgado et al., 2015), but no HABs have occurred.
Band-Schmidt et al. (2010), reviewed the reports in the Mexican Pacific, including the GOLCA. They concluded that this dinoflagellate is more abundant during March/April, associated with a SST from 18 to 25°C, and an increase of nutrients from coastal upwelling and river runoffs. Also, that blooms are inhibited by El Niño. Later studies have confirmed these assertions.
Other Latin American Countries
In the coasts of Ecuador several HAB events occurred in the 1990s (Torres, 2000). Within the list of species, Gymnodinium sp. proliferated in February 1993 in the Península of Santa Elena, extending for several kilometers, with a SST of 25°C and a wind speed of 8 kn. HABs of Gymnodinium sp. were reported in March 1993, in Estero Salado (Golfo de Guayaquil, GGUAY) and Apri 1997 in Isla del Muerto (Santa Clara). In August of the same year, in island Santa Clara, this species proliferated (<5 × 104 cell L−1) and remained during 5 months. In February/March 1999, abundances of 3.5 × 105 cell L−1 were reported in GGUAY, with phosphates concentrations of 10.1 μg-at L−1 and silicates of 170.69 μg-at L−1. Nutrient values were high in comparison with previous data recorded in the same area. In January 1998 a bloom of Gymnodinium sp. occurred at a SST of 25–29.7°C, and was related with ENSO and associated with a bird mortality. In 1999 (April/May) blooms of Gymnodinium sp. were reported near shrimp farms and associated to turtle mortality (Torres, 2000). Torres-Chuquimarca (2011) reviewed HABs events from 1968 to 2009, and concluded that from March to May, HABs are recurrent in the coasts of Ecuador and again, the most frequent genus was Gymnodinium sp. Areas with the highest incidence of blooms and mortality of organisms were in the GGUAY (80%), particularly Estero Salado, and Río Guayas, Puna island and the coastline of the province El Oro, a region where shrimp farming is an important activity. The mortality of organisms associated to these blooms suggests that the responsible species could be G. catenatum.
In Brazil, this species was recorded in 1997 (Proença et al., 2001) in the estuarine complex of Paranaguá (Southern Brazil), where fisheries and aquaculture are important economic activities. From 1913 to 2000 several HAB species that impacted wildlife off the coast of Brazil were reported, including Gymnodiniun sp., although G. catenatum was also reported (Odebrecht et al., 2002). From August 2002 to October 2003, it was reported in Paranaguá, among various toxic species, with a maximum abundance of 6.4 × 103 cell L−1 in February (spring-fall) at 29°C (Mafra-Junior et al., 2006).
In Chile, during March-April 1999, in the archipelago of Chiloé and inland waters, a massive fish mortality that extended >1,330 km took place, from the latitude 42° to 54° S, affecting salmon farming (a loss of ~1,500 t), eels, sea stars, sea urchins, mussels, octopuses and snails (Clément et al., 2001). Mortalities were associated with Gymnodinium sp. (4–8 × 106 cell L−1). Also, densities of 3 to 43 × 104 cell L−1 were recorded in the Magellan region (MAGR). The bloom was more intense during neap tides and strong solar radiation. A higher abundance was found in surface waters during the day, and at night at 10 m depth. The HAB originated outside the fjords and channels in the hydrographic front between the open waters (>30 salinity) and the inland waters (salinity15–25) (Clément et al., 2001). In the archipelago of Chiloé, temperature was 1.5°C higher (15°C) than the expected, suggesting that the growth of dinoflagellates could be stimulated with high irradiance, additionally to prolonged minimal amounts of discharged fresh water in the channels and fjords, providing suitable conditions for oceanic species. HABs were associated with the front structure of the body of water masses.
In Argentina, this species was recorded for the first time in 1964 (Balech, 1964). Until 1995, during March-April, it was observed in low densities (80 cell L−1) in Mar del Plata (MPlat), but in April 1997, an abundance of 1.9 × 103 cell L−1 was reported (Akselman et al., 1998). This species was also recorded on the coast of Uruguay in 1991 at the end of summer, and in autumn 1993 it was reported in both, Uruguay and MPlat. From 1991 to 2004 blooms occurred in Uruguay at a SST between 21.8 and 24.0°C and salinities between 18.4 and 32 (Méndez, 2006). In March 2003, in coastal waters of MPlat, the species was found in high abundances (8.9 × 104 cell L−1) (Montoya et al., 2006). As toxin profiles differed within both locations, authors suggested that the MPlat HAB was a local event and was not caused by the transportation of the population from the estuarine region.
In Cuba, G. catenatum was found in the NE region coinciding with higher nutrient inputs from rivers and anthropogenic discharges. Nevertheless, during dry season, at a SST between 26.8 and 27.3°C, salinity 36.3, abundances were reported between 400 and 800 cell L−1 (Leal et al., 2001). In the southern region, in Bahía de Cienfuegos, it was also reported (2.3 × 103 cell L−1) at a wider SST range (between 23.6 and 27.0°C) (Moreira-González et al., 2013).
Toxin Profiles in Environmental Samples
In Mexico, two review papers have been published regarding the toxicity and toxin profile of phytoplankton and shellfish samples during HABs of G. catenatum. The first report included information from the Mexican Pacific coast (Band-Schmidt et al., 2010), and the second only considered data from the GOLCA (Bustillos-Guzmán et al., 2016). As in many other geographic zones, natural phytoplankton PSTs analysis linked to this species are scarce in LAm, and reports have only been done in Uruguay, Argentina and Mexico (Méndez et al., 2001; Gárate-Lizárraga et al., 2006; Montoya et al., 2006; Quijano-Scheggia et al., 2012).
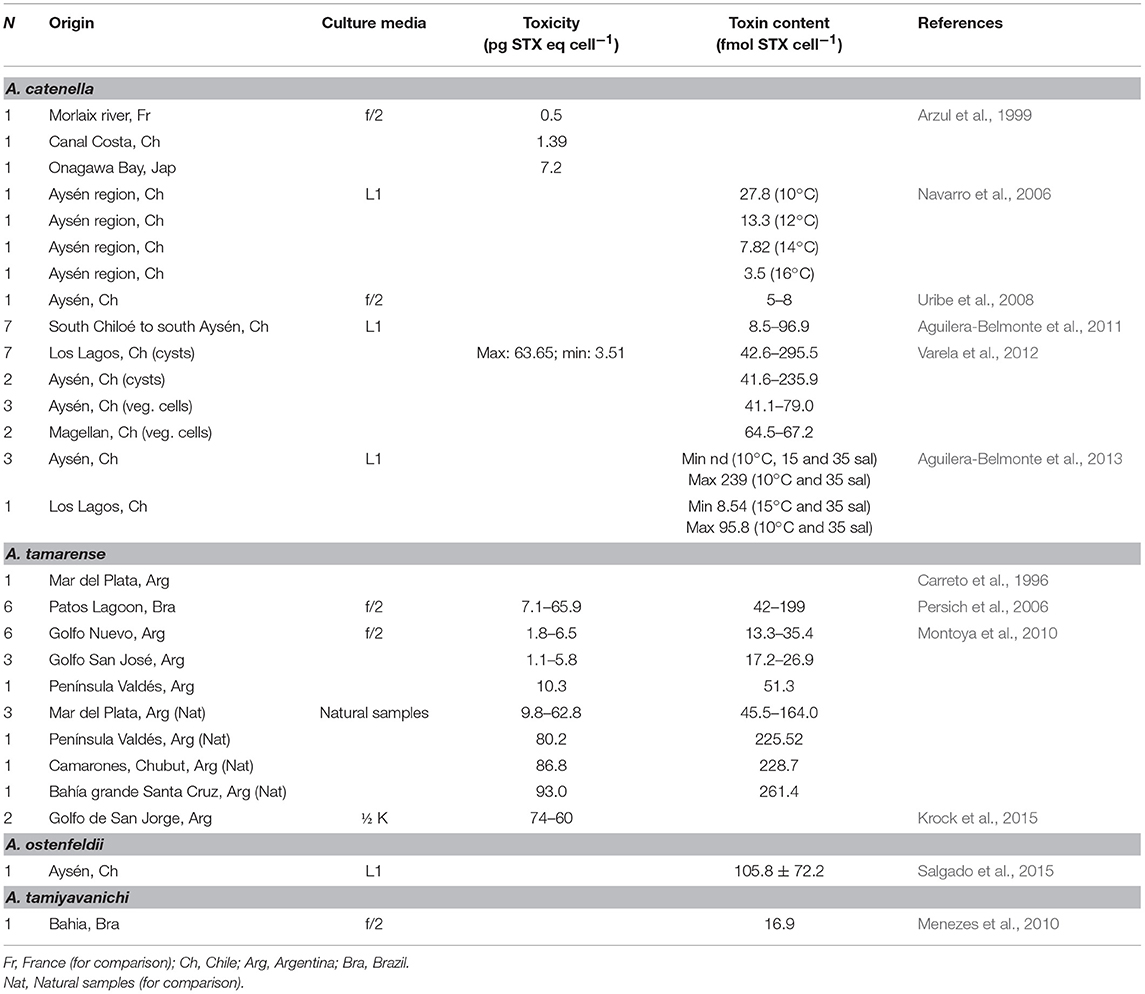
Toxicity of this species is relatively low in natural phytoplankton samples and, according to reported analysis, it oscillates between 1 and 3.7 pg STXeq cell−1 in BAPAZ (Gárate-Lizárraga et al., 2006), and in BMANZ from 1.4 to 10.9 pg STXeq cell−1, with an average of 4.2 pg STXeq cell−1 (Quijano-Scheggia et al., 2012). The PSTs analogs are predominantly sulfocarbamoyl C1/2 (73.0% in molar basis), followed by carbamoyl, STX (16.2%) and NEO (3.3%). Decarbamoyl analogs (dcGTX2/3, dcSTX) are less represented (<5%). In coastal waters of MPlat, toxin content was 122 fmol cell−1 (Montoya et al., 2006), and presented a profile dominated by C1/2 (82 mol%), followed by GTX2/3 (9%), GTX4 (6%), and dcGTX2/3 (4%). The calculated toxin cell content was similar to that reported previously for Uruguayan isolates (Méndez et al., 2001). These values also agreed with the calculated cell toxin concentration related to the first lethal poisoning in Mexico, of 10 pg cell−1 (Mee et al., 1986). Both reviews emphasized the low quantities of PSTs in natural phytoplankton samples of the dinoflagellate. This remark, as shown in this work, could also be extended to other zones in LAm.
Concerning the toxin profile of phytoplankton samples, two patterns can be clearly distinguished: One where sulfocarbamoyl analogs (B, C) are dominant (>50% in molar basis), and a second one, where decarbamoyl analogs (dcGTX2/3, dcSTX) dominate, representing >70% (Gárate-Lizárraga et al., 2004, 2006). The first pattern has been reported in bloom samples of Argentina (Montoya et al., 2006) and Mexico (Quijano-Scheggia et al., 2012). The second was found in samples from Mexico: Bahía Concepción (BACO) and BAPAZ (Gárate-Lizárraga et al., 2004, 2006) in the GOLCA. However, the liquid chromatography with fluorescence detection (LC-FLD) method commonly used for these analyses has some withdrawals, and miss-identification may occur depending on the extraction methods, if two or more toxins have the same retention time or if phantom peaks are present they can compromise the identification of analogs (Bustillos-Guzmán et al., 2015). These patterns have to be further confirmed and analyzed by confirmatory methods such as LC coupled to mass spectrometry detectors alone or in tandem (LC-MS or LC-MS/MS). Benzoyl analogs should also be analyzed in natural phytoplankton samples of G. catenatum, since it has been reported that at least 15 of them have been identified in cultured strains (Durán-Riveroll et al., 2017).
During HABs of this species in Mexico, PSTs content of several species of mollusks have been reported, including oysters, clams, and scallops (Bustillos-Guzmán et al., 2016; Quijano-Scheggia et al., 2016). Dominance of B and C, or dc analogs (dcGTX2/3, dcSTX) are usually found in mollusks (Gárate-Lizárraga et al., 2004; Hernández-Sandoval et al., 2009). In Panopea globosa analogs could be linked to the bloom phase, with a higher content of C toxins during the HAB occurrence, and a higher content of GTX5, dcGTX2, and dcSTX several weeks afterwards (Medina-Elizalde et al., 2018).
In Venezuela, from August to December 1991, in mussels (Perna perna) from Isla Margarita, the most abundant analog was dcSTX; NEO and GTX1-4 were also registered (La Barbera-Sánchez et al., 2004). The presence of dcGTX2/3 was attributed to G. catenatum, however, during this bloom cells of A. tamarense were also documented. In Uruguay, during a HAB, the toxin profile in Mytilus edulis and the clam Donax hanleyanus presented N-sulfocarbamoyl analogs (C1/2, 53% in molar basis) and significant amounts of GTX2/3 and STX for the former bivalve and dc toxins, mainly dcGTX2 (~63%), for the later species (Méndez et al., 2001). Analog variation was also linked to the bloom phase, as well as the cell abundance, ingested cells or specific biotransformations of each bivalve.
Autoecology Studies
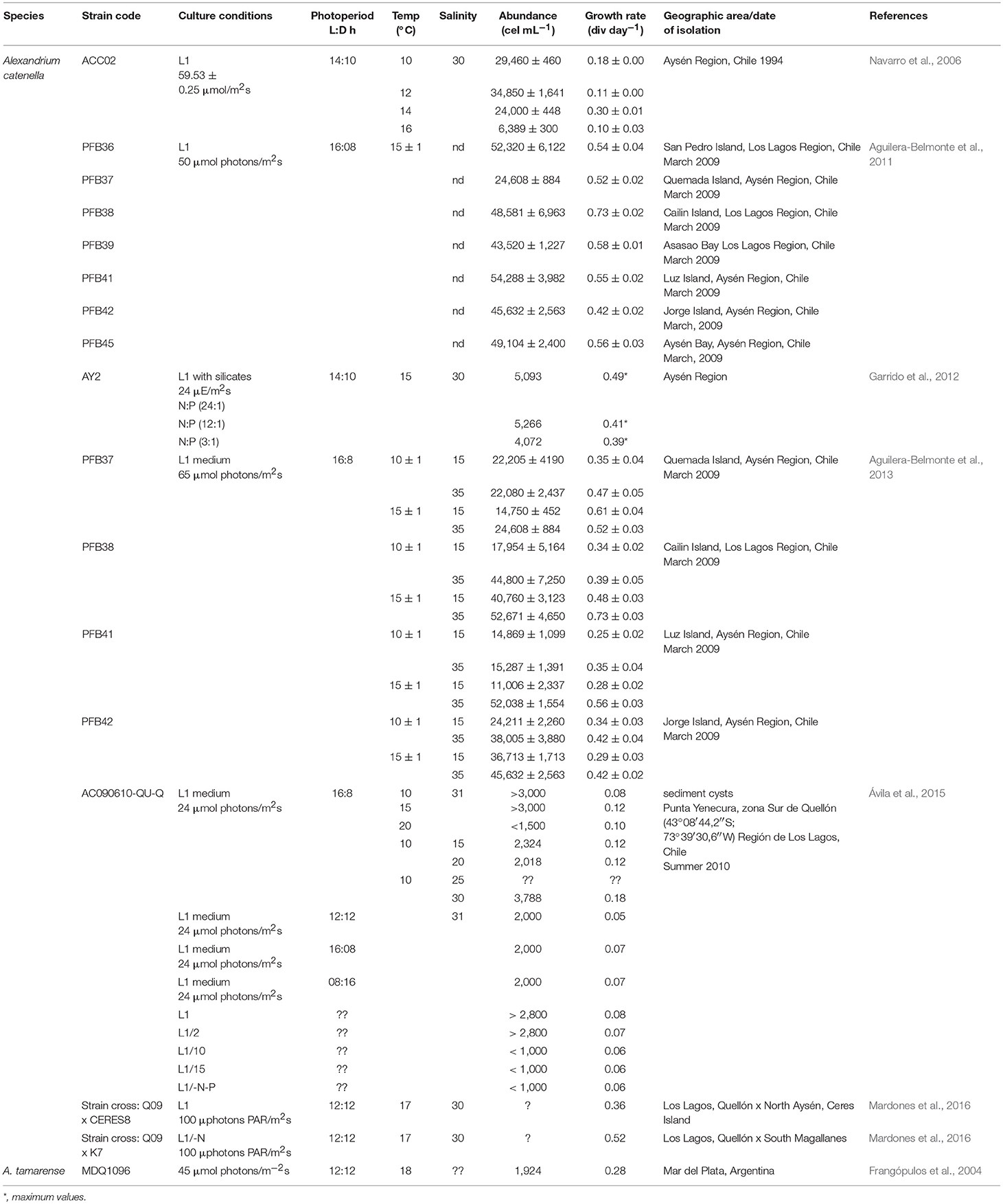
Several autoecology studies have been performed with strains of G. catenatum from the GOLCA and the Pacific coast of Mexico (reviewed by Band-Schmidt et al., 2010, 2016). Under laboratory conditions, this species tolerates a wide salinity, temperature, and N:P ratio. Strains are able to grow at salinities from 15 to 40; the optimal range changes with seawater source. In general, highest growth rates (from 0.28 to 0.31 div day−1) occur between 28 and 38 (Band-Schmidt et al., 2004). Strains can grow at temperatures between 11.5 and 33°C, with higher growth rates (from 0.32 to 0.39 div day−1) between 21 and 24°C, and maximum cell concentration between 4,700 and 5,500 cell mL−1 (Band-Schmidt et al., 2004, 2014). This species also tolerates a high range of N:P ratio (from 5.4 to 74.3 μM) with growth rates between 0.20 and 0.24 div day−1, and a maximum cell concentration (5,852 cell mL−1) at a N:P ratio of 23.5 (Bustillos-Guzmán et al., 2011). This wide tolerance to environmental factors probably explains partially the wide distribution of this species along the Mexican Pacific coast.
There are only two studies in LAm where photosynthetic pigments are reported. The first one determined the effect of two light intensities (120 and 350 μmol quanta m−2s−1) on a strain from BACO [Murillo-Martínez, 2015, in (Paredes-Banda et al., 2016)], finding that, at the highest light intensity (HL) growth rates were of 0.28 ± 0.06 div day−1, with maximum cell concentration of 5902 cell mL−1; at a lower light intensity (LL) growth decreased in 50% (0.14 div day−1) and the maximum abundance decreased by 45%. At LL the concentration of chl a and peridinine was higher than at HL. Also, at HL diadinoxanthine and diatoxanthine increased (18%) indicating the photoprotection in cells is higher compared with LL conditions. No differences where observed in the concentration and toxin profile under both light conditions indicating that toxin synthesis is not related with light intensity. The second study, in an isolate from Santa Catarina, Brazil, determined the pigment profile, detecting chl a, chl c2, peridinine, diadinoxanthine, and minor carotenoids (Proença et al., 2001).
Few studies have been performed in LAm to understand the effect of planktonic species on its growth and survival. In the GOLCA, grazing studies have been performed with the copepod Acartia clausi and the dinoflagellate Noctiluca scintillans, demonstrating that both are important predators of the dinoflagellate, suggesting they have an important ecological role in the regulation of its blooms (Palomares-García et al., 2006; Bustillos-Guzmán et al., 2013). It was also demonstrated the allelopathic effect of the raphidophyte Chattonella marina var. marina and the dinoflagellate Margalefidinium polykrikoides toward G. catenatum. Both species inhibited the growth of G. catenatum and caused changes in its morphology, and chain formation. Inhibition was stronger and occurred in a shorter time with Chattonella; mortality occurred with and without direct cell contact, indicating that toxic metabolites are released to the culture medium (Fernández-Herrera et al., 2016; Band-Schmidt et al., 2017). These results suggested that biotic factors affect its growth providing new insights on the interactions between this dinoflagellate and co-occurring planktonic species.
Gymnodinium catenatum Toxin Profiles Under Laboratory Conditions
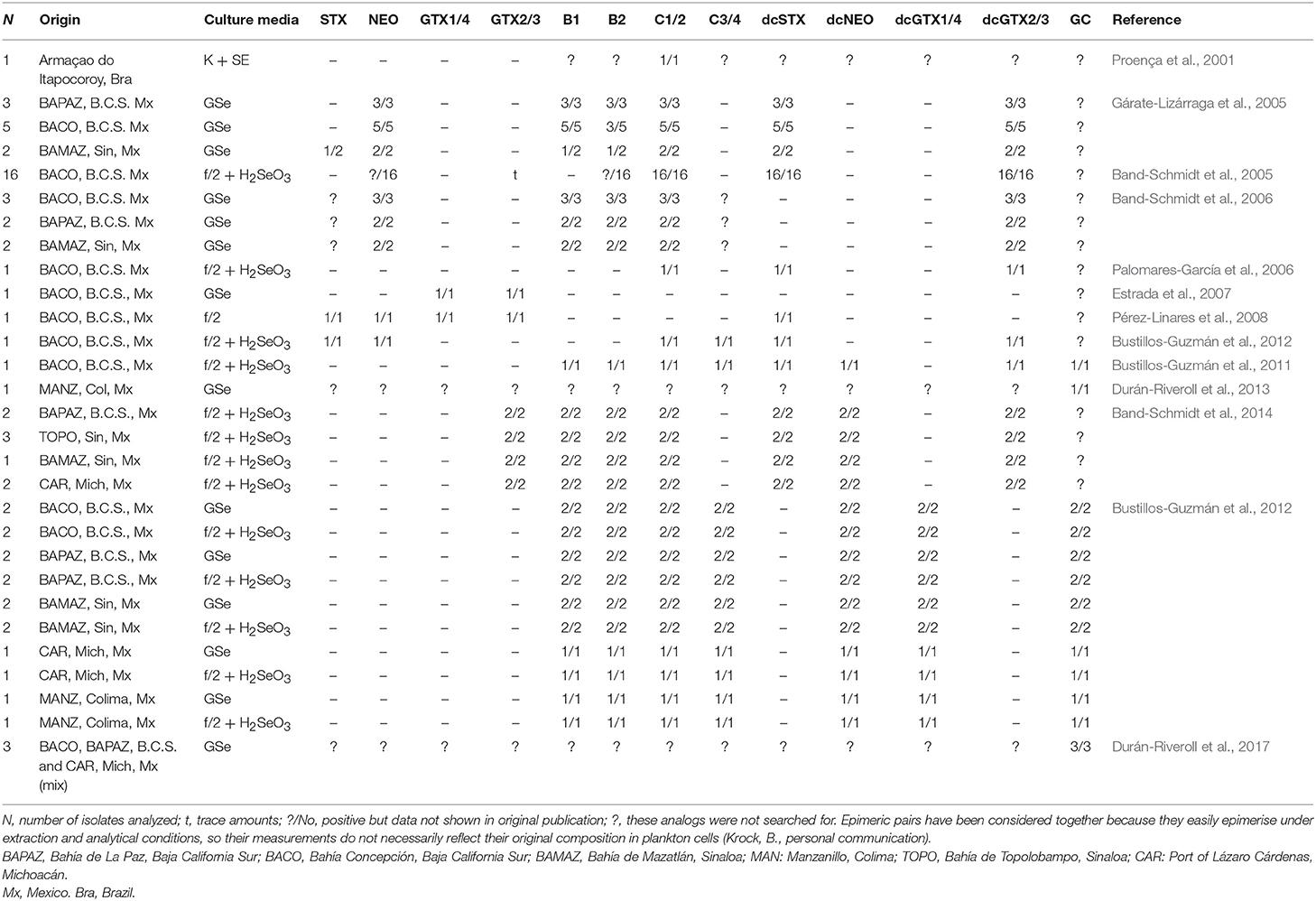
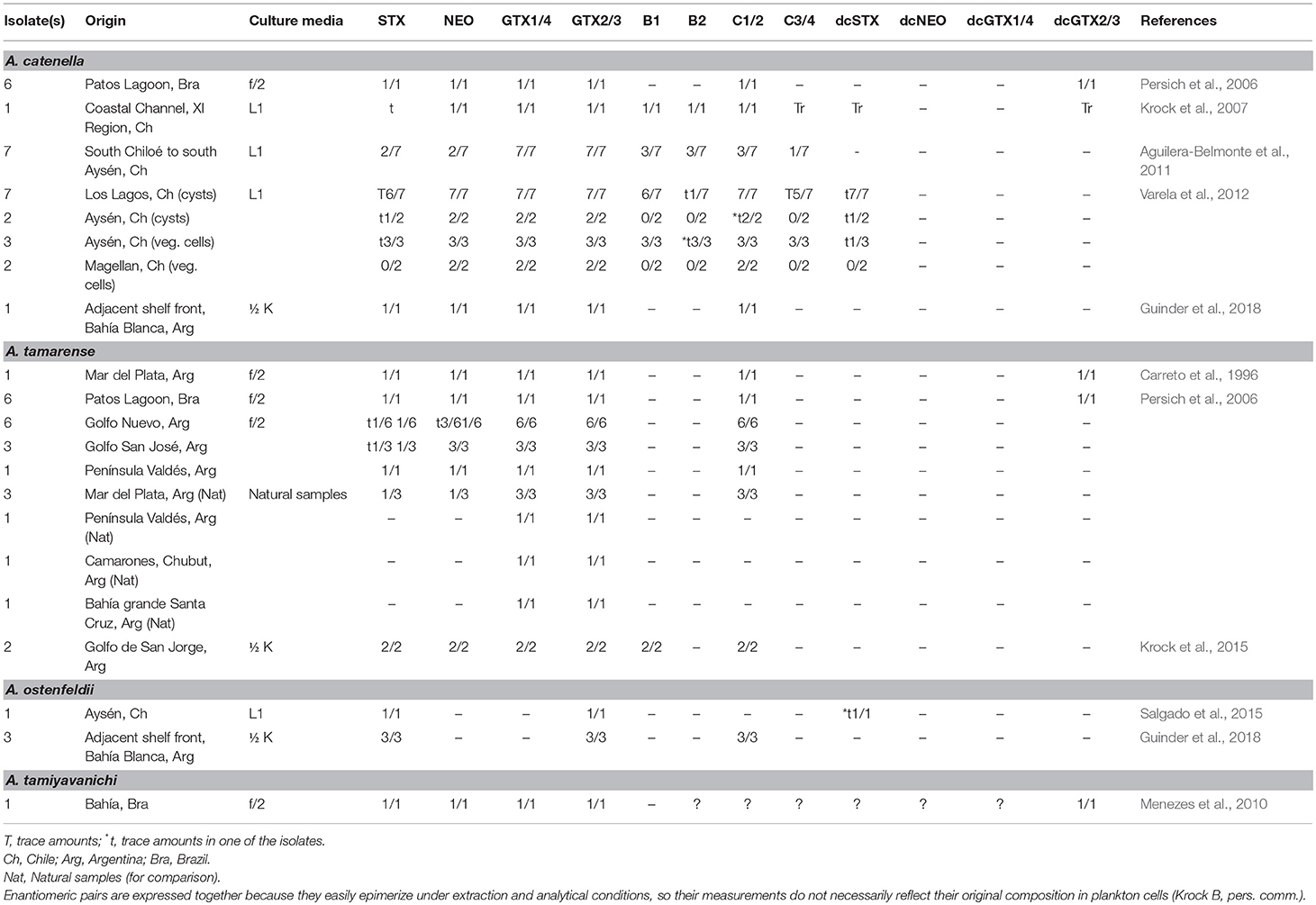
In LAm, several strains of G. catenatum have been isolated and maintained in culture for laboratory studies (Figure 3). Toxin profiles in laboratory conditions, however, have been reported only from Brazil and Mexico. In Brazil, one isolate has been studied, isolated from Armaçao do Itapocoroy (AdI). In Mexico, the isolates used for autoecological studies come from Baja California Sur (BAPAZ, BACO); Sinaloa (BAMAZ); Colima (BMANZ); Bahía de Topolobampo, Sinaloa (TOPO) and Lázaro Cárdenas, Michoacán (CAR). In these studies, toxin analyses have been performed mostly by HPLC-FLD. This technique, though reliable for most of the common STX analogs, has some drawbacks that have been mostly reduced by several improvements of the methods. Nevertheless, it is important to keep in mind that some of these drawbacks are persistent and have led to important confusions in toxin analysis.
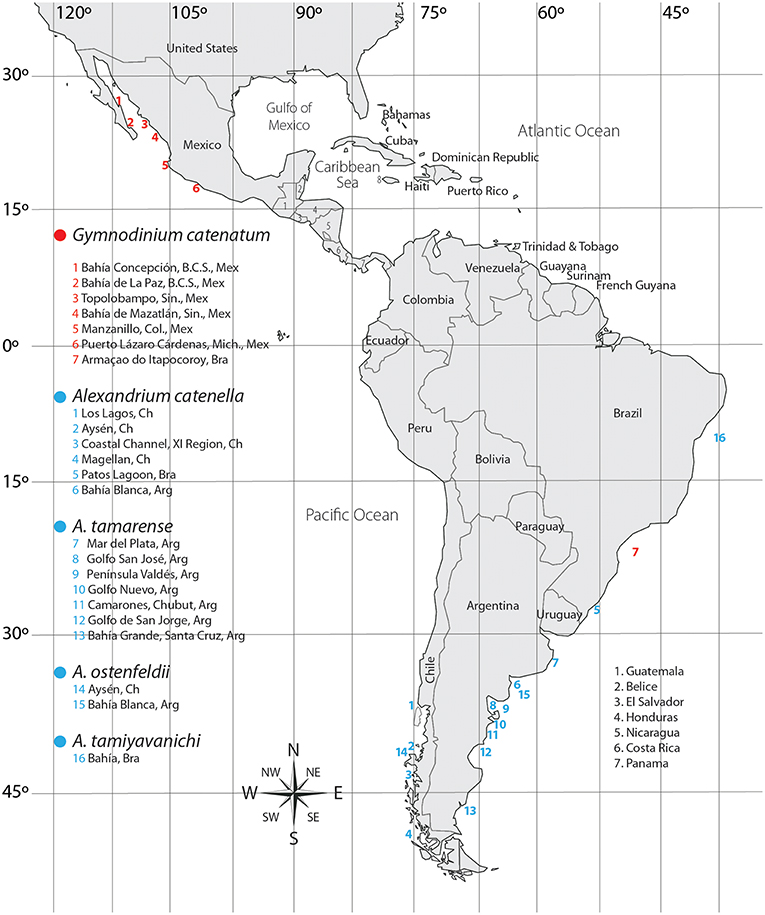
Figure 3. Origin of isolates from where toxin production in laboratory conditions have been analyzed.
In 2001, laboratory studies started in Brazil. The only available standards for the analysis were GTX1-4, NEO, and STX, but none of these analogs were found in the culture samples, and peaks eluted in retention times previously reported for N-sulfocarbamoyl toxins. The production of these analogs was later proven by acidic hydrolysis of the extract and further analysis, showing a peak for GTX2/3, equivalent to the C1/2 toxins, characteristic of this species (Proença et al., 2001).
Since 2005, toxin profiles from Mexican isolates started to be studied. These isolates have been mainly from the GOLCA region, and the Mexican Pacific. Their toxin profile included NEO*1, dcSTX, dcGTX2/3, B1, and C1/2 (Table 2). The low molar percentages of B toxins was considered a distinctive characteristic of Mexican strains, compared to reports from Portugal and Spain (Negri et al., 2001; Ordás et al., 2004; Gárate-Lizárraga et al., 2005), but a huge variation in profiles and toxin content among strains was found, and it was also proven that they can vary with culture age and culture conditions (Gárate-Lizárraga et al., 2005).
It has been reported that the toxin content was slightly variable with culture age, but differences were not always significant (Band-Schmidt et al., 2005). As cultures aged, a decrease in the number of isolates producing NEO*, GTX2/3, C1/2, and B2 was registered, and a there was significant increase of decarbamoyl analogs (dcSTX, dcGTX2/3) (Table 2). Nevertheless, in another study, no significant differences in toxin content or toxicity related to culture age were found, neither differences between strains isolated from vegetative cells and cysts. They also reported STX during certain days of the culture, a toxin that is almost never reported in Mexican isolates, which could indicate that the rest of the time it is produced in non-detectable concentrations (Band-Schmidt et al., 2006).
Later, the toxin profile of one isolate from BACO was analyzed, though the main goal was to determine its toxic capacity when fed to the copepod A. clausi. Toxin profile of the isolate showed only the commonly reported analogs C1/2, dcSTX, and dcGTX2/3, and no B1, B2, dcNEO or any carbamoyl toxins that were previously reported (Palomares-García et al., 2006) (Table 2). Also, the effects of this dinoflagellate were demonstrated on the clam Nodipecten subnodosus. In this case, toxins were extracted with a different acetic acid concentration (0.1 N vs. 0.03 N in former experiments) (Estrada et al., 2007). The authors found a vast amount of GTX toxins, but did not specify the analogs, and no N-sulfocarbamoyl toxins, such as B1, B2, and C1-4 were reported (Table 2), though these analogs have been often described as the main toxin component for the species. A year later, the effect of the PSTs were studied in the white leg shrimp, Litopenaeus vannamei, using an isolate from BACO (Pérez-Linares et al., 2008). Cultures were grown in f/2 medium and the toxin profile was analyzed prior to injecting the shrimp with the toxic extracts. The profile was very different from previous reports from the same isolate, probably related to differences in culture media (f/2 vs. GSe), temperature (21°C vs. 26 ± 1°C), and toxin extraction, that was performed with hydrochloric acid instead of acetic acid. The described profile for this isolate was composed by STX, NEO, GTX1/4, GTX2/3, and dcSTX (Pérez-Linares et al., 2008), and no N-sulfocarbamoyl toxins or other decarbamoyl toxins (dcNEO, dcGTX1-4) (Table 2), were reported. Probably, the acidic conditions of the extraction could modify the toxin profile, or the lack of N-sulfocarbamoyl standards prevented the detection of these toxins. As reported in many other countries, the presence of dcSTX, dcGTX2/3, and C1/2 are considered usual components of G. catenatum strains from the Pacific Mexican coasts, also, being the decarbamoilated analogs the most important in terms of toxin potency while the latest are usually the most important in terms of molar contribution (Band-Schmidt et al., 2010).
In the search for a better understanding of how the environmental factors affect the toxin profile and toxicity, in vitro experiments have been performed using different nutrient concentrations and temperature regimes. In 2012, the analysis of an isolate from BACO in the GOLCA, with different N:P ratios was reported (Bustillos-Guzmán et al., 2012). Toxins were extracted with 0.03 N acetic acid and a subsample was hydrolized to detect N-sulfocarbamoyl toxins. No significant differences in cell toxicity or toxin profile were recorded, and by the end of the experiment, an increase in total toxicity was evident in all treatments, as reported previously (see Band-Schmidt et al., 2005, 2006; Gárate-Lizárraga et al., 2005). The toxins found in these experiments were STX, NEO*, dcSTX, dcGTX2/3, B1, B2, and C1-3 (Table 2). This toxin profile varied slightly with previous reports of isolates from the same geographical area, where STX and/or GTX1/4 and no N-sulfocarbamoyl toxins were described (Estrada et al., 2007; Pérez-Linares et al., 2008).
The effect of temperature in toxin profile, toxicity and growth was studied in 2014 in eight Mexican isolates from four locations in the Pacific coast: BAPAZ, BAMAZ, TOPO, and CAR (Band-Schmidt et al., 2014). All isolates produced the same ten analogs (Table 2), though significant differences were found among them in terms of GTX2/3 and B toxins, all of them produced higher proportions of B and C toxins. At lower temperatures (16–19°C) the production of C toxins was greater and at higher temperatures (30–33°C) B toxins represented up to 63.4% of the toxin profile, whereas at lower temperatures the maximum was 12.7% in molar basis. Decarbamoyl toxins (dcSTX, dcGTX2) were more abundant at 21°C, but since the main changes in toxin profile were within the low-potency toxins, no changes in cell toxicity related to temperature were found. An interesting relationship between dcSTX and dcGTX2 was found since both increased or decreased simultaneously (Band-Schmidt et al., 2014). This toxin profile change related to temperature, from mostly C1/2 toxins in lower temperatures to mostly B1/2 at high temperatures could be explained by enzyme activity, but more studies are necessary.
In 2001, new analogs reported for G. catenatum that brought attention to this species (Negri et al., 2001). Chromatographic peaks eluted late in the C toxin chromatograms. These peaks were later confirmed in Spanish isolates (Ordás et al., 2004), and reported as C5 and C6 compounds or possible artifacts, but it was later confirmed to be new toxin analogs containing a benzoyl ring in the lateral chain. These toxins were named GC1-3 toxins or “hydrophobic toxins” (Negri et al., 2003b). These peaks were previously noted in isolates from different countries, and in 2007 Negri and collaborators reported the widespread presence of these analogs in isolates from Australia, China, Japan, Portugal, Uruguay, and Spain, comprising between 10 and 63 mol% of the total toxin in cultured vegetative cells or cysts (Negri et al., 2007). One year later, a Portuguese strain was analyzed and several unknown oxidation products were found, in addition to the three GC toxins previously reported (Vale, 2008). Further mass spectrometry analysis confirmed the existence of a wide range of GC analogs (Figure 1) (Vale, 2008). In 2011, these analogs were searched for and found in Mexican isolates (Bustillos-Guzmán et al., 2011). The isolate used for these analysis was from BACO, and the toxin detection was done by HPLC-FLD, using the methodology previously described by Vale (2008) and comparing the retention times of the later peaks. The GC toxins found in this isolate were abundant and mostly composed by the sulfobenzoylated, the C-11 sulfated and the non-sulfated analogs (corresponding to the GCb series, GC1/2 and GC3 analogs, respectively). Apart from GC toxins, this analysis found the commonly reported B and C analogs but no STX or NEO, bringing out the question about previous analysis and the possibility of having misidentified dcSTX and dcNEO for STX and NEO (Bustillos-Guzmán et al., 2011). A second report of GC toxins in Mexico was from an isolate from the Pacific coast (MANZ), using nuclear magnetic resonance (NMR) in semi-purified water-methanol fractions, where the para-hydroxylated benzoyl ring was visible (Durán-Riveroll et al., 2013).
In 2015, Bustillos-Guzmán and collaborators analyzed the toxin profile of G. catenatum from five locations along the Mexican Pacific coast that were cultivated in two different media (GSe, f/2 + H2SeO3). Analyses were performed by hydrophilic interaction liquid ion chromatography (HILIC) coupled to tandem mass spectrometry (MS/MS). In these analysis, C1/2 analogs were the most abundant, with >85% on molar basis. Decarbamoyl toxins, such as dcSTX and dcNEO represented a mean average content <5% and no differences were found between the two culture media (Bustillos-Guzmán et al., 2015). The richness of GC analogs in Mexican isolates was confirmed but due to the lack of standards, they could not be quantified, but in terms of relative abundance (peak area cell−1) an interesting geographical increasing trend from strains from northern to southern regions was found (Bustillos-Guzmán et al., 2015). Also, the study was interesting since it was not possible to confirm the presence of GTX2/3 that had been reported in previous analysis done by HPLC-FLD. The same occurred for STX, and NEO, which led to the question on the existence of phantom peaks, particularly in dcNEO, which probably had been misreported as NEO. These findings raised doubts on the reliability of the most used technique in Mexico and LAm in general, especially for analogs for which reference standards are unavailable, such as GC toxins (Bustillos-Guzmán et al., 2015). For these reasons, it has been proposed that a second detection method, such as MS, should be performed in order to overcome the normal drawbacks of the identification methods.
The most recent research on GC analogs has been performed by Durán-Riveroll et al. (2017). They confirmed the production of fifteen GC analogs in the mixture of three Mexican isolates from the GOLCA, and the coasts of Michoacán, in the Pacific coast. The microalgal extract was fractionated by reverse-phase column chromatography and a MS/MS-guided fractionation was performed. Confirmation was done using MS on the search of the precursor ions and their fragments, using HILIC-MS/MS. For the analogs with same mass and mass fragments, 1H-NMR spectroscopy was used to discriminate between them (GC4/5 and GC1a/2a). Fifteen GC analogs were identified: GC1-6, GC1a-5a, GC1b/2b, and GC4b/5b. During this study, the chromatographic behavior of the previously named “hydrophobic analogs” was analyzed, and it was revealed that GC analogs are as hydrophilic as many of the “common hydrophilic” analogs. In light of these results, authors proposed a better designation as “benzoyl analogs” and subdivide them into para-hydroxybenzoyl, di-hydroxybenzoyl, and sulfo-benzoyl analogs (Durán-Riveroll et al., 2017). The combination of high resolution techniques, such as HILIC-MS/MS and 1H-NMR represented an important progress on the GC analogs identification and proved the need of the use of confirmatory techniques, mostly when relatively unknown analogs are investigated.
Cell Toxin Content
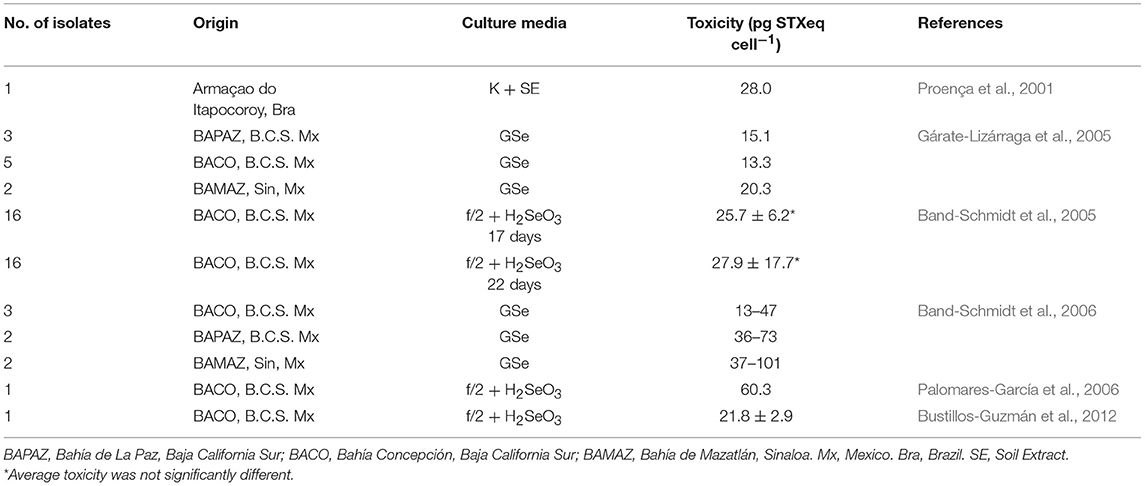
Reported toxicities from different studies are shown in Table 3. Differences in toxicity have been found among isolates (Band-Schmidt et al., 2006), related mainly to the toxin profile: isolates from BAMAZ have been found to be the most toxic with a higher amount of the more potent carbamoyl toxins; isolates from BAPAZ were in second place and the ones from BACO, where the least toxic. A wide variation in toxicity among isolates from the same area have also been found, which has been reported previously in other geographical areas (Bolch et al., 2001; Seok Jin et al., 2010). Also, toxicity in all isolates reached their highest point during the exponential growth phase (Band-Schmidt et al., 2006), as described for other isolates. The only other report of the toxin content in cultured cells is from a Brazilian isolate were the toxicity was estimated by mouse bioassay to be 29 pg STXeq cell−1 (Proença et al., 2001).
According to previous studies, cultured dinoflagellates tend to produce less toxin per cell (cell toxin quota) due to the “forced growth” by high nutrient conditions, compared to natural populations (Cembella, 1998). In nature, cell division could take longer and toxins tend to concentrate inside the cell. Nevertheless, in vitro observations from G. catenatum cultures in Mexico have shown higher cell toxin quotas than natural populations, probably related to differences in nutrients between natural and culture conditions (Gárate-Lizárraga et al., 2005; Band-Schmidt et al., 2006). However, the experiments with different N:P ratios did not shown effects in toxicity and/or toxin profile (Bustillos-Guzmán et al., 2012).
Docking Studies
Since the properties of GC analogs, such as mammalian toxicity, receptor-binding affinities and biological action mechanisms are mostly unknown due to the lack of analytical standards and the difficulties in producing and purifying these toxins, computational tools were used as an alternative strategy for their exploration. Only one docking study on G. catenatum toxins has been done in LAm, by Durán-Riveroll et al. (2016). Docking modeling is a useful in silico tool that can reveal molecular interaction mechanisms in great detail, generating information that cannot be deduced from electrophysiological approaches (Gordon et al., 2013). Molecular docking approaches have been key in the description of the interactions of several toxins with NaV channels, like with μ-conotoxins (Li et al., 2001). This approximation, even though it allows screening of many ligands for a given protein (Halperin et al., 2002; Brooijmans and Kuntz, 2003), also has certain disadvantages, related to its accuracy (Warren et al., 2006). In any case, it is possible to obtain important clarifying information about the mode of action and affinity of toxins in a protein target.
Members of the NaV channels family are the membrane-protein targets for STX and its analogs. These interactions are mediated by the guanidinium groups in the toxin molecules, which are formed by a central carbon and three nitrogen atoms, and have a positive charge at physiological pH, directly implicated in their binding capacity to the NaV, and allows the toxins to block the Na+ influx to the cell (Durán-Riveroll and Cembella, 2017). Docking studies have been useful to gain a better understanding of the blocking capacity, and due to the absence of information on GC analogs, theoretical studies are considered essential to increase the knowledge about their properties. In this study, authors found that all eighteen GC analogs theoretically interacted with the NaV 1.4 (muscular NaV channel) residues in the two protein models used for the experiment. They also reported high affinity values (as low binding energies ΔG), for some of the GC toxins, raising the hypothesis that at least some of them could be toxic to mammals because they are able to reach key protein residues by electrostatic interactions (Durán-Riveroll et al., 2016). As the model of ion channel of NaV eukariotic structure continues improving (Shen et al., 2017) instead of using homologation models of the bacterial NaV, as in this analysis, docking studies will generate more precise information. The greatest obstacle, however, is that docking programs, in order to reduce computational costs, consider the protein as a rigid body, which is far from real. The omission of conformational protein changes due to ligand binding can yield unrealistic results, and for that reason, the use of molecular dynamics (MD) is highly recommended to be used in combination with docking approach (Deeb et al., 2010), though the later greatly increases computational costs and it is more time consuming than molecular docking simulations.
Molecular Studies
The first study of genetic variation among strains of G. catenatum from Asia, Europe, and Australia was published by Bolch and collaborators in 1999. Populations from Japan, Spain, and Portugal showed higher genetic similarities than with those from Australia, where a recent dispersal could have happened (Bolch et al., 1999). In LAm, molecular analyses of the species are scarce. In 2008 the LSUrDNA sequences and morphology of strains from the GOLCA were reported, where a single nucleotide polymorphism was identified in sequences from strains from BACO, suggesting a mutation or genetic isolation (Band-Schmidt et al., 2008). Authors proposed that the Western Pacific population could be an ancestral population of this species. In 2013, the gene expression and histological injuries in the Japanese oyster Crassostrea gigas when exposed to G. catenatum toxins were analyzed, determining changes in the transcription level cell-cycle regulation genes and epithelial damages associated to inflammatory responses, concluding that the toxins induced DNA damage in this mollusk (García-Lagunas et al., 2013).
The origin of STX genes and the relationship between copy number and genome size in G. catenatum has not been well established, recently Mendoza-Flores et al. (2018) carried out a study to determine the origin of sxtA (domains sxtA1 and sxtA4) and their gene copy number. The phylogenetic tree with partial sequences of sxtA1 of G. catenatum showed a separated subclade of toxic and non-toxic Alexandrium species, while sequences of sxtA4 showed two well-supported clades within the dinoflagellates group, separating G. catenatum from Alexandrium species. Authors concluded that G. catenatum did not acquire sxtA gene by horizontal gene transference from Alexandrium. Moreover, differences in copies number of sxtA1 and sxtA4 between G. catenatum and Alexandrium species were observed.
Pyrodinium bahamense
Pyrodinium bahamense is an important member of PST-producing marine dinoflagellates, especially in tropical waters, and has caused more human illnesses and fatalities than any other PST producing dinoflagellate (Usup et al., 2012). This species was originally described from New Providence Island, Bahamas (Plate, 1906). For many years, two varieties were assigned to the genus: the Indo-Pacific variety designated “compressum,” and the Atlantic-Caribbean variety “bahamense.” However it was demonstrated that the range of distribution of both varieties extended beyond these original locations (Martínez-López et al., 2007; Morquecho, 2008), and that both varieties co-occur in several areas (Glibert et al., 2002; Gárate-Lizárraga and González-Armas, 2011). In addition to several morphological attributes, one of the primary differences between both varieties was the absence of toxin production in var. bahamense (Steidinger et al., 1980). However, a recent re-evaluation found no consistent morphological or molecular traits that could be used to separate the varieties, since toxin production was also demonstrated in var. bahamense (Landsberg et al., 2006; Mertens et al., 2015).
Its distribution in the Pacific coast extends from southern GOLCA to Colombia (Martínez-López et al., 2007; Rodríguez-Salvador and Meave del Castillo, 2007; Morquecho, 2008; Gárate-Lizárraga and González-Armas, 2011; Usup et al., 2012). In the Atlantic coast it has been reported from the Gulf of Mexico to Uruguay (Licea et al., 2013; Limoges et al., 2015; Mertens et al., 2015; Poot-Delgado et al., 2015; Cusick et al., 2016). HABs have been linked in several occasions to human poisoning (Rosales-Loessener, 1989; Rodríguez et al., 1990; Saldate-Castañeda et al., 1991; Núñez-Vázquez et al., 2011; Gárate-Lizárraga et al., 2012; Callejas et al., 2015) as well as to epizootic events (fish and sea turtles) (Núñez-Vázquez et al., 2011; Amaya et al., 2018) (Table 1). The first HAB of this dinoflagellate in LAm was reported in the coast of Guatemala in 1987, causing 175 intoxications and 26 fatalities (Rosales-Loessener, 1989). PSP cases produced by this species have affected several Latin American countries, notably in the southern Mexican Pacific, and Central America (Figure 2).
Bloom Dynamics
Mexico
Vegetative cells of P. bahamense were reported for first time in 1942 in Mexico (Osorio-Tafall, 1943). In several occasions, this species has been registered from the coasts of Michoacán to Chiapas (Figure 2) (Cortés-Altamirano et al., 1993; Orellana-Cepeda et al., 1998). In winter 1995, a HAB was recorded on the SW coast, affecting invertebrates, fish, and causing the death of 145 turtles (Orellana-Cepeda et al., 1998).
In the GOLCA, it was recorded for the first time in May 2005, in the lagoon system of Topolobampo-Santa María-Ohuira, with an abundance of 100 cell L−1. Cysts were also recorded (Martínez-López et al., 2007). Shortly afterwards, vegetative cells in the island San José in BAPAZ were reported (Morquecho, 2008). The author mentioned that the relatively low frequency of cysts of P. bahamense in this zone may indicate the influence of warm water from the tropical Pacific, and therefore it is likely that El Niño event contributed to the transport of vegetative cells or cysts from Central America. High temperatures prevailed in summer (July-October) when outbreaks occurred close to San José Island. From July to November 2010, in the southern peninsula of Baja California, it was found with abundances between 800 and 1110 cell L−1 at a SST of 28–28.5°C and salinity of 35.2 (Gárate-Lizárraga and González-Armas, 2011). These contributions widen the northern distribution of this species in the Pacific coastline of LAm. The presence of this dinoflagellate from July to November was considered unusual, and again authors attributed this to El Niño event. It was suggested that this is an invasive species and that it was probably transported by surface tropical waters moving within the GOLCA (Cortés-Altamirano et al., 2006; Gárate-Lizárraga and González-Armas, 2011). Also, the species was recorded in coastal lagoons of Sinaloa (Alonso-Rodríguez et al., 2015). To date, no HABs of P. bahamense have been reported in this region.
During December 1989 and February 2002, in the southern Pacific coast of Mexico, HABs of this species were registered (Licea et al., 2008). Environmental conditions based on satellite images reported a positive thermal anomaly of 1.5°C between southern Mexico and Costa Rica. The dinoflagellate was recorded on the coast of Oaxaca from September 2009 to June 2010, with abundances from April to June 2010 of 4 × 103 cell L−1 and 3.3 × 104 cell L−1, respectively. The SST ranged from 27.4 to 31.3°C and from 28.4 to 30°C, respectively (Alonso-Rodríguez et al., 2015).
In 2001, in BACA, cysts were reported (Meave del Castillo et al., 2006). Ten years later, in July 2010 vegetative cells were recorded at low abundances, from 1 × 103 to 1462 × 106 cell L−1 (Gárate-Lizárraga et al., 2012).
Based on a cyst study in the Gulf of Tehuantepec, it was suggested that the species has been present in this region since 1860, and that cells have been transported recently from the Indo-Pacific population (Sánchez-Cabeza et al., 2012). They mentioned that, since 1950, its influx increased in this region, due to La Niña events, however historical data of strong El Niño anomalies, recorded from 1892 to 1983, indicated there was a minimum cyst flow. Emphasizing that in 81% of cases these fluxes coincided with heavy rain (>400 mm) and high nutrients, and that high cyst flow was correlated with lower temperatures (<24.5°C). The authors proposed that low SST (conditions during La Niña) could favor the dinoflagellate growth. When a rapid transition to high SST conditions occur, rainfall increased with an input of large amounts of nutrients, making it possible to maintain the bloom. At the termination of the bloom, cysts could be incorporated into the sediments. They suggested that this situation may occur during strong upwelling conditions, hypothesis that needs to be confirmed. It was also suggested that the exceptional records in the coastline and offshore waters of the Mexican central Pacific during the 1999–2000 La Niña could suggest a link with the recurrence of HABs (Hernández-Becerril et al., 2007).
In the southern Gulf of Mexico, according to data recorded from 1966 to 1996, in coastal lagoons this species is widely distributed throughout the year (Gómez-Aguirre, 1998). In Laguna de Términos, the highest reported abundance has been 3 × 103 cell mL−1, and blooms are more frequent in autumn-winter. HAB formation could be associated with seawater fluxes and northern winds that remove the water column. Mangrove forests could also provide suitable conditions for cell accumulation (Gómez-Aguirre, 1998). In the Gulf of Mexico, this dinoflagellate is limited to the SE region (Aké-Castillo and Poot-Delgado, 2016).
Other Latin American Countries
In November 2005 and March 2006, in the coast of El Salvador, satellite images showed a positive thermal anomaly of 1.5°C between southern Mexico and Costa Rica during a HAB of P. bahamense (Licea et al., 2008). In December 2005, its abundance reached 489 × 105 cell L−1 and in March 2006 it decreased to 15 cell mL−1. The authors mentioned that blooms could be influenced by SST, strong winds and heavy precipitation. From November to June 2010 in the same area, a bloom of ~13 km was recorded with abundances of 15.3 × 106 cell L−1. Offshore concentrations were of 22 × 103 cell L−1 (Licea et al., 2012). They stated that HABs of this dinoflagellate in Central America are influenced by smaller scale gyres, upwelling and local hydrographic conditions. Cysts were detected in March and August 2012 in the Gulf of Fonseca (Alvarado et al., 2014).
In November 2005, a HAB was detected on the Pacific coast of Nicaragua, with a maximum abundance of 17.3 × 106 cell L−1, being likely that this HAB was a reflection of a major climate event scale that covered a wide area of the Central Pacific (Chow et al., 2010).
Toxin Profiles in Environmental Samples
Data regarding toxicity and toxin content for P. bahamense is scarce. Only two reports on toxin profiles in natural samples exist. The first one is from El Salvador, during a bloom in 2005–2006 that caused human intoxications and mortality of sea turtles (Licea et al., 2008). The second report is from phytoplankton samples of a HAB in the coast of Guerrero, Mexico in 2010. Sulfocarbamoyl analogs dominated (>80 mol%), followed by STX and GTX3, with a contribution of 12.0 and 6.8 mol%, respectively (Gárate-Lizárraga et al., 2012).
In 1987, during a HAB in Guatemala, the clam Amphichaena kindermanni presented mainly B1 toxin, accompanied by small amounts of STX and NEO (Rodríguez et al., 1990). The net toxicity calculated from the concentrations of these three toxins corresponded to 7,500 mg STX dihydrochloride 100 g−1; 93.7 times above the maximum level for most regulations in Latin American countries that limits PSTs content at 80 μg STXeq 100 g−1 (400 MU 100 g−1) for human consumption. During this event, 175 people were intoxicated, and 26 people died (Rosales-Loessener, 1989).
In 1991 and 1993, in Uruguay, toxin profile of M. edulis was linked to HABs of this species. The mollusk presented C1/2 (up to 53 mol%), with significant amounts of GTX2/3, and STX, while D. hanleyanus presented decarbamoyl analogs, mainly dcGTX2 (~63 mol%) (Méndez et al., 2001).
In Mexico, most of the human intoxications related with this species have been due to the consumption of diverse bivalve species (Saldate-Castañeda et al., 1991; Cortés-Altamirano et al., 1993; Gárate-Lizárraga et al., 2012). In 1995, in BACA, STX, GTX2, dcGTX2/3, dcSTX as well as B1 were reported in the oyster C. iridescens (Núñez-Vázquez et al., 2007a). In 2010, a dominance of sulfocarbamoyl analogs (59 mol%), mainly B1/2 and C1, followed by STX (12%) and GTX3 (6.8%) were recorded in C. mexicana, in the same bay (Gárate-Lizárraga et al., 2012), with toxin concentrations ranging from 579.0 to 894.7 μgSTX 100 g−1. In 2001, in the coasts of Chiapas, STX, NEO, GTX2/3, B1 were detected in D. gracilis, and STX, GTX2/3 and B1 in M. capax (Núñez-Vázquez et al., 2007a). No data on toxin concentrations were given.
During a bloom in Nicaragua, 45 people developed symptoms of PSP with one fatal case in 2005. The toxin profile of A. tuberculosa presented STX as the dominant toxin with small quantities of NEO and B1 (Callejas et al., 2015).
Molecular Studies
Limited genetic information exists regarding P. bahamense. Morphological differences, as well as the LSUrDNA sequences were analyzed in vegetative cells and cysts of both varieties: compressum and bahamense, from distinct geographic regions such as Jamaica, Puerto Rico, Guatemala and Colombia (Mertens et al., 2015). No morphological differences between vegetative cells of the isolates were found. Cyst morphology, however, showed differences between specimens from Indo-Pacific and Atlantic-Caribbean regions. Results agreed with ribosomal sequences, where a distinct ribotype was identified for each geographical region. Based on these results, the authors suggested that it is a species complex.
As many other dinoflagellates, this species has bioluminescence capacity. Molecular diversity based on 18S rRNA and on the luciferase gen (lcf) was examined, using single cell isolates from Florida, USA and Puerto Rico. Phylogenetic analysis revealed that all sequences clustered together and were closely related to Alexandrium spp. With lcf sequences from Florida and Puerto Rico, two distinct clusters formed, defined by a set of core amino acid substitutions, and the inclusion of lcf sequences from the Indo-Pacific strain resulted in a third cluster. Lcf sequences of P. bahamense were more closely related to Pyrocystis spp. than Alexandrium spp. suggesting a much greater variation than that seen in bioluminescent species with known gene variants (Cusick et al., 2016).
Alexandrium Species
One of the most studied harmful algal blooming genera worldwide is the genus Alexandrium. More than 30 species have been defined and at least half of them are known to be toxic or have harmful effects (Anderson et al., 2012). Three different families of known toxins are produced by species of this genus: STX, spirolides, and goniodomins. In LAm this genus is widely distributed (Figure 2), and several toxic species have been reported: A. catenella, A. minutum, A. monilatum, A. ostenfeldii, A. tamarense, and A. tamiyavanichi. There are also reports of A. peruvianum, however Kremp et al. (2014) proposed that A. peruvianum should be considered as a synonym of A. ostenfeldii due to inconsistent morphological and gradual genetic divergence of groups, together with no evidence of compensatory base changes indicating reproductive isolation (Kremp et al., 2014). For the purpose of this review A. peruvianum is considered as a synonym of A. ostenfeldii.
HABs of Alexandrium are mostly reported in South America and the main responsible species are A. catenella (Chile) and A. tamarense (Uruguay, Argentina). The intensity and frequency of these HABs have increased since 1990, causing severe economic losses in the fishing and aquaculture sector (Álvarez et al., 2009; Menezes et al., 2010; Aguilera-Belmonte et al., 2011, 2013; Mardones et al., 2015).
A total of 350 cases have corresponded to the consumption of seafood associated with HABs caused by Alexandrium species in South America (Table 1). Chile is the most affected country; HABs in this area have also caused massive mortalities of invertebrates, fish, both wild and cultivated, as well as seabirds and whales.
Bloom Dynamics
Chile
In Chile, the main Alexandrium species reported is A. catenella. From 1972 to 1994, outbreaks in the MAGR and Aysén regions (AYSR) were attributed to this species. HABs have increased in their frequency, extension, duration and intensity (Guzmán et al., 2002, 2010). Until this date the distribution limits of this species were: Cailin in the southern sector of Los Lagos region, and in the north Seno Ponsonby, in the MAGR. In October 1972, after a period of high irradiance and thermohaline stratification, a HAB lasted for 5 weeks, with densities from 2.4 to 6 × 105 cell L−1 at the surface, and 157 × 102 and 3201 × 102 cell L−1 in the sub-surface layer (5–10 m), respectively. The same hydrographic conditions were recorded in Bahía Bell in 1973, when this species bloomed. In subsequent years (1972–1994) HABs continued emerging (Guzmán et al., 2002). The authors suggested that blooms occurred in response to global changes related to El Niño, which causes a decrease in salinity and stronger stratification, proposing that blooms are related to the terminal period of La Niña and the initiation of intense El Niño episodes. The authors concluded that this dinoflagellate is able to bloom under calm conditions, high insolation and a stable water column.
There is a list of data of HABs of this species from 1994 in the MAGR, with maximum abundance values of 30,902 cell L−1, and in the AYSR of 5,808 cell L−1. Cells are found from spring to autumn, when high temperatures trigger the blooms (Guzmán et al., 2002). From 1993 to 1998, also in AYSR, several dinoflagellates were identified, including A. catenella. Temperature ranged from 5.2 to 16.2°C; the lowest temperatures were recorded in 1996, and the highest in 1994 and 1998. HABs coincided with a temperature from 11 to 14°C, mainly between December and April, being more frequent in February (Cassis et al., 2002). Data suggested that 1998 was an anomalous year that presented an extensive HAB that covered the entire south of the country in March, overlapping with positive anomalous values. This event caused 20 human poisonings, two fatalities and losses of over USD 10 M to the salmon industry (Mardones et al., 2015).
From 1995 to 2002, several outbreaks with marked seasonality were reported in internal waters of the NW Patagonia (Molinet et al., 2003). Blooms occurred mainly from January to March, with a tendency to expand its distribution northwards. These events were attributed to the presence of cysts banks, and associated to thermal oscillations in adjacent ocean waters that affected the circulation in inland waters. Cyst dispersion was associated to the drift of surface waters, caused mainly by winds and circulation of inland waters (Molinet et al., 2003). Also, the structure of the water column suggested that these HABs originated from the mixture of Antarctic and sub-Antarctic surface waters, derived by wind action. Cyst germination is considered relevant in the recurrent HABs in inland seas, as well as in the fjords and MAGR channels (Uribe et al., 2010). In 2005 and 2006, another bloom in the X region occurred at 12°C (Fuentes et al., 2008). Data suggested that stratification conditions favored dinoflagellates growth and coincided with the presence of PSTs (Díaz et al., 2014).
In March 2009, a HAB covered a wide geographical area from 46° to 43° 45′ S. During late February in Easter Island, this dinoflagellate reached a maximum of 2230 cell mL−1; in March, in the same geographic region it reached 12 × 102 cell mL−1, and in Cuptana 6 × 103 cell mL−1. These data demonstrated an expansion of the northern distribution of this species with respect to their historical distribution range, being the first record of A. catenella in oceanic waters of the Pacific coast SE outside Chiloé Island. The new distribution suggested a cell transport toward inland waters through Chacao channel. Cells probably also reached Chiloé water fjord and channels, by oceanic water masses (Mardones et al., 2010). Northern distribution of this species continues expanding, during the last intense bloom of A. catenella, in the late summer of 2016, PSP-affected areas reached for the first time as far north as Los Ríos (39°S) (Hernández et al., 2016).
In the Southern Austral Ecosystem, this species was associated to temperatures between 9 and 13°C and salinities of 28 and 33 (Cruzat et al., 2018), with higher abundances at 10°C. The high genetic diversity in terms of population differentiation suggested a possible recent dispersion. Many factors could be influencing this dispersion, as the frequent use of transportation related with aquaculture operations, ocean currents, and natural dispersion. In the AYSR there has been a tendency of intense HABs in summer. Cysts play a key factor for repopulation during spring-summer and they can be re-suspended by the advection of the water column (Pizarro et al., 2018). In southern Chile fjords, resting cyst of A. catenella are sparce and there have been doubts on their importance in the recurrence of massive toxic dinoflagellate blooms in the region. Mardones et al. (2016) under laboratory conditions demonstrated with strains isolated from this region that moderate vegetative cell abundances (>400 cell mL−1) can produce high amounts of cysts which have a short dormancy period (minimum 69 days). These results are in agreement with the finding by Díaz et al. (2014) of empty cysts found a few months (~3 months) after a bloom event, representing less than 10%. Mardones et al. (2016) suggest that the short cyst dormancy for Chilean strains explains the rapid cyst depletion from the sediments of the inner fjords. However, dense cyst aggregation cysts can be accumulated and preserved in selected areas of the fjords. Authors point out the need to investigate the oceanographic conditions that lead to massive outbreaks.
Argentina
In the Argentine sea, A. tamarense is the most frequently reported harmful dinoflagellate species. During 1980–1984, several HABs of this species were reported. In 1980 a high abundance (1.8 × 106 cell L−1) restricted to a hydrographic frontal system was found outside PVAL, characterized by high nutrients and turbulence (Carreto et al., 1985). The initial outbreaks expanded widely, and covered most of the Argentine coasts, in the frontal area, where vegetative cells, cysts, and high levels of toxicity are found. Also, a decoupling zooplankton-phytoplankton arrangement was found, being the main predator the dinoflagellate Polykrikos schwartzii (Carreto et al., 1985). In 1981, a HAB lasted 4 months (September to December), and was also restricted to a frontal system. In areas with stratified waters, cysts were found in sediments and were re-suspended during the mixing of the water column. Also seedbeds of hypnozygotes were observed which are known to serve as dispersion centers and increase the extension of toxic areas (Carreto et al., 1985). In 1995, during an annual cycle, cysts were found most of the year in coastal waters in MPlat (151–2758 cysts cm−3) (Carreto et al., 1998a). In situ germination was the main source of vegetative cells that were initially found in August at 30 m (80 cells L−1) depth and in September at 0 m (15 × 103 cells L−1). A broad temperature range was recorded, from 9°C to 20°C in summer, during a well-developed thermocline. It has been suggested that the presence of vegetative cells in winter could be attributed to the biological mechanism that controls cyst germination before temperature rises (Carreto et al., 1998a). A retrospective analysis of HAB and toxicity concluded that most records of this species occur in a wide coastal strip from 34° to 47° S at a depth between 50 and 100 m. Maximum abundances are reported in the frontal system of the Patagonian tidal region. Advection transport from external waters toward the coast explained the accumulation of cells in the intertidal zone. The Patagonian current contributed to the northward spread of cysts, the thermocline, hydrographic fronts and ocean currents were also key factors for their growth and distribution (Carreto et al., 1998a).
In Golfo Nuevo (GNVO) this species was recorded in August 1995 (Gayoso, 2001), at a lower temperature (9°C), with a peak in September of the same year of 6.1 × 103 cell L−1. Cysts were recorded in surface sediments (300 cysts cm−3). Heavy precipitation in winter and high solar radiation during spring could have influenced the formation of HABs. However, during blooms in July and December 2000, with a maximum abundance of 21.8 × 103 cell L−1, and temperature from 9 to 12.5°C, a multiple linear regression of the cell abundance with environmental variables (solar radiation, temperature, wind speed, rainfall, and nutrients), did not show a high correlation (Gayoso and Fulco, 2006), remaining to determine the factors that influence its blooms in this region.
Waters in the north coast of the Patagonian region, are ecosystems poor in nitrates (Montoya et al., 2010). On the other hand, phosphate concentrations are relatively high throughout the year, reason why the N:P ratio is heavily imbalanced with respect to the Redfield ratio. On the contrary, nitrate concentration is high in the sub-Antarctic waters and the Patagonian current mainly in southern latitudes, where N limitation was not found during summer. Therefore, it is assumed that the nutrient ratio and cell toxin content from natural populations of A. tamarense are correlated with the availability of nutrients in the southern region of South America, and the toxin content is inversely correlated with water temperature.
Other Alexandrium species, such as A. catenella, A. excavatum and A. ostenfeldii have been reported in Argentina. During spring/summer of 1980 PSTs were detected for the first time in GNVO (Esteves et al., 1992). The responsible species was A. excavatum, with abundances from 7.5 × 105 to 3.1 × 103 cell L−1 during an upwelling event. The SST was 15°C and prevailing winds were from the SW (>20 km h−1). In late December northern winds dominated, the average temperature was 18.3 ± 0.9°C and salinity was 33.9. Nitrate concentration remained constant, 0.3 ± 0.1 μg-at L−1. However, in February nitrates, phosphates and ammonium raised to 1.9, 7.1, and 9.3 μg-at L−1, respectively.
In the Beagle Channel, during 2006 and 2007 A. catenella, A. tamarense and A. ostenfeldii were observed (Almandoz et al., 2011). Temperature ranged from 4.9 to 10.1°C, and salinity from 30.7 to 31.2. In 2006, nitrate and phosphate were higher in winter (15.2 and 1.4 μM, respectively). The highest abundance of A. tamarense was detected in spring 2006 with 11.7 × 105 cell L−1 (Almandoz et al., 2011). Outbreaks in the Beagle Channel of this dinoflagellate, have been proposed to have a relationship with the decrease in the ozone layer (Benavides et al., 1995). In the same region, in July 2005 and December 2006, A. ostenfeldii had a low abundance (5860 and 3850 cell L−1, respectively). Temperature and salinity varied between 7.5 and 10°C and 30–30.5. Considering the apparent absence of records of this species in the Antarctic waters, the Beagle channel is considered as the most southerly distribution of A. ostenfeldii (Almandoz et al., 2014).
Other Latin American Countries
In El Salvador, in September 2014 an intense HAB of M. cf. polykrikoides co-occurred with Alexandrium cf. globosum. Fish deaths were reported and attributed to M. polykrikoides (Alvarado et al., 2015).
In the Pacific coast of Colombia, in Bahía Tumaco and in the island Gorgona, in April/May 2001, an A. tamarense bloom with maximum abundances of 7.5 × 106 cell L−1 occurred (García-Hansen et al., 2004). A year afterwards, in March 2002, another event was recorded from northern Cabo Corrientes to Bahía Solano, with a lower abundance (1.6 × 106 cell L−1). In both occasions, temperature was between 24 and 24.6°C, and salinities between 33 and 34. These parameters were abnormal, since temperature was three degrees lower and salinity was 2.5 higher than the average for this region. The authors assumed that these conditions reflected upwelling events, and suggested that this could be a recently introduced species.
In Venezuela, A. tamarense was recorded forming HABs since 1972, during March and June with abundances of 1 × 106 cell L−1. Cells were also detected in mussel's viscera, in raft-cultures in the Gulf of Cariaco. In 1975, in April-March, this species proliferated with other dinoflagellates. This HAB was associated with the predominance of trade winds that influenced the water circulation, causing coastal upwellings. Another bloom occurred in the NW coast of island Margarita in May 1977, intoxicating 171 people.
In February 2010 and May 2014, in Peruvian shellfish extraction areas, HABs of Alexandrium were reported. In May 2012 A. ostenfeldii bloomed at a temperature between 18 to 23°C, phosphate concentration ranged from 0.7 to 11.6 μM and nitrate concentrations from 0.8 to 9.8 μM. In 2015, A. minutum was reported for the first time off the coast of Peru, with a maximum abundance of 16 × 106 L−1, suggesting that this species was transported by ballast water or that its presence had not been detected previously (Baylón et al., 2015).
In Brazil, the distribution of A. tamarense apparently expanded into the southern hemisphere during the last two decades. The first HAB was documented in 1996 (Persich et al., 2006). Through satellite images, a cold water front that moved from northern Uruguay to Brazil was detected, which may have been the transportation vector. The high similarity among toxic profiles of Alexandrium strains from Brazil and Uruguay, suggested a Uruguayan origin of the Brazilian strain. Also, the low number of cysts in Brazilian sediments supported the hypothesis of a recent dispersal from Uruguay. The authors concluded that the southern Brazilian coastline may have experienced additional outbreaks of PSP originated from local cyst germination or by recent populations that were transported by the Uruguayan coastal current (Persich et al., 2006). In 2007, A. minutum was registered on the coast of Río de Janeiro, with abundances from 4.3 × 105 to 1 × 102 cell L−1; cysts were also registered (Menezes et al., 2007).
In Uruguay, frequent HABs of A. tamarense were registered during the 90s. From 1991 to 1993, recurrent blooms were reported in early spring, near La Paloma, with abundances of 80 × 103 cell L−1. In the coastal area, in a transition zone between the stream and the Malvinas current, abundance was of 355 × 102 cell L−1 with a SST from 11 to 15°C (Méndez et al., 2001). A wide and strong thermal-saline front during spring-winter coincided with the HABs; blooms initiated when the discharge of the Río de la Plata decreased, as well as the front of the Falklands current (Méndez et al., 1996). From 1991 to 2004, HABs of A. tamarense and G. catenatum have been reported, blooms of Alexandrium occurred at abundances of 10 × 103 cells L−1, temperatures between 11 and 14°C, and salinities of 32.2.
Toxin Profiles in Environmental Samples
Alexandrium catenella
Paralytic toxins have affected ~35% of the Southern Pacific coast of Chile (Oyaneder-Terrazas et al., 2017). In 1996, samples of the mussel M. chilensis from the regions XI and XII, were analyzed, and in general they showed a molar dominance of GTX analogs, with a dominance of GTX2/3 in samples collected in the XI region, whereas GTX1/4 were the major analogs in the XII region (Lagos et al., 1996). The more potent analogs STX and NEO represented, together, 12 and 16 mol%, for the XI and XII region, respectively. A clear contrasting pattern was observed for the XII region when comparing M. chilensis profiles between years with a dominance of STX and NEO (45–46 mol%) in 1992 (Lagos et al., 1996). In several bivalves collected in 2012 in the AYSR (Region XI) a clear dominance of GTXs analogs was evident in most bivalves (García et al., 2015). Record toxicity values have been found in the AYSR with maximal values oscillating between 22,000 and 28,340 μg STX eq 100 g−1 (Guzmán et al., 2002; Molinet et al., 2003).
Compartmentalization of PSTs showed that GTX analogs are mainly found in the digestive tract. The case of M. chilensis is relevant since in the adductor muscle 99% (in molar basis) NEO was found, whereas in the mantle and digestive glands, GTX1/4 were detected. In a recent study of the PSTs content in regulated and non-regulated aquatic organisms from regions with a variable presence of A. catenella (Oyaneder-Terrazas et al., 2017), the analogs GTX1/4, GTX2/3, NEO, dcSTX, STX were detected. Dominant analogs in rocky strata-dwelling species were 58.8 mol% STX, followed by 15.4 mol% GTX2/3, 4.8 mol% NEO, and 3.3 mol% dcSTX; while in sandy bottom-dwelling species, 77.7 mol% of GTX2/3 were detected, 19.3 mol% STX, 2.1 mol% NEO and 0.9 mol% dcSTX. Data clearly showed the variability of toxin profiles in this zone and that this variation is not only associated to the sampling season, but also to the distinct basal analogs produced by this dinoflagellate as reported previously (Varela et al., 2012) and the bivalve species.
In phytoplankton samples, sulfocarbamoyl toxins C1/2 and B1 represented 70% in molar basis of the total analogs, followed by carbamoyl analogs GTX1/4 (24 mol%), contributing in smaller proportions GTX2/3 (≈3 mol%). NEO, STX, dcGTX2/3 and dcSTX were found in trace levels. Cell toxicity was estimated in ≈15 pg STXeq cell−1 (Oyaneder-Terrazas et al., 2017). The authors pointed out that this was a characteristic toxin profile for species blooming during January and March in the austral fjords of Chile. In other seasons the toxin profile differed, for instance, toxin profiles in spring predominantly had, in molar basis, β-epimers (C2, GTX4, GTX3), and in autumn α-epimers (C1, GTX1, GTX2) (Oyaneder-Terrazas et al., 2017), which is a bit surprising since it has been proven that these enantiomers do not reflect the original composition in plankton due to their easy epimerization under extraction and analytical conditions (Bustillos-Guzmán et al., 2015). Size fractionated plankton samples obtained from the XI region of Chile only presented sulfocarbamoyl analogs C1/2 in concentrations <0.7 ng μL−1 (Pizarro et al., 2018). The authors pointed out the low abundance of A. catenella during the cruise. In autumn, during an exceptional toxic event in the Beagle Channel in 1991/1992, the cell toxicity was calculated, also via mouse bioassay, to be 325 pg STXeq cell−l (Benavides et al., 1995).
In El Rincón, Argentina in 2015 during a bloom of Pseudonitzschia, cells of A. catenella and A. ostenfeldii were found in low numbers (up to 1 × 103 cell L−1). Phytoplankton net tows (NT) samples presented PST in low concentrations ranging between 114.4 ng NT−1 and 2593.8 ng NT−1. Toxin profiles were dominated by GTX1/4; C1/2 and GTX2/3 were also detected (Guinder et al., 2018).
Alexandrium tamarense
In Argentinian coasts, based on historical data and on shellfish toxicity, it was suggested that A. tamarense could grow offshore and that cells were transported inshore toward shellfish populations. This phenomena also occurred in the Uruguayan coast, where shellfish reached toxicity values between 80 and 10,000 μg STXeq 100 g−1, whereas in the Argentinian coast the toxicity values were higher, with exceptional values up to 50,000 μg STXeq 100 g−l (Carreto et al., 1998b). Toxin profile in phytoplankton samples during a bloom from MPlat to Bahía Grande, were dominated by GTX1/4 analogs (from 69.1 to 93.6 mol%), with an exception of a sample from MPlat, where GTX2/3 represented 88.5 mol% (Montoya et al., 2010). Sulfocarbamoyl toxins (C1/2) were scarce, and their contribution was <8.5 mol%. Cell toxicity varied between 9.8 and 93.0 pg STXeq cell−1. Toxin profiles contrasted with those obtained from culture strains, where sulfocarbamoyl analogs represented >60 mol%. Therefore, cell toxicity from field samples, in general, was higher when compared with cultured strains (1.8–10.3 pg STXeq cell−1) grown in nutrient replete conditions. These results were confirmed with NT samples from the Argentinian coast containing A. tamarense cells and minor quantities of A. aff. minutum, which again showed a higher concentration of GTXs (Fabro et al., 2017). Gonyautoxin concentrations (GTX1/4) ranged between 0.3 and 1104 ng NT−1 and GTX2/3 ranged between 0.1 and 31.7 ng NT−1. Cell quotas of total GTX estimated for A. tamarense species complex plus A. minutum were <0.8 pg cell−1. N-sulfocarbamoyl toxins (C1/2) were also detected at low concentrations. The high potency carbamoyl analogs STX and NEO were the least frequent analogs (Fabro et al., 2017).
Mussels (M. edulis) were sampled during two blooms, in spring and autumn. In both occasions, the toxin profile in mussels was dominated by GTX1/4 and to a lesser extent by C1/2 that constituted >80 mol% (Carreto et al., 2004). However, toxin profile changed according to the mussel toxin content. Thus whereas GTX1/4 represented more than 60 mol% during the maximum toxicity and detoxification rate period, an important increase of STX and GTX2/3 was observed at a low toxin content. Toxin profile changes reflected the compositional shifts resulting from differential toxin retention and toxin bioconversions within the mussel (Oshima, 1995). Maximum values of toxicity in the mussel were between 450 and 500 μg STXeq 100 g−1.
Other Alexandrium Species
In Trinidad and Tobago, PSTs were first reported in 1999 in the mussel Perna viridis from Chaguaramas (Yen et al., 2004). STX, dcSTX, GTX1/4, GTX2/3 and dcGTX2/3 were detected, with the last three toxins being the most abundant. During 2000, STX, dcSTX, NEO, GTX2/3, and dcGTX2/3 were detected, with the last three analogs in the highest concentrations. No causative organism was identified. Known PSTs-producing dinoflagellates in this region are A. catenella, A. tamarense, G. catenatum, and P. bahamense (Heileman and Siung-Chang, 1990; Sánchez-Suárez and Troncone-Osorio, 1994; La Barbera-Sánchez et al., 2004).
Although A. minutum has been reported in Peru and Argentina, to our knowledge, no further toxin research on this species has been performed. In Brazil only one bloom in the coast of Río de Janeiro in 2007 was analyzed, being NEO (73.8 mol%), GTX2/4 (22.6 mol%), and STX (3.6 mol%) the reported toxins (Menezes et al., 2007).
In the northern coast of Chile, phytoplankton toxin profiles related to Alexandrium blooms did not present PSTs (Álvarez et al., 2009). However, the mollusks Semimytilus algosus and Argopecten purpuratus presented low quantities of PSTs, between 27.5 and 34.4 μg STXeq 100 g−1 (determined by mouse bioassay). The toxin profile was characterized by the presence of C2 and GTX2/3 in both bivalves, concluding that A. catenella was unlikely to be the responsible species for these PSP events.
Autoecological Studies
Few strains of the genus Alexandrium have been isolated and autoecological studies of the group are scarce in LAm (Figure 3). Since Chile has been historically adversely affected by their blooming off its coasts, it is in this country where most of studies have been performed, followed by Argentina and Brazil.
Alexandrium catenella
The effect of temperature, salinity, L:D cycle, cyst or vegetative strains, and nutrients on the growth of A. catenella from diverse regions of Chile have been determined (Table 4) (Navarro et al., 2006; Aguilera-Belmonte et al., 2011, 2013; Ávila et al., 2015). In general, this species tolerates temperatures between 10 and 16°C, producing cell concentrations above 50,000 cell mL−1 at 15°C with growth rates from 0.54 to 0.73 div day−1 (Navarro et al., 2006; Aguilera-Belmonte et al., 2011, 2013); indicating that growth is temperature-dependent. Studies for the same species from other geographical sites have reported a higher optimal temperature for growth 20–25°C (Siu et al., 1997), the optimal temperature in this study being 14°C demonstrated its great capacity of this species to adapt to a wide range of temperatures. Maximum biomasses are reached between 15 and 65 days; this wide time difference could be related to the L:D cycle used in the different culture conditions or could be inherent to strain differences (Table 4) (Navarro et al., 2006; Aguilera-Belmonte et al., 2011, 2013). Their salinity tolerance is also wide; growing under laboratory conditions at salinities from 15 to 35. Chain formation was observed mainly during the first week of culture, reducing their presence (>7%) when cultures reached ~5,000 cell mL−1 (Navarro et al., 2006). With these studies, a wide range of tolerance to salinity and temperature was proved, which helps explain, at least partially, its ecological success.
The effect of temperature on the cell dry weight was also determined. Maximum biomass was inversely proportional to temperature, with values of 117.3 ± 0.9, 120.3 ± 3.2, 90.9 ± 0.6 and 23.3 ± 1.4 μg mL−1 at 10, 12, 14, and 16°C, respectively, with a direct relationship between cell dry weight and cell abundance. Cell dry weight was greater in the initial phase, decreased during the exponential phase, and increased again toward the terminal phase. The highest values for individual cell dry weights were obtained at 14°C (9.6 ± 1.1 ng cell−1, day 3) and at 10°C (9.5 ± 0.9 ng cell−1 day 3). The lowest were obtained at 12°C (5.9 ± 0.3 ng cell−1, day 3) and at 16°C (5.8 ± 1.7 ng cell−1, day 6). The authors suggested that the increase of cell weight during the growth phase could be explained by the presence of thecae of dead cells that added to the total weight of the culture and also to the increase in cell size (Navarro et al., 2006).
These results suggested this species has also a wide photo-acclimation and that it presents a lower range of thermal tolerance in relation to strains from other geographic regions. The optimal temperature range correlated well with ambient water temperatures in southern Chile, in which important blooms have been reported. Noteworthy differences have been detected in growth among populations from different geographical origins obtained from the same toxic event, concluding that abiotic factors can differentially affect the population dynamics of toxic genotypes, making it extremely difficult to predict the ecological behavior of this species in the field in terms of the intensity of a potential outbreak (Aguilera-Belmonte et al., 2011, 2013).
In 2015, Ávila and collaborators evidenced the effects of salinity, temperature, photoperiod and nutrients in cultures established from cysts. Cysts were isolated from Punta Yenecura, Los Lagos region, in Chile. Different experiments were designed, and only one parameter was tested at the time (salinity, temperature, photoperiod, nutrients) (details in Table 4). Maximum biomasses were never above 4,000 cell mL−1 and was reached between 28 and 51 days of culture; maximum abundances were found at 10 and 15°C and salinities of 30-31 with growth rates from 0.12 to 0.18 div day−1. No clear effect of the L:D cycle was observed on cell abundance, whereas growth rate was higher at 16:08 and 08:16 L:D. Higher abundances where obtained with L1 and L1/2 media, but where much lower than biomasses obtained with strains obtained from vegetative cells in other studies. This difference could also be explained by the lower light intensity used (Ávila et al., 2015) (Table 4). With strain crosses obtained from cysts collected in Los Lagos and AYSR during the massive HAB event, Mardones et al. (2016) using the same medium with and without N, and higher light intensities also obtained high growth rates (0.36 and 0.52 div day−1) (Table 4).
There is only one report of allelopathy regarding Alexandrium species from LAm. This study was performed with a strain of A. catenella from southern Chile (Arzul et al., 1999). The allelopathic and hemolytic effect of this, and other toxic Alexandrium species from Europe and Asia were determined on Gymnodinium mikimotoi, Scrippsiella trochoidea, and Chaetoceros gracile. Filtrates of A. catenella reduced the growth of G. mikimotoi and C. gracile, particularly in the exponential phase of culture, which was related with toxicity and haemolytic activity. No effect on S. trochoidea was observed. This information supported the environmental observations, where blooms of the later usually follow a PSP outbreak caused by A. catenella (Arzul et al., 1999).
Alexandrium tamarense
The growth rate under different N and P regimes in A. tamarense from MPlat was determined. The strain was compared with strains of A. tamarense, A. minutum, and A. anderssoni from Spain. The Argentinian isolate presented the lowest affinity for P. The half saturation coefficient (Ks) and the P concentration to which the specific culture cell growth rate was zero (Kmin) under P limitation were 1.68 and 0.48, respectively, which were higher than the Ks and Kmin of the other strains. The lower affinity for P indicated that the Argentinian isolate has a lower competitive ability under P limitation. The average abundance for this strain under non-nutrient limitation was 1,924 cell mL−1, with growth rates from 0.22 to 0.28 div day−1 (Table 4). These values were also lower than those reported previously for A. catenella, with the exception of a strain obtained from cyst germination (Frangópulos et al., 2004).
Other Alexandrium Species
Another study reported a negative experimental effect on copepod nauplii larvae, and larvae of gastropods and tintinnids. Exudates of A. tamiyavanichii decreased the survival of copepod larvae, suggesting that the dinoflagellate has an impact on the trophic chain (Silva et al., 2013).
Toxin Profiles in Alexandrium Species Under Laboratory Conditions
Several studies on toxin profiles have been performed with Alexandrium species in LAm, mostly in countries where the blooms of species of this genus are common and are an important threat for the economic activities.
Alexandrium catenella
This species is particularly important along the Chilean coasts, and many research groups have performed toxin analysis in cultures for more than one decade. Data from toxin profiles, toxicity and toxin content are available in Tables 5, 6, and the origins of the isolates are indicated in Figure 3.
One of the first autoecological studies performed in LAm was done to determine the role of temperature in growth rate, cell density and toxicity (Navarro et al., 2006). Cells of A. catenella isolated from the AYSR were cultured in L1 medium at four temperatures: 10, 12, 14, and 16°C. Toxins were extracted with 0.1 M HCl and later HCl 1 M was added. Toxicity was measured using patch clamp electrophysiology with human embryonic kidney cells expressing STX-sensitive rat skeletal muscle NaV channels. Toxin content was variable during the experiment, but an inverse relationship was noted between temperature and toxin content, with the highest toxin content found at 10°C, and the lowest at 16°C. According to Cembella (1998), low temperatures contribute to higher PSTs levels, making Alexandrium strains from higher latitudes more toxic, which is probably related to effects on metabolic processes (Navarro et al., 2006).
Among the toxinological studies performed on Alexandrium species in LAm, Brazilian isolates have been analyzed. Persich et al. (2006) worked on Alexandrium isolates and reported the species as A. tamarense, but later molecular studies confirmed them as A. catenella (Menezes et al., 2018). Five isolates were cultured from cysts and one from vegetative cells, in f/2 medium at 20°C. Toxins were extracted with 0.05 M acetic acid and analyzed by LC-FLD with post-column derivatization. Toxin content ranged from 42 to 199 fmol cell−1 and toxicity varied between 7.1 to 65.9 pg STXeq cell−1, except for the culture originated from vegetative cells, which showed higher toxicity, mainly due to the higher GTX4 content (Persich et al., 2006).
Krock et al. (2007) analyzed the toxin composition of an isolate from the coastal Channel, XI Region of Chile. Toxins were extracted in 0.03 M acetic acid and analyzed by LC-FLD and LC-MS/MS. For the first time both techniques were used for comparison and precision. Detected toxins were C1/2, GTX1/7, B1, GTX2/3, NEO, and B2, in descending order according to their concentration. STX, dcSTX, dcGTX2/3, and C3/4 were present only in trace amounts (<1 mol%). Alexandrium catenella is considered the predominant, or even exclusive, source of PSTs in central Chile, and its toxin profile agreed with other profiles from populations from the Pacific Ocean in the northern hemisphere (Krock et al., 2007).
Aguilera-Belmonte et al. (2011) compared the phenotypic and genotypic characteristics, including toxin profiles of seven isolates from the same HAB event, but from different geographical zones. Isolates were cultured in L1 medium at 15 ± 1°C. Toxin analysis was performed by LC-FLD on 0.05 M acetic acid extracts. N-sulfocarbamoyl analogs were detected after hydrolysis. All isolates had different toxin profiles, but most of them showed high proportions of GTX1/4 followed by GTX2/3. In three out of seven isolates they reported B1, B2, and C toxins (43 mol%). In two, NEO and STX (29 mol%) (Aguilera-Belmonte et al., 2011), showing large intraspecific variability, as reported within a variety of dinoflagellates, even among clones from the same geographic area (Tillmann et al., 2009; Seok Jin et al., 2010). In addition, they found a cosmopolitan distribution of the morphospecies within the North American Clade, though interesting differences were found in their toxin profiles (Aguilera-Belmonte et al., 2011).
Research on these isolates continued, but the aim of this study was to analyze four combined conditions: two temperatures (10 and 15°C) and two salinities (15 and 35). Samples were extracted with 0.05 M acetic acid. Toxins were analyzed by UPLC-FLD, and samples from the four replicates were pooled to cover intrinsic variability among them (Aguilera-Belmonte et al., 2013). Toxin content varied widely among isolates, treatments, salinities and culture age. During stationary phase, most isolates accumulated toxins (from 1.4 to 4.5 times) compared to the exponential phase, with three exceptions of the sixteen analyses (19%). Concerning the toxin profile, NEO, GTX2/3 and GTX1/4 represented more than 85 mol% of the toxin content in all strains and culture conditions. B1 was detected only in one isolate from the AYSR, and this analog was detected only when cultured at 10°C. Interestingly, the highest toxin contents were reached at this temperature (in agreement with Navarro et al., 2006) and a salinity of 35. This isolate also presented the lowest (non-detectable) and the highest toxin content (239 fmol cell−1) at those culture parameters, being the culture age the only difference (exponential and stationary, respectively) (Aguilera-Belmonte et al., 2013). The scale of the combined effects of temperature and salinity was not clear, and differences were found among strains during all the experimental process, even though, two of the isolates were apparently genetically identical at least in their nuclear ribosomal ITS sequences (Aguilera-Belmonte et al., 2011). Results showed differential effects of temperature and salinity on growth parameters and toxin content, being the first more affected by salinity and the second by temperature.
Toxin profiles research continued, and toxin profiles from cultures originated from cysts and vegetative cells from different Chilean zones (Los Lagos, AYSR, and MAGR) and different years of isolation were compared. Analyses were carried out in 0.05 M acetic acid extracts and separated by LC, but the detector is not reported. Identified toxins were STX, dcSTX, NEO, GTX1/4, GTX2/3, B1, B2, C1-3, though STX and dcSTX were reported only in trace amounts and not in all isolates (Varela et al., 2012). The authors recognized two toxin production patterns in the strains, according to their toxin profile: one constituted by a group where analogs C1/2 accounted for most of the toxin content (47–74%), and a second one, where GTX1/4 accounted for the main analogs (54–87%). This second group was also conspicuous because of the absolute absence of B1, previously reported as distinctive for Chilean isolates (Montoya et al., 2010). They also reported morphological, toxinological and genetic differences among strains of this species, in accordance to other studies that have also characterized the diversity of the species in other regions (MacKenzie et al., 2004; Orlova et al., 2007).
A recent work from an oceanographic expedition in 2015, reports the isolation of four Alexandrium strains from offshore the Argentine coasts, near Bahía Blanca Estuary, south Mar del Plata. The cells were isolated in 1/10 K medium, later transferred to 1/2 K medium flasks, and incubated at 15°C and 50 μmol photons m−2s−1 on a 16:8 LD photocycle. One of the four isolates was identified as A. catenella/tamarense morphotype, but further morphological and phylogenetic analysis conformed to the description of A. catenella. Toxin profile from this one culture was dominated by C1/2 (72%), GTX1/4 (15%), NEO (9%), GTX2/3 (2%), and STX (2%) (Guinder et al., 2018).
These studies confirm that the only way to differentiate species from the Alexandrium tamarense/fundyense/catenella complex is by the use of molecular tools, and also, that more studies on toxin production are needed in the area. The presence of toxigenic planktonic species has been reported, and this indicates a hazard for fisheries, shellfish production and harvest and for human and animal health.
Alexandrium tamarense
Carreto et al. (1996) isolated vegetative cells from MPlat and compared their toxin profile with the toxin profile of mussels (M. edulis) and marine snails [Zidona angulata and Adelomedon (Pachycymbiola) brasiliana]. Toxin profiles were analyzed in 0.1 M acetic acid extracts from dinoflagellate cells by LC-FLD with post column derivatization. They reported C1/2 toxins as the most abundant, followed by GTX1/4, NEO, GTX2/3, and dcGTX2/3; STX was also reported, but as a small fraction. Toxin profiles in gastropods were highly dissimilar to those from the dinoflagellate, confirming metabolic changes upon the ingested toxin cell content, though all presented high toxin levels, but mainly from STX with the exception of M. edulis that presented mostly GTX analogs (Carreto et al., 1996). This study evidenced the importance of toxin analysis in gastropods.
In Brazil, toxin content of isolates were determined. Five strains were cultured from cysts and one from vegetative cells, in f/2 medium at 20°C. Toxins were extracted with 0.05 M acetic acid and analyzed by LC-FLD with post-column derivatization. Toxin content ranged from 42 to 199 fmol cell−1 and toxicity varied between 7.1 to 65.9 pg STXeq cell−1, except for the culture originated from vegetative cells, which showed higher toxicity, mainly due to the higher GTX4 content (Persich et al., 2006).
In 2010, a comparative toxin profile study from cultured strains and natural populations was performed. Cultures were established from vegetative cells isolated from MPlat, GNVO, Golfo de San José and off Península Valdés (PVAL), and maintained in f/2 medium at 15°C and 12:12 L:D. At the same time, they sampled in MPlat, close to PVAL, Bahía Camarones and Bahía Grande. They found toxins in all isolates and in natural samples, though those from natural samples were significantly more toxic, and the highest toxicity was found in natural samples from open coastal waters in comparison with semi-enclosed water bodies such as bays or gulfs (Montoya et al., 2010). In general, natural samples produced more carbamoyl analogs whereas cultures produced more of the less potent N-sulfocarbamoyl toxins C1/2. A clear correlation (r2 = 0.993) between nitrate concentration and GTX1/4 in natural samples was found, while GTX2/3 decreased significantly (r2 = 0.987). Nevertheless, in culture, where nitrate concentration is higher than in natural conditions, the production of GTXs was considerably lower. When temperature and toxin content was compared, an inverse linear correlation (r2 = 0.989) was found, in agreement with previous studies on the genus.
The fact that natural populations were notably more toxic than those in culture raises the question about the natural factors involved, and one could be associated to bacteria (Uribe and Espejo, 2003), and that natural conditions are far from being imitated in culture. Also, the fact that populations from open waters (e.g., out from PVAL) were more toxic than those from enclosed water habitats remains enigmatic, and is probably related to specific responses to environmental conditions (Montoya et al., 2010). In many cases, cultured Alexandrium cells are less toxic than those from natural environments, and they can lose toxin production during routine culture maintenance, which has been stated previously, and this has led to the hypothesis that some aspects on toxin production might not be genetically determined (Ogata et al., 1987). Nevertheless, field samples displayed important variability just as in other Alexandrium species and other toxin-producing dinoflagellates, like G. catenatum. Authors stated a clear need of further research on toxin-producing species to reach a better understanding on toxin metabolism, changes within culture conditions, and significance of environmental conditions and associated bacteria (Uribe and Espejo, 2003).
In 2012, during a research cruise, Krock et al. (2007) sampled 48 stations along the Argentinian coast. From one station, outside Golfo de San Jorge, they isolated A. tamarense and established two clonal cultures using half strength K medium, 15°C and 16:08 L:D. PSTs were analyzed from 0.03 M acetic acid extraction by LC-FLD, according to previous methodology. Toxin profiles of the cultures were almost identical, and consisted of GTX1/4, C1/2 and minor proportions of NEO, GTX2/3, and STX (Krock et al., 2015). This profile has been previously reported for Argentinean strains in culture and natural populations (Carreto et al., 1996; Montoya et al., 2010). In comparison, toxin profiles in field samples consisted only of GTX2/3, while this toxin accounted for minor proportion in cultures. One possible explanation for these results could be that a chemical conversion due to storage conditions, since field samples were analyzed 3 months after the sampling and were transported from Argentina to Germany, where the analysis was performed. Nevertheless, it is interesting that not only the lateral chain was lost in toxins C1/2 to be converted into GTX2/3, but a dehydroxilation implying the loss of the N-1 hydroxyl group on GTX1/4 and NEO happened, and were converted into GTX2/3 and STX, respectively. Undeniably, further studies are needed under controlled conditions to clarify these processes (Krock et al., 2015).
Alexandrium ostenfeldii
During the expedition carried out in 2015 by Guinder et al. (2018), three of the isolates were identified as A. ostenfeldii. Cells from this species were isolated as mentioned (in 1/10 K medium and transferred to 1/2 K medium flasks and incubated at 15°C and 50 μmol photons m−2s−1 on a 16:8 LD photocycle). These cultures were specifically analyzed for spiroimines and also for PSTs (Guinder et al., 2018).
Toxin analysis from these cultured strains showed GTX2/3, C1/2 and STX as the most abundant toxins (no percentages reported). The three isolates produced six spirolides, being SPX-1 the most abundant (83–93% of the total). The authors reported a novel analog, and named it spirolide M. The other four are still uncharacterized (named “compounds 1–4”) (Guinder et al., 2018). The novel spirolides produced by the A. ostenfeldii isolates highlight the richness and structure variability of these toxins, and there are probably more analogs than previously thought, as often seen in toxin-producing plankton. This is the first toxinological research on A. ostenfeldii at higher latitudes of the Patagonian shelf, and the other isolated strain from a southern geographical area (Beagle Channel) did not produce PSTs, but only spirolides (Almandoz et al., 2014).
Nevertheless, during this expedition the PST profiles from field plankton samples and those from the isolates did not match. The dominant toxins in field samples were GTX1/4, while the dominant toxins in cultures, including A. catenella and A. ostenfeldii, were C1/2 and GTX2/3, respectively. According to the authors, this discrepancy could be the result of a lack of detection of GTX1/4 due to the high limit of detection (LOD) for this epimeric pair. Other possibilities are that the profile changed due to the growth conditions in culture or due to other PSTs producing organisms that have not been reported in the SW Atlantic (Guinder et al., 2018), which highlights the importance of enhanced research in the area.
Salgado et al. (2015) studied a Chilean strain of A. ostenfeldii. The authors analyzed and compared morphology and toxin production of three strains from the Baltic Sea (Finland), the Mediterranean Sea (Spain) and the Chilean coast. Strains were cultured L1 medium with a photoperiod of 12:12 L:D and different temperatures and salinities were tested, in a total of nine treatments for each isolate. Paralytic shellfish toxins were extracted with 0.05 M acetic acid, and analyzed by LC-FLD. For detection of N-sulfocarbamoyl toxins, a fraction of the acid extract was hydrolyzed. Less polar toxins SPX and GYM, were extracted with 100% methanol and LC coupled to high resolution mass spectrometry (LC-HRMS) was used. Analysis showed PSP toxins in Chilean and Baltic isolates, but no detectable amounts in the Mediterranean strain; PSP toxin profiles were similar in both strains but the Chilean isolate had a greater toxin content than the Baltic strain (4.0 ± 1.9 pg cell−1). These results showed a significant correlation of toxin content and cell biovolume; as stated in previous studies with A. catenella from Chile, A. ostenfeldii showed higher toxin values when cultured at lower temperature (10°C), also at this temperature the cell volume was larger. In both isolates, detected toxins were GTX2/3 and STX. Trace amounts of dcSTX were found in all treatments except at 10°C and at a salinity of 32, where toxin content and cell biovolume were highest. Toxin profile and proportions did not change significantly among treatments (Salgado et al., 2015). Only the Mediterranean isolate produced detectable amounts of SPX, and only the Baltic isolate produced GYM toxins. Treatments affected mostly cell growth and toxin content, but no changes in the toxin profile were observed. The significantly high PSP toxin content detected in the Chilean isolate (max. 279.8 pg cell−1) suggested that this species could be more toxic than isolates previously reported, and should be further analyzed given the natural conditions of the region, where water temperatures of 10°C and salinity of 32 are common (Almandoz et al., 2014). However, the three isolates showed great variations in toxin profiles in all treatments, suggesting this species has a great capability of adaptation, as reported for A. catenella. So far, information on this species complex in LAm is still scarce and there is a great need for more studies (Salgado et al., 2015).
Alexandrium tamiyavanichi
Only one study on A. tamiyavanichi toxin analysis has been reported in LAm. Toxins were extracted with 0.03 M acetic acid, and analyzed with LC-FLD. Analogs STX, NEO, GTX1-5, and dcGTX2/3 were searched for, but N-sulfocarbamoyl toxins were not analyzed. Reported toxins were STX, NEO, and GTX4 as the main components, followed by GTX3 and dcGTX2/3 (Menezes et al., 2010). In their study, despite general morphological characters agreed with those reported for A. tamiyavanichi, they detected important variability that could also match with the morphology of A. cohorticula, suggesting the possibility of being a conspecific species, pointing out the need of genetic studies on wild and cultured populations to confirm their taxonomic identity, given that the use of morphological characters as the only tool for identification has caused previous misidentifications. Their phylogenetic analysis supported their identification as A. tamiyavanichi, even when the species has not been reported in the Atlantic coasts of South America. Previously A. tamiyavanichi has been reported in Asian countries (see Menezes et al., 2010 and references therein), and the possible explanation for this finding, given the lack of previous records of the species, could be that it has been part of the cryptic flora and that this zone has been part of their natural biogeography. The report of this toxin-producing species account to four PSTs producing dinoflagellates in the Brazilian coasts, where more studies are needed (Menezes et al., 2010).
Molecular Studies
Molecular markers have been particularly useful for discerning Alexandrium species when morphological traits are not sufficient, to know the genetic diversity among populations, or to determine phylogenetic relationships among species geographically distant. In LAm the few genetic studies in Alexandrium species have allowed to corroborate taxonomic questions, biogeographic history, and changes in distribution patterns. A classic study related with molecular phylogeny of Alexandrium genus in North America was published by Scholin et al. (1995). Alexandrium catenella and A. tamarense belong to the species complex A. catenella/tamarense/fundyense; this group has reached relevance in South America due to its impact on fishing and aquaculture activities. The molecular markers developed by these authors are still used to answer current taxonomic and phylogenetic issues (Scholin et al., 1995).
The first attempt to obtain sequences of LSUrDNA of A. catenella was carried out in Chile. Sequences showed at least two different strains of the species that bloom in this region, with a proximity to the North American ribotype and distant proximity from the Asian ribotype (Córdova and Müller, 2002). Six years later, Uribe et al. (2008), constructed cDNA libraries from axenic strains from the AYSR in Chile, obtaining 10,850 expressed sequence tags (ESTs), where the most expressed genes corresponded to proteins coding for bioluminescence, carbohydrates, and those associated with photosynthesis (Uribe et al., 2008). They also carried out a detailed analysis of bioluminescent proteins: luciferin-binding protein and luciferase, additionally two unigenes presented 100% identity with a toxic strain-specific sequence of A. tamarense. Moreover, ESTs of A. catenella were closely related to A. tamarense. Taking into account that bioluminescence proteins were among the most expressed genes in A. catenella, the authors suggested that probably bioluminescence could be related with physiological responses such as predation avoidance and cell communication (Uribe et al., 2008).
Aguilera-Belmonte et al. (2011) compared physiological and genetic variability in populations of A. catenella from southern Chile isolated from the same toxic event. A remarkable variability at the genetic and physiological level among strains was observed. Chilean strains showed a higher genetic diversity (3%) than other strains of the world, and differences on PSTs analogs were detected between strains. Intraregional diversity of this species, partial sequences of the LSU gene, and ITS regions of rDNA, as well as toxicological and morphological analysis, have been evaluated. The analysis of rDNA sequences of Chilean strains were separated as part of the Clade I (North American) of the A. tamarense species complex (Varela et al., 2012). However, a significant genetic diversity was observed between Chilean strains with ITS sequences. Although morphological variations within and between strains were observed, some features were absent, such as a ventral pore in the 1' plate, which was a distinctive characteristic in Chilean strains. These approaches indicate a significant intraregional variability, however this genetic diversity does not agree with the supposed northward expansion along the west coast of South America.
Sequences from the ITS1, 5.8S rDNA, ITS2, and D1-D5 LSUrDNA regions, were used to identify strains of the morphospecies A. catenella as A. tamarense. Species-specific primers designed for real time PCR were a good molecular tool to detect this dinoflagellate in bivalves such as M. edulis (Jedlicki et al., 2012).
Persich et al. (2006) investigated the probable origin of A. tamarense in Brazil, and found a close relationship between this species with A. tamarense, A. fundyense and A. catenella from North America, northern Europe and northern Asia (Scholin et al., 1994; Medlin et al., 1998). Interestingly, they did not find any close relationship to any of the A. tamarense complex from the southern hemisphere. According to the molecular information, they proposed that the isolates used for this study were probably transported from Uruguayan waters during coastal fronts in Argentina and Uruguay; they considered the possibility of further outbreaks from transported cells or resting cysts in Brazilian coasts (Persich et al., 2006). Even though the origin of the so-called “North American type” Alexandrium is not clear, DNA results in this study support the hypothesis of an early establishment of these populations during a period of cooler global oceans, when natural boundaries did not exist and transport across hemispheres was possible. This hypothesis was proposed since the LSUrDNA sequences from South American isolates have enough differences from the North American modern populations that may indicate that these populations have been separated for a long time, and evolutionary processes have occurred (Persich et al., 2006).
Recently, Fabro et al. (2017) carried out morphological and genetic analyses of three morphospecies of Alexandrium from the Argentinean Sea. Their results showed some variations in morphological characteristics, but were consistent with classical descriptions of A. tamarense and A. ostenfeldii, however cells of A. minutum had morphological traits that did not agree completely with the description from Balech (Balech, 1995; Fabro et al., 2017). Using qPCR method, it was possible to corroborate the presence of A. minutum and A. ostenfeldii in NT samples. While partial sequences of the LSUrRNA of the A. tamarense complex clustered within the Alexandrium ribotype Group I, and were consistent with A. catenella. This was the first report of A. minutum and A. ostenfeldii on this area of Argentinean Sea (Fabro et al., 2017).
Cruzat et al. (2018) determined genetic variability of A. catenella analyzing ITS1-5.8S-ITS2 sequences of ribosomal DNA of environmental samples from the Southern Austral ecosystem. The authors found 33 haplotypes, three of these highly frequent, increasing the genetic diversity from 2.8 to 3.1%, for this species in the study area. All sequences agreed with the morphological identification for A. catenella, with sub-clades that correspond to haplotypes from distinct geographic regions (Cruzat et al., 2018).
Toxin profiles, morphology and phylogeny were also investigated by Salgado et al. (2015) in three strains of A. ostenfeldii from the Baltic, Mediterranean, and southern Chile. The phylogenetic reconstruction was performed with LSUrRNA sequences, showing a geographic distribution congruent with the selected strains. Strain AOA32 from Chile only produced PSTs, and had a close phylogenetic relationship with a strain from Callao, Peru (Salgado et al., 2015).
Using morphological traits and LSU and ITS sequences Menezes et al. (2010), identified A. tamiyavanichi and general morphological characters agreed with those reported previously; however, they detected important variability that could also match with the morphology of A. cohorticula. The most important feature to identify cells of this species is the anterior sulcal plate, while less than 10% of the analyzed specimens did not agree with the morphology of A. tamiyavanichi. Therefore, they suggested the possibility of them being conspecific species, and pointed out the need of genetic studies on wild and cultured populations to confirm their taxonomic identity, given that the use of morphological characters as an only tool for identification has caused misidentifications. Phylogenetic reconstruction showed a monophyletic clade, which included Brazilian and Asiatic strains, with enough genetic distance between them. Moreover, based on their results they propose a fraterculus group (A. tamiyavanichi/tropicale/fraterculus) as a sister group of A. tamarense species complex (Menezes et al., 2010). New morphological and molecular data (28S and ITS rDNA) for Alexandrium species from Brazil, confirm a close phylogenetic relationship among A. tamiyavanichi and A. fraterculus (Menezes et al., 2018), while A. catenella sequences were grouped with con-specifics from North America, Chile and Japan, moreover a genetic variability within A. tamutum clade was found, including a new record of this species for Brazil. Two strains isolated from Guanabara Bay formed a monophyletic clade with both molecular markers; suggesting it could be a new species of Alexandrium closely related to A. minutum and A. tamutum.
In the Mexican Pacific coast A. tamiyavanichii was found and described less than a decade ago (Esqueda-Lara and Hernández-Becerril, 2010), due to the difficulty for identifying and quantify this species Hernández-Becerril et al. (2018) analyzed ITS2 rDNA sequences obtained by real-time quantitative PCR (qPCR), managing to detect cell abundances of <30 cells L−1 in field samples. Differences in cell densities and their distribution in water column were observed, and two explanations were proposed: A. tamiyavanichii could be transported off the coast due to physical forces such as winds and upwelling, or when environmental conditions are favorable, the physiological characteristics of this species allows it to maintain high cell abundances.
Associations With Bacteria
Dinoflagellates are characterized by different ecological relationships. A relationship that has acquired a great interest is the one established between bacteria and dinoflagellates. This interest relies since bacteria are capable of regulating the different HAB phases, in addition that they are considered to have a role in cell toxicity, growth, and other physiological aspects of dinoflagellates (Hold et al., 2001; Uribe and Espejo, 2003; Croft et al., 2005; Maas et al., 2007).
Alexandrium strains from the southern coast of Chile grow in association with heterotrophic bacteria that mostly affect its growth by the synthesis of algicidal substances that promote cell lysis (Vasquez et al., 2001; Córdova et al., 2002; Uribe and Espejo, 2003). The proliferation of A. catenella in the decade of 1980 in Chile, allowed to report the presence of intracellular bacteria in vegetative cells, and to describe the process of bacterial phagocytosis (Córdova et al., 2002). Also, this was the first time that bacteria of the genus Moraxella sp. were isolated from the cell surface of the dinoflagellate, and related with the production of small amounts of STX (Guzmán et al., 1975; Silva, 1982; Kodama et al., 1990; Orr et al., 2013).
The genera Cytophaga, Pseudoalteromonas, and Ruegeria released alga-lytic compounds among other compounds when grown in absence of phytoplankton cells, causing high mortality (>50%) in A. catenella. When the same bacteria, free of organic nutrients, were returned to the algal culture they displayed no detrimental effects on the dinoflagellate and recovered their symbiotic characteristics. Thus, bacterial-derived lytic activities are expressed only in the presence of high-nutrient media and it is likely that in situ environmental conditions modulate their expression (Amaro et al., 2005). Another aspect to consider is the geographical distribution of strains, since despite isolating the bacteria from different regions, the associated bacteria community is similar, recording the presence of the genera Psychrobacter, Sulfitobacter, Aeromonas, Flavobacterium, Pseudomonas, Proteus and Moraxella in isolates of A. catenella and A. fundyense from different regions of Chile and the Bay of Fundy, respectively (Ferrier et al., 2002).
Bacterial phagocytosis by Alexandrium has also been documented (Silva, 1982). In total absence of bacteria, cell toxicity of A. catenella decreased five times as the majority of the STX analogs decreased significantly, with the exception of NEO. Also, as the culture of the dinoflagellate reached the stationary phase, the cell abundance proportion of bacteria:dinoflagellate remained constant (3,000 bacteria cell−1 of Alexandrium), however, bacterial size tended to increase, suggesting they could be saprophytic bacteria that feed on dinoflagellate by-products (Schut et al., 1993; Dantzer and Levin, 1997; Uribe and Espejo, 2003).
Conclusion
In LAm, the three main marine dinoflagellate genera that produce PSTs are widely distributed and represent an important risk for human health, economic and ecological reasons. PSTs have been also related to epizoic events, fish and aquaculture losses, though most of the times the impacts have not been calculated in economic terms. Despite these problems and risks, few countries have established and maintained monitoring programs, and losses due to PSTs are becoming an important public concern. HABs research efforts have been concentrated in North America (G. catenatum in Mexico) and in South America (A. catenella, A. tamarense mainly in Chile and Argentina). Few studies regarding P. bahamense exist, probably because this species is found mainly in regions with low resources for research, which could explain partially why this is the PST species that has caused greater human impacts.
Studies related to bloom dynamics, autoecology and toxins of G. catenatum are mostly from Mexico, however, this species has been reported in several countries, but in some regions, the species identification remains to be confirmed. Blooms have been associated with a narrow temperature window (18–25°C), high irradiance, transitional hydrographic conditions, El Niño events, and nutrient increase, mostly from river runoffs and coastal upwelling (Table 7). Even though natural plankton samples have a low toxicity, and a profile dominated by the less toxic sulfocarbamoyl analogs, this profile can change in mollusks tissues due to their digestive metabolism and become highly toxic for consumers. Interestingly, autoecology studies have demonstrated that the cell toxin content is higher under in vitro conditions than in the natural environment, which can be partly explained by the higher nutrient concentrations used in culture media. It has also been proposed that the toxin composition is not a conservative feature in G. catenatum, however some populations appear to be pretty consistent in their toxin profile, such as strains from LAm, but strain studies from other regions of LAm need to be carried out to confirm this. It has also been demonstrated that under culture conditions, G. catenatum tolerates a wide range of temperature, salinity, irradiance and N:P ratios, which probably explains its wide distribution along the Mexican Pacific coast. Nevertheless, some variations exist, which have been related to strain origin, temperature, and culture age.
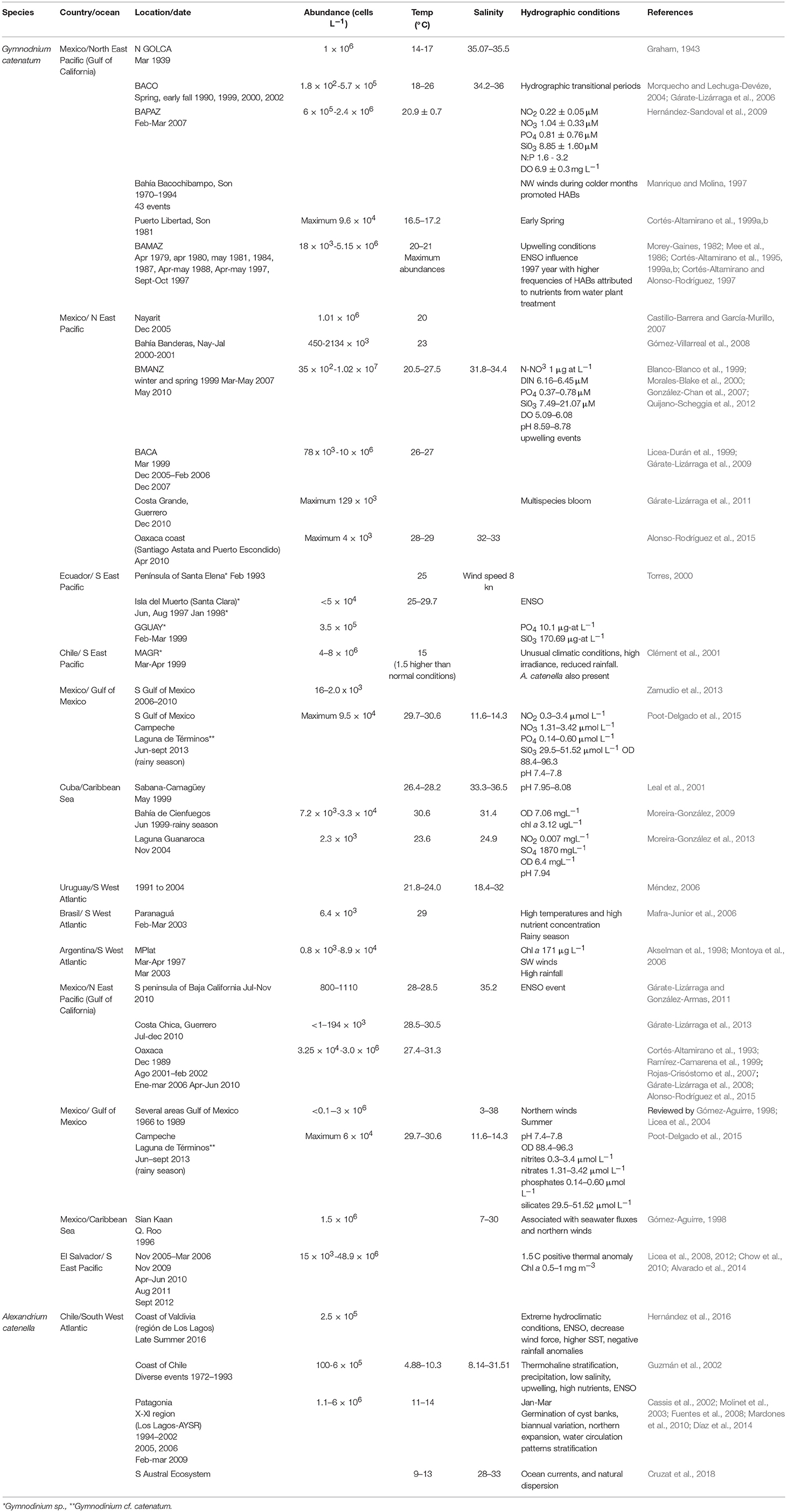
Table 7. Abiotic factors during HABs of G. catenatum, P. bahamense, and A. catenella in different geographic regions of Latin America.
The distribution of P. bahamense is restricted to tropical latitudes. Blooms have been related to warm waters (27–31°C), heavy rains, strong winds, and to El Niño events (Table 7). This species is found in a broad salinity range from 7 to 35. It has been suggested that it could be an invasive species in the northern boundary of its distribution (GOLCA region). Data regarding toxicity and toxin profile of natural phytoplankton samples are scarce and contradictory, carbamoyl or sulfocarbamoyl analogs can dominate. Toxin profiles in mollusks is also quite variable. It is clear that more research and close monitoring is needed for the understanding of the bloom ecology on this species.
HABs of Alexandrium toxic species are mostly reported in South America, being the main responsible species A. catenella (Chile) and A. tamarense (Uruguay-Argentina), which have had severe social impacts, and for this reason research has been concentrated on these two species. In general, Alexandrium species bloom at colder temperatures (5–14°C) than G. catenatum and P. bahamense. HABs of A. catenella are related to high irradiances, water stratification, and extreme climatic conditions such as a higher SST, decreased salinity and precipitation, higher nutrients and El Niño events (Table 7). Deep water upwelling also trigger blooms and cyst beds play an important role in the initiation of blooms. Also, an important expansion in the northern limits of these species has been registered, but the reason for this remains to be understood. Seasonal differences in cell toxicity and in toxin analogs in A. catenella have been found in environmental samples, and contrary to G. catenatum cell toxin content is higher in environmental samples than cultured strains, indicating that toxin metabolism in both species is regulated by different factors.
Regarding toxin analysis in PSTs producers, an important factor to be considered is the different techniques used to extract and analyze toxins which have led to different and confusing results in some cases samples from the same species and region. Different studies have used two acids (hydrochloric acid or acetic acid) in various concentrations, sometimes even using thermic processing of samples. Post-column and pre-column oxidations methods are used, with different drawbacks such as lack of separation of some analogs or the inability to detect some others, and even the appearance of phantom peaks that could be mistaken for toxin analogs. Also, GC toxins are not yet considered in normal analysis and their presence is often neglected due to the longer retention times needed to elute them, and to the lack of commercial standards. It is clear that only a few countries in LAm have the sufficient technological and technical capacity to analyze paralytic toxins a factor that needs to be considered to support both research and monitoring programs in this region.
Other studies such as molecular biology, in silico analyses in order to assess toxicity, nutrient assimilation, trophic interactions, and bacterial community studies are still incipient in LAm, but given the great importance of PSTs producing species in LAm, undoubtedly they will continue to increase in the next few years.
This review evidences that studies have been concentrated in relatively few countries, species and topics. And in some regions studies are only supported for a few years by specific research groups. In general, there is equipment and technical limitation in the different regions of LAm. A regional HAB research program is needed in order to have a more complete understanding of the environmental conditions that favor the PST blooms in this region.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was aided by institutional projects of CIBNOR (PPAyC, Planeación Ambiental y Conservación) and from the Instituto Politécnico Nacional (IPN grants SIP 2018-0662). Consejo Nacional de Ciencia y Tecnología (FORDECyT grant 260040, grant A1-S-14968), and Red Temática sobre Florecimientos Algales Nocivos, CONACyT (RedFAN). Authors thank Dr. J. Vinuesa for providing historical HABs epizootic data from Argentina and MF Sánchez-Bernal for the graphic design.
Footnotes
1. ^In 2015, Bustillos-Guzmán and collaborators, using LC-MS/MS found that the analog reported as NEO in previous analysis could have corresponded to dcNEO.
References
Aguilera-Belmonte, A., Inostroza, I., Carrillo, K. S., Franco, J. M., Riobo, P., and Gómez, P. I. (2013). The combined effect of salinity and temperature on the growth and toxin content of four Chilean strains of Alexandrium catenella (Whedon and Kofoid) Balech 1985 (Dinophyceae) isolated from an outbreak occurring in southern Chile in 2009. Harmful Algae 23, 55–59. doi: 10.1016/j.hal.2012.12.006
Aguilera-Belmonte, A., Inostroza, I., Franco, J. M., Riobó, P., and Gómez, P. I. (2011). The growth, toxicity and genetic characterization of seven strains of Alexandrium catenella (Whedon and Kofoid) Balech 1985 (Dinophyceae) isolated during the 2009 summer outbreak in southern Chile. Harmful Algae 12, 105–112. doi: 10.1016/j.hal.2011.09.006
Aké-Castillo, J. A., and Poot-Delgado, C. A. (2016). “FAN en el Golfo de México: panorama general sobre eventos y especies,” in Florecimientos Algales Nocivos en México, eds E. García-Mendoza, S. I. Quijano-Scheggia, A. Olivos-Ortíz, E. J. Núñez-Vázquez (Ensenada: CICESE), 298–307.
Akselman, R., Carreto, J., and Montoya, N. (1998). “Gymnodinium catenatum and autumn toxicity in northern shelf waters of Argentina,” in Harmful Algae, eds B. Reguera, J. Blanco, M. L. Fernández, and T. Wyatt, (Vigo: Xunta de Galicia, IOC-UNESCO), 122–123.
Almandoz, G. O., Hernando, M. P., Ferreyra, G. A., Schloss, I. R., and Ferrario, M. E. (2011). Seasonal phytoplankton dynamics in extreme southern South America (Beagle Channel, Argentina). J. Sea Res. 66, 47–57. doi: 10.1016/j.seares.2011.03.005
Almandoz, G. O., Montoya, N. G., Hernando, M. P., Benavides, H. R., Carignan, M. O., and Ferrario, M. E. (2014). Toxic strains of the Alexandrium ostenfeldii complex in southern South America (Beagle Channel, Argentina). Harmful Algae 37, 100–109. doi: 10.1016/j.hal.2014.05.011
Alonso-Rodríguez, R., Gárate-Lizárraga, I., Luckas, B., Reinhardt, K., and Bustillos-Guzmán, J. (2004b). “Mortalidad de larvas de camarón en cultivo en Sinaloa, México, asociado a mareas rojas de Gymnodinium catenatum,” in Book of abstracts XIII Reunión Nacional de la Sociedad Mexicana de Planctología (Nuevo Vallarta), 54–55.
Alonso-Rodríguez, R., Mendoza-Amézquita, E., Velásquez-López, S. A., Seim, J. A., and Martínez-Rodríguez, V. M. (2015). Florecimientos algales nocivos producidos por Pyrodinium bahamense en Oaxaca, México (2009-2010). Sal Pub Mex 57, 343–351. doi: 10.21149/spm.v57i4.7578
Alonso-Rodríguez, R., and Páez-Osuna, F. (2003). Nutrients, phytoplankton and harmful algal blooms in shrimp ponds: a review with special reference to the situation in the Gulf of California. Aquaculture 219, 317–336. doi: 10.1016/S0044-8486(02)00509-4
Alonso-Rodríguez, R., Páez-Osuna, F., and Gárate-Lizárraga, I. (2004a). El fitoplancton en la Camaronicultura y Larvicultura: Importancia de un Buen Manejo. Mexico, Inst Cienc Mar Limnol-UNAM/CESASIN.
Alvarado, Y., Espinoza, J., and Amaya, O. (2014). First report of Pyrodinium bahamense cysts in marine sediments from the Gulf of Fonseca, El Salvador. Harmful Algae News 40, 10–11.
Alvarado, Y., Quintanilla, R., and Amaya, O. (2015). First report of Alexandrium globosum on the Pacific coast of Central America. Harmful Algae News 51:3.
Álvarez, G., Uribe, E., Vidal, A., Ávalos, P., González, F., Mariño, C., et al. (2009). Paralytic shellfish toxins in Argopecten purpuratus and Semimytilus algosus from northern Chile. Aquat. Living Resour. 22, 341–347. doi: 10.1051/alr/2009028
Amaro, A. M., Fuentes, M. S., Ogalde, S. R., Venegas, J. A., and Suárez-Isla, B. A. (2005). Identification and characterization of potentially algal-lytic marine bacteria strongly associated with the toxic dinoflagellate Alexandrium catenella. J. Eukaryot. Microbiol. 52, 191–200. doi: 10.1111/j.1550-7408.2005.00031.x
Amaya, O., Dechraoui-Bottein, M. Y., Leighfield, T., and Ruiz, G. (2017). “Five years of application of the Receptor Binding Assay (RBA) on seafood products and threatened turtles during outbreaks HABs in El Salvador,” in Marine and Fresh-Water Harmful Algae, Proceedings 17th International Conference on Harmful Algae, eds L. A. O. Proença and G. M. Hallegraeff (Florianópolis: ISSHA, IOC-UNESCO), 130–132.
Amaya, O., Quintanilla, R., Stacy, B. A., Dechraoui-Bottein, M.-Y., Flewelling, L., Hardy, R., et al. (2018). Large-scale sea turtle mortality events in El Salvador attributed to paralytic shellfish toxin-producing algae blooms. Front. Mar. Sci. 5:411. doi: 10.3389/fmars.2018.00411
Amaya, O., Ruiz, G., Espinoza, J., and Rivera, W. (2014). Saxitoxin Analyses With a Receptor Binding Assay (RBA) Suggest PSP Intoxication of Sea Turtles in El Salvador. Harmful Algal News IOC-UNESCO.
Anderson, D. M., Alpermann, T. J., Cembella, A. D., Collos, Y., Masseret, E., and Montresor, M. (2012). The globally distributed genus Alexandrium: multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 14, 10–35. doi: 10.1016/j.hal.2011.10.012
Arzul, G., Seguel, M., Guzman, L., and Erard-Le Denn, E. (1999). Comparison of allelopathic properties in three toxic Alexandrium species. J. Exp. Mar. Biol. Ecol. 232, 285–295. doi: 10.1016/S0022-0981(98)00120-8
Ávila, M., de Zarate, C., Clement, A., Carbonell, P., and Pérez, F. (2015). Efecto de factores abióticos en el crecimiento vegetativo de Alexandrium catenella proveniente de quistes en laboratorio. Rev. Biol. Mar. Oceanog. 50, 177–185. doi: 10.4067/S0718-19572015000200004
Balech, E. (1964). El plancton de Mar del Plata Durante el Período 1961-1962 Buenos Aires: Boletín del Instituto de Biología Marina.
Balech, E. (1995). The Genus Alexandrium Halim (Dinoflagellata). County Cork: Sherkin Island Marine Station.
Band-Schmidt, C., Bustillos-Guzmán, J., Morquecho, L., Gárate-Lizárraga, I., Alonso-Rodríguez, R., Reyes-Salinas, A., et al. (2006). Variations of PSP toxin profiles during different growth phases in Gymnodinium catenatum (Dinophyceae) strains isolated from three locations in the Gulf of California, Mexico. J. Phycol. 42, 757–768. doi: 10.1111/j.1529-8817.2006.00234.x
Band-Schmidt, C. J., Bustillos-Guzmán, J., Gárate-Lizárraga, I., Lechuga-Devéze, C., Reinhardt, K., and Luckas, B. (2005). Paralytic shellfish toxin profile in strains of the dinoflagellate Gymnodinium catenatum Graham and the scallop Argopecten ventricosus GB Sowerby II from Bahía Concepción, Gulf of California, Mexico. Harmful Algae 4, 21–31. doi: 10.1016/j.hal.2003.10.004
Band-Schmidt, C. J., Bustillos-Guzmán, J. J., Durán-Riveroll, L. M., López-Cortés, D. J., Hernández-Sandoval, F. E., and Núñez-Vázquez, E. J. (2016). “Autoecología de microalgas nocivas aisladas del Golfo de California,” in Florecimientos Algales Nocivos en México., eds E. García-Mendoza, S. I. Quijano-Scheggia, A. Olivos-Ortíz, E. J. Núñez-Vázquez (Ensenada: CICESE), 57–71.
Band-Schmidt, C. J., Bustillos-Guzmán, J. J., Hernández-Sandoval, F. E., Núñez-Vázquez, E. J., and López-Cortés, D. J. (2014). Effect of temperature on growth and paralytic toxin profiles in isolates of Gymnodinium catenatum (Dinophyceae) from the Pacific coast of Mexico. Toxicon 90, 199–212. doi: 10.1016/j.toxicon.2014.08.002
Band-Schmidt, C. J., Bustillos-Guzmán, J. J., López-Cortés, D. J., Gárate-Lizárraga, I., Núñez-Vázquez, E. J., and Hernández-Sandoval, F. E. (2010). Ecological and physiological studies of Gymnodinium catenatum in the Mexican Pacific: a review. Mar. Drugs 8, 1935–1961. doi: 10.3390/md8061935
Band-Schmidt, C. J., Bustillos-Guzmán, J. J., López-Cortés, D. J., Núñez-Vázquez, E. J., and Hernández-Sandoval, F. E. (2011). El estado actual del estudio de florecimientos algales nocivos en México. Hidrobiólogica 21, 381–412.
Band-Schmidt, C. J., Fernández-Herrera, L. J., Ramírez-Rodríguez, D. V., Zumaya-Higuera, M. G., Hernández-Sandoval, F. E., Núñez-Vázquez, E. J., et al. (2017). “Effect of different taxonomic groups on the growth and toxin content in Gymnodinium catenatum cultures from the Pacific coast of Mexico,” in Proceedings 17th International Conference on Harmful Algae, eds L. A. O. Proença and G. M. Hallegraeff (Florianópolis: IOC-UNESCO), 54–57.
Band-Schmidt, C. J., Morquecho, M. L., Lechuga-Devéze, C. H., and Anderson, D. M. (2004). Effects of growth medium, temperature, salinity and seawater source on the growth of Gymnodinium catenatum (Dinophyceae) from Bahía Concepción, Gulf of California, Mexico. J. Plankton. Res. 26, 1459–1470. doi: 10.1093/plankt/fbh133
Band-Schmidt, C. J., Rojas-Posadas, D. I., Morquecho, L., and Hernández-Saavedra, N. Y. (2008). Heterogeneity of LSU rDNA sequences and morphology of Gymnodinium catenatum dinoflagellate strains in Bahía Concepción, Gulf of California, Mexico. J. Plankton Res. 30, 755–763. doi: 10.1093/plankt/fbn035
Barraza, J. E. (2009). Food poisoning due to consumption of the marine gastropod Plicopurpura collumellaris in El Salvador. Toxicon 54, 895–896. doi: 10.1016/j.toxicon.2009.06.026
Baylón, M., Sánchez, S., Bárcena, V., López, J., and Mamani, E. (2015). Primer reporte del dinoflagelado potencialmente tóxico Alexandrium minutum Halim 1960 en el litoral peruano. Rev. Peru Biol. 22, 113–118. doi: 10.15381/rpb.v22i1.11129
Benavides, H., Prado, L., Días, S., and Carreto, J. (1995). “An exceptional bloom of Alexandrium catenella in the Beagle Channel, Argentina,” in Harmful Marine Algal Blooms, eds P. Lassus, G. Arzul, E. Erard, P. Gentien, and C. Marcaillou (Paris: Technique et Documentation-Lavoisier), 113–119.
Blanco-Blanco, M., Aguilar-Olguín, S., and Morales-Blake, A. (1999). “Caracterización de una marea roja en la Bahía de Manzanillo, Colima, México,” in Proceedings VIII Congreso Latinoamericano sobre Ciencias del Mar, COLACMAR, eds A. E. Tresierra-Aguilar and Z. G. Culquichicón-Malpica (Trujillo), 338–339
Bolch, C., Negri, A., and Blackburn, S. (2001). “Life cycle variation in PST content and cell toxicity in PST-producing dinoflagellates,” in Proceedings of the LIFEHAB: Life Histories of Harmful Algal Bloom Species European Union Workshop, eds E. Garcés, A. Zingone, M. Montresor, and B. Reguera (Calviá), 37–42.
Bolch, C. J., Blackburn, S. I., Hallegraeff, G. M., and Vaillancourt, R. E. (1999). Genetic variation among strains of the toxic dinoflagellate Gymnodinium catenatum (Dinophyceae). J. Phycol. 35, 356–367. doi: 10.1046/j.1529-8817.1999.3520356.x
Brooijmans, N., and Kuntz, I. D. (2003). Molecular recognition and docking algorithms. Annu. Rev. Biophys. Biomol. Struct. 32, 335–373. doi: 10.1146/annurev.biophys.32.110601.142532
Bustillos-Guzmán, J., Band-Schmidt, C. J., López-Cortés, D., Gárate-Lizárraga, I., Núñez-Vázquez, E., and Hernández-Sandoval, F. E. (2012). Variaciones en el crecimiento y toxicidad en Gymnodinium catenatum Graham del Golfo de California bajo diferentes proporciones de nitrógeno y fósforo. Cienc Mar. 38, 101–117. doi: 10.7773/cm.v38i1A.1916
Bustillos-Guzmán, J., Vale, P., and Band-Schmidt, C. J. (2011). Presence of benzoate type toxins in Gymnodinium catenatum Graham isolated from the Mexican Pacific. Toxicon 57, 922–926. doi: 10.1016/j.toxicon.2011.02.023
Bustillos-Guzmán, J. J., Band-Schmidt, C. J., Durán-Riveroll, L. M., Hernández-Sandoval, F. E., López-Cortés, D. J., Núñez-Vázquez, E. J., et al. (2015). Paralytic toxin profile of the marine dinoflagellate Gymnodinium catenatum Graham from the Mexican Pacific as revealed by LC-MS/MS. Food Addit. Contam A. 32, 381–394. doi: 10.1080/19440049.2014.1000978
Bustillos-Guzmán, J. J., Band-Schmidt, C. J., López-Cortés, D. J., Hernández-Sandoval, F. E., Núñez-Vázquez, E., and Gárate-Lizárraga, I. (2013). Grazing of the dinoflagellate Noctiluca scintillans on the paralytic toxin-producing dinoflagellate Gymnodinium catenatum: Does grazing eliminate cells during a bloom? Cienc Mar. 39, 291–302. doi: 10.7773/cm.v39i3.2242
Bustillos-Guzmán, J. J., Leyva-Valencia, I., Hernández-Sandoval, F. E., Band-Schmidt, C. J., López-Cortés, D. J., and Núñez-Vázquez, E. J. (2016). “Ficotoxinas en aguas del Golfo de California: Una revisión,” in Florecimientos Algales nocivos en México, eds E. García-Mendoza, S. I. Quijano-Scheggia, A. Olivos-Ortíz, and E. J. Núñez-Vázquez (Ensenada: CICESE), 38–55.
Callejas, L., Darce, A. C. M., Amador, J. J., Conklin, L., Gaffga, N., Rogers, H. S., et al. (2015). Paralytic shellfish poisonings resulting from an algal bloom in Nicaragua. BMC Res Notes. 8:74. doi: 10.1186/s13104-015-1012-4
Carreto, J., Elbusto, C., Sancho, H., Carignan, M., Yasumoto, T., and Oshima, Y. (1996). Comparative studies on paralytic shellfish toxin profiles of marine snails, mussels and an Alexandrium tamarense isolate from the Mar del Plata coast (Argentina). Rev. Invest. Des. Pesq. 10, 101–107.
Carreto, J. I., Akselman, R., Montoya, N. G., Negri, R. M., Benavides, H. R., Carignan, M. O., et al. (1998a). “Alexandrium tamarense bloom dynamics and Mytilus edulis toxicity in the coastal waters off Mar del Plata (Argentina),” in Harmful Algae, eds B. Reguera, J. Blanco, M. L. Fernández, T. Wyatt (Vigo: Xunta de Galicia), 135–138.
Carreto, J. I., Montoya, N. G., Akselman, R., Negri, R. M., Carignan, M. O., Cucchi-Colleoni, A. D., et al. (2004). “Differences in the PSP toxin profiles of Mytilus edulis during spring and autumn blooms of Alexandrium tamarense off Mar del Plata coast, Argentina,” in Proceedings Xth Harmful Algal Blooms Conference, eds K. A. Stedinger, J. H. Lansberg, R. T. Tomar, and G. A. Vargo (St. Petersburg, FL: Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, and Intergovermental Oceanographic Commission of UNESCO), 100–102.
Carreto, J. I., Montoya, N. G., Colleoni, A. D., and Akselman, R. (1998b). “Alexandrium tamarense blooms and shellfish toxicity in the Argentine sea: a retrospective view,” in Harmful Algae, eds B. Reguera, J. Blanco, M. L. Fernández, and T. Wyatt (Vigo: IOC-UNESCO), 131–134.
Carreto, J. I., Negri, R. M., Benavides, H. R., and Akselman, R. (1985). “Toxic dinoflagellate blooms in the Argentine Sea,” in Toxic Dinoflagellates, eds D. M. Anderson, A. W. White, and D. G. Baden (New York, NY: Elsevier Science Publishing Co.), 147–152.
Cassis, D., Muñoz, P., and Avaria, S. (2002). Variación temporal del fitoplancton entre 1993 y 1998 en una estación fija del seno Aysén, Chile (45°26'S 73°00'W). Rev. Mar. Oceanog. 37, 43–65. doi: 10.4067/S0718-19572002000100007
Castillo-Barrera, E., and García-Murillo, A. (2007). “Relación de organismos planctónicos presentes en florecimientos algales nocivos en bahía de Matachén, San Blas, Nayarit,” in Recursos y Medio Ambiente: Proceedings XIV Congreso Nacional de Ciencia y Tecnología (Nuevo Vallarta).
Cembella, A. (1998). “Ecophysiology and metabolism of paralytic shellfish toxins in marine microalgae,” in Physiological Ecology of Harmful Algal Blooms, eds D. M. Anderson, A. D. Cembella, G. M. Hallegraeff (Springer-Verlag; Heidelberg: NATO-Advanced Study Institute Series), 381–404.
Cembella, A., and Band-Schmidt, C. J. (2018). “Gymnodinium catenatum. Harmful algal species fact sheets,” in Harmful Algal Blooms: A Compendium Desk Reference, eds S. E. Shumway, J. M. Burkholder, and S. L. Morton (Holboken, NJ: Wiley Blackwell), 605–611.
Chow, N., Vammen, K., and Reguera, B. (2010). First report of PSP on Pacific coast of Nicaragua associated with Pyrodinium bahamense. Harmful Algae News 41, 6–7.
Clément, A., Seguel, M., Arzul, G., Guzmán, L., and Alarcon, C. (2001). “A widespread outbreak of a haemolytic, ichthyotoxic Gymnodinium sp. in southern Chile,” in Harmful Algal Blooms 2000, eds G. M. Hallegraeff, S. I. Blackburn, C. J. Bolch, R. J. Lewis (Paris: IOC-UNESCO), 66–69.
Córdova, J. L., Cárdenas, L., Cárdenas, L., and Yudelevich, A. (2002). Multiple bacterial infection of Alexandrium catenella (Dinophyceae). J Plankton Res. 24, 1–8. doi: 10.1093/plankt/24.1.1
Córdova, J. L., and Müller, I. (2002). Use of PCR and partial sequencing of the large-subunit rRNA gene to identify Alexandrium catenella (Dinophyceae) from the South of Chile. Harmful Algae 1, 343–350. doi: 10.1016/S1568-9883(02)00066-5
Cortés-Altamirano, R. (1987). Observaciones de mareas rojas en la Bahía de Mazatlán, Sinaloa, México. Cienc Mar. 13, 1–19. doi: 10.7773/cm.v13i4.557
Cortés-Altamirano, R. (2002). “Mareas rojas: Biodiversidad de microbios que pintan el mar,” in Atlas de Biodiversidad de Sinaloa, eds J. L. Cifuentes and J. Gaxiola-López (Colegio de Sinaloa), 442.
Cortés-Altamirano, R., and Alonso-Rodríguez, R. (1997). Mareas rojas durante 1997 en la Bahía de Mazatlán, Sinaloa, México. Cien Mar. UAS. 15, 31–37.
Cortés-Altamirano, R., Lavín, M., Sierra-Beltrán, A., and Cortés-Lara, M. C. (2006). Hipótesis sobre el transporte de microalgas invasoras desde el Pacífico oeste tropical hasta el Golfo de California por las corrientes marinas. Ciencias del. Mar. U. A. S. 18, 19–26.
Cortés-Altamirano, R., Licea, D. S., and Gómez-Aguirre, S. (1999b). “Evidencias de aumento de microalgas nocivas en la bahía de Mazatlán, Sin., México,” in VII Congreso Latinoamericano sobre Ciencias del Mar, 17 -21 de octubre de 1999, eds A. E. Tresierra and Z. G. Culquichicón Malpica (Trujillo), 343–345.
Cortés-Altamirano, R., Manrique, F. A., and Luna-Soria, R. (1995). Presencia de mareas rojas en el costa este del Golfo de California. Rev. Lat-Amer. Microbiol. 37, 337–342.
Cortés-Altamirano, R., Muñoz-Cabrera, L., and Sotomayor-Navarro, O. (1993). Envenamiento paralítico por mariscos (PSP), causado por el dinoflagelado Pyrodinium bahamense var. compressum en la costa suroeste de México. An. Inst. Cienc. Mar. Limnol. 1010, 43–54.
Cortés-Altamirano, R., and Núñez-Pasten, A. (1992). Doce años (1979-1990) de registros de mareas rojas en la Bahía de Mazatlán, Sinaloa, México. An. Inst. Cienc. Mar. Limnol. 19, 113–121.
Cortés-Altamirano, R., Núñez-Pasten, A., and Pasten-Miranda, N. (1999a). Abundancia anual de Gymnodinium catenatum Graham dinoflagelado tóxico de la costa este del Golfo de California. Ciencia y Mar. Universidad del Mar. 3, 50–56.
Costa, P. R., Pereira, P., Guilherme, S., Barata, M., Nicolau, L., Santos, M. A., et al. (2012). Biotransformation modulation and genotoxicity in white seabream upon exposure to paralytic shellfish toxins produced by Gymnodinium catenatum. Aquat. Toxicol. 106, 42–47. doi: 10.1016/j.aquatox.2011.08.023
Croft, M. T., Lawrence, A. D., Raux-Deery, E., Warren, M. J., and Smith, A. G. (2005). Algae acquire vitamin B 12 through a symbiotic relationship with bacteria. Nature 438:90. doi: 10.1038/nature04056
Cruzat, F. A., Muñoz, C., González-Saldía, R. R., Inostroza, A., and Andree, K. B. (2018). High genetic variability of Alexandrium catenella directly detected in environmental samples from the Southern Austral Ecosystem of Chile. Mar. Poll. Bull. 127, 437–444. doi: 10.1016/j.marpolbul.2017.12.022
Cusick, K. D., Wilhelm, S. W., Hargraves, P. E., and Sayler, G. S. (2016). Single-cell PCR of the luciferase conserved catalytic domain reveals a unique cluster in the toxic bioluminescent dinoflagellate Pyrodinium bahamense. Aquat. Biol. 25, 139–150. doi: 10.3354/ab00664
Dantzer, W. R., and Levin, R. (1997). Bacterial influence on the production of paralytic shellfish toxins by dinoflagellated algae. J. Appl. Microbiol. 83, 464–469. doi: 10.1046/j.1365-2672.1997.00246.x
de la Garza-Aguilar, J. (1983). Intoxicación alimentaria por ingestión de mariscos contaminados. Sal. Pub. Mex. 25, 145–150.
Deeb, O., Rosales-Hernández, M. C., Gómez-Castro, C., Garduño-Juárez, R., and Correa-Basurto, J. (2010). Exploration of human serum albumin binding sites by docking and molecular dynamics flexible ligand–protein interactions. Biopolymers 93, 161–170. doi: 10.1002/bip.21314
Deeds, J. R., White, K. D., Etheridge, S. M., and Landsberg, J. H. (2008). Concentrations of saxitoxin and tetrodotoxin in three species of puffers from the Indian River Lagoon, Florida, the location for multiple cases of saxitoxin puffer poisoning from 2002 to 2004. T. Am. Fish. Soc. 137, 1317–1326. doi: 10.1577/T07-204.1
Díaz, P. A., Molinet, C., Seguel, M., Díaz, M., Labra, G., and Figueroa, R. I. (2014). Coupling planktonic and benthic shifts during a bloom of Alexandrium catenella in southern Chile: Implications for bloom dynamics and recurrence. Harmful Algae 40, 9–22. doi: 10.1016/j.hal.2014.10.001
Durán-Riveroll, L., Krock, B., Cembella, A., Peralta-Cruz, J., Bustillos-Guzmán, J. J., and Band-Schmidt, C. J. (2017). Characterization of benzoyl saxitoxin analogs from the toxigenic marine dinoflagellate Gymnodinium catenatum by hydrophilic interaction liquid ion-chromatography-tandem mass spectrometry. Nat. Prod. Chem. Res. 5:275. doi: 10.4172/2329-6836.1000275
Durán-Riveroll, L. M., and Cembella, A. D. (2017). Guanidinium toxins and their interactions with voltage-gated sodium ion channels. Mar. Drugs 15:303. doi: 10.3390/md15100303
Durán-Riveroll, L. M., Cembella, A. D., Band-Schmidt, C. J., Bustillos-Guzmán, J. J., and Correa-Basurto, J. (2016). Docking simulation of the binding interactions of saxitoxin analogs produced by the marine dinoflagellate Gymnodinium catenatum to the voltage-gated sodium channel NaV1. 4. Toxins 8:129. doi: 10.3390/toxins8050129
Durán-Riveroll, L. M., Peralta-Cruz, J., Bustillos-Guzmán, J. J., and Band-Schmidt, C. J. (2013). Presencia de toxinas tipo benzoato en una cepa de Gymnodinium catenatum (Dinophyceae) aislada de Manzanillo, Colima, México. Hidrobiológica 23, 169–175.
Enrique Barraza, J., Armero-Guardado, J., and Valencia de Toledo, Z. M. (2004). The red tide event in El Salvador, August 2001-January 2002. Rev. Biol. Trop. 52(Suppl. 1):1–4.
Esqueda-Lara, K., and Hernández-Becerril, D. U. (2010). Dinoflagelados Microplanctónicos Marinos del Pacífico Central de México (Isla Isabel, Nayarit y Costas de Jalisco y Colima). Mexico City: Universidad Nacional Autónoma de México. 206.
Esteves, J. L., Santinelli, N., Sastre, V., Díaz, R., and Rivas, O. (1992). A toxic dinoflagellate bloom and PSP production associated with upwelling in Golfo Nuevo, Patagonia, Argentina. Hydrobiologia 242, 115–122. doi: 10.1007/BF00018067
Estrada, N. A., Lagos, N., García, C., Maeda-Martínez, A. N., and Ascencio, F. (2007). Effects of the toxic dinoflagellate Gymnodinium catenatum on uptake and fate of paralytic shellfish poisons in the Pacific giant lions-paw scallop Nodipecten subnodosus. Mar. Biol. 151, 1205–1214. doi: 10.1007/s00227-006-0568-x
Fabro, E., Almandoz, G. O., Ferrario, M., John, U., Tillmann, U., Toebe, K., et al. (2017). Morphological, molecular, and toxin analysis of field populations of Alexandrium genus from the Argentine Sea. J. Phycol. 53, 1206–1222. doi: 10.1111/jpy.12574
FAO (2004). Marine Biotoxins. Food and Agriculture Organization of the United Nations. Rome: FAO Food and Nutrition paper 80.
Fernández-Herrera, L. J., Band-Schmidt, C. J., López-Cortés, D. J., Hernández-Guerrero, C. J., Bustillos-Guzmán, J. J., and Núñez-Vázquez, E. (2016). Allelopathic effect of Chattonella marina var. marina (Raphidophyceae) on Gymnodinium catenatum (Dinophycea). Harmful Algae 51, 1–9. doi: 10.1016/j.hal.2015.10.009
Ferrari, G. (2001). “HABs in the southwestern atlantic ocean,” in Proceedings 2nd International Conference on Harmful Algae Management and Mitigation (November 2001, Qingdao, China), eds M. Zhu, Y. Zou, L. Cheong, S. Hall, (Paris: IOC-UNESCO), 34–35.
Ferrier, M., Martin, J., and Rooney-Varga, J. (2002). Stimulation of Alexandrium fundyense growth by bacterial assemblages from the Bay of Fundy. J. Appl. Microbiol. 92, 706–716. doi: 10.1046/j.1365-2672.2002.01576.x
Frangópulos, M., Guisande, C., and Maneiro, I. (2004). Toxin production and competitive abilities under phosphorus limitation of Alexandrium species. Harmful Algae 3, 131–139. doi: 10.1016/S1568-9883(03)00061-1
Fuentealba, R., Lozic, J., and Zegpi, A. (1981). Observaciones clínicas de una intoxicación masiva con veneno paralítico de los mariscos (Puerto Natales, Magallanes, Chile). Ans. Inst. Patag. Pta. Arenas. 12, 289–293.
Fuentes, C., Clement, A., and Aguilera, A. (2008). “Summer Alexandrium catenella bloom and the impact on fish farming, in the XI Aysén Region, Chile,” in Proceedings 12th International Conference on Harmful Algae, eds Ø. D. Moestrup, G. Doucette, H. Enevoldsen, A. Godhe, G. Hallegraeff, and B. Luckas (Copenhaguen: IOC-UNESCO), 183–186.
Gárate-Lizárraga, I., Bustillos-Guzmán, J., Erler, K., Muñetón-Gómez, M., Luckas, B., and Tripp-Quezada, A. (2004). Paralytic shellfish toxins in the chocolata clam, Megapitaria squalida (Bivalvia: Veneridae), in Bahía de La Paz, Gulf of California. Rev. Biol. Trop. 52, 133–142.
Gárate-Lizárraga, I., Bustillos-Guzmán, J., Morquecho, L., Band-Schmidt, C. J., Alonso-Rodríguez, R., Erler, K., et al. (2005). Comparative paralytic shellfish toxin profiles in the strains of Gymnodinium catenatum Graham from the Gulf of California, Mexico. Mar. Poll. Bull. 50, 208–236. doi: 10.1016/j.marpolbul.2004.11.034
Gárate-Lizárraga, I., Bustillos-Guzmán, J. J., López-Cortés, D. J., Hernández-Sandoval, F., Erler, K., and Luckas, B. (2006). Paralytic shellfish toxin profiles in net phytoplankton samples from Bahía Concepción, Gulf of California, Mexico. Mar. Poll. Bull. 52, 800–815. doi: 10.1016/j.marpolbul.2006.03.003
Gárate-Lizárraga, I., Díaz-Ortíz, J., Pérez-Cruz, B., Alarcón-Tacuba, M., Torres-Jaramillo, A., Alarcón-Romero, M., et al. (2009). Cochlodinium polykrikoides and Gymnodinium catenatum in Bahía de Acapulco, Mexico (2005-2008). Harmful Algae News 40, 8–9.
Gárate-Lizárraga, I., Díaz-Ortíz, J. A., Pérez-Cruz, B., Alarcón-Romero, M. A., Chávez-Almazán, L. A., García-Barbosa, J. L., et al. (2011). A multi-species dinoflagellate bloom and shellfish toxicity in Costa Grande, Guerrero, Mexico (December, 2010). CICIMAR Oceánides 26, 67–71.
Gárate-Lizárraga, I., and González-Armas, R. (2011). Occurrence of Pyrodinium bahamense var. compressum along the southern coast of the Baja California Peninsula. Mar. Poll. Bull. 62, 626–630. doi: 10.1016/j.marpolbul.2011.01.009
Gárate-Lizárraga, I., Pérez-Cruz, B., Díaz-Ortíz, J., Alarcón-Tacuba, M., Chávez-Almazán, L., Alarcón-Romero, M., et al. (2012). Toxicity and paralytic toxin profile in Pyrodinium bahamense var. compressum and violet oyster in Bahía de Acapulco. Harmful Algae News 45, 2–3.
Gárate-Lizárraga, I., Pérez-Cruz, B., Díaz-Ortiz, J. A., Alarcón-Tacuba, M. A., Alarcón-Romero, M. A., Chávez-Almazán, L. A., et al. (2013). Blooms of Pyrodinium bahamense var. compressum and rock oyster toxicity in Costa Chica, Guerrero, Mexico. CICIMAR Océanides 28, 37–42.
Gárate-Lizárraga, I., Pérez-Cruz, B., Díaz-Ortíz, J. A., and Band-Schmidt, C. J. (2008). Microalgas y biotoxinas marinas en las costas mexicanas. Conversus 9, 22–26.
Gárate-Lizárraga, I., Verdugo-Díaz, G., and Okolodkov, Y. B. (2016). “Florecimientos algales nocivos en la costa occidental de Baja California Sur,” in Florecimientos Algales Nocivos en México, eds E. García-Mendoza, S. I. Quijano-Scheggia, A. Olivos-Ortíz, and E. J. Núñez-Vázquez (Ensenada: CICESE), 44–59.
García, C., del Carmen Bravo, M., Lagos, M., and Lagos, N. (2004). Paralytic shellfish poisoning: post-mortem analysis of tissue and body fluid samples from human victims in the Patagonia fjords. Toxicon 43, 149–158. doi: 10.1016/j.toxicon.2003.11.018
García, C., Pérez, F., Contreras, C., Figueroa, D., Barriga, A., López-Rivera, A., et al. (2015). Saxitoxins and okadaic acid group: accumulation and distribution in invertebrate marine vectors from Southern Chile. Food Addit. Contam. A. 32, 984–1002. doi: 10.1080/19440049.2015.1028107
García-Hansen, I., Cortés-Altamirano, R., and Sierra-Beltrán, A. (2004). La marea roja causada por el dinoflagelado Alexandrium tamarense en la costa Pacífica colombiana (2001). Rev. Biol. Trop. 52(Suppl. 1): 59–68.
García-Lagunas, N., Romero-Geraldo, R., and Hernández-Saavedra, N. Y. (2013). Genomics study of the exposure effect of Gymnodinium catenatum, a paralyzing toxin producer, on Crassostrea gigas defense system and detoxification genes. PLoS ONE 8:e72323. doi: 10.1371/journal.pone.0072323
García-Mendoza, E., Medina, J., Rivas, D., Ruiz, M. C., Bustillos-Guzmán, J., Núñez-Vázquez, E. J., et al. (2016). “Paralytic shellfish toxins cause seabirds and marine mammals massive mortalities in the upper gulf of California,” in 17th International Conference on Harmful Algae Brazil, 9–14 October, (Florianópolis), 83.
Garrido, C., Frangópulos M., and Varela, D. (2012). Efecto de diferentes proporciones de nitrógeno/fósforo en el crecimiento y toxicidad de Alexandrium catenella (Dinoflagellata). An. Inst. Patagon. 40, 113–123. doi: 10.4067/S0718-686X2012000200010
Gayoso, A. M. (2001). Observations on Alexandrium tamarense (Lebour) Balech and other dinoflagellate populations in golfo Nuevo, Patagonia (Argentina). J. Plank Res. 23, 463–468. doi: 10.1093/plankt/23.5.463
Gayoso, A. M., and Fulco, V. K. (2006). Occurrence patterns of Alexandrium tamarense (Lebour) Balech populations in the Golfo Nuevo (Patagonia, Argentina), with observations on ventral pore occurrence in natural and cultured cells. Harmful Algae. 5, 233–241. doi: 10.1016/j.hal.2004.12.010
Glibert, P. M., Landsberg, J. H., Evans, J. J., Al-Sarawi, M. A., Faraj, M., Al-Jarallah, M. A., et al. (2002). A fish kill of massive proportion in Kuwait Bay, Arabian Gulf, 2001: the roles of bacterial disease, harmful algae, and eutrophication. Harmful Algae 1, 215–231. doi: 10.1016/S1568-9883(02)00013-6
Gómez-Aguirre, S. (1998). First record of Pyrodinium bahamense (Dinoflagellata) in brackish waters of the Mexican Caribbean coast. An. Inst. Biol. 69, 121–123.
Gómez-Villarreal, M. C., Martínez-Gaxiola, M. D., and Peña-Manjarrez, J. L. (2008). Proliferaciones algales 2000-2001 en Bahía de Banderas, México según el sensor SeaWiFS. Rev. Biol. Trop. 56, 1653–1664.
González-Chan, R. B., Hernández-Silva, L., Navarro-Ornelas, J. R., and Blanco Padilla, M. A. (2007). “Proliferación algal nociva en las Bahías del puerto de Manzanillo, Colima (marzo-mayo 2007),” in Recursos y Medio Ambiente: Memorias del XIV Congreso Nacional de Ciencia y Tecnología, 29–31 october 2007 (Nuevo Vallarta)
Gordon, D., Chen, R., and Chung, S.-H. (2013). Computational methods of studying the binding of toxins from venomous animals to biological ion channels: theory and applications. Physiol. Rev. 93, 767–802. doi: 10.1152/physrev.00035.2012
Graham, H. W. (1943). Gymnodinium catenatum, a new dinoflagellate from the Gulf of California. T. Am. Microsc. Soc. 62, 259–261. doi: 10.2307/3223028
Guinder, V. A., Tillmann, U., Krock, B., Delgado, A., Krohn, T., Garzón Cardona, J., et al. (2018). Plankton multiproxy analyses in the Northern Patagonian Shelf, Argentina: community structure, phycotoxins and characterization of Alexandrium strains. Front. Mar. Sci. 5:394. doi: 10.3389/fmars.2018.00394
Guzmán, L., Campodónico, I., and Hermosilla, J. (1975). Estudios sobre un florecimiento tóxico causado por Gonyaulax catenella en Magallanes. III. Fitoplancton asociado. An. Inst. Patagonia 6, 173–183.
Guzmán, L., Pacheco, H., Pizarro, G., and Alarcón, C. (2002). “Alexandrium catenella y veneno paralizante de los mariscos en Chile,” in Floraciones Algales Nocivas en el Cono sur Americano, eds E. A. Sar, M. E. Ferrario, B. Reguera (Madrid: Instituto Español de Oceanografía), 235–255.
Guzmán, L., Vivanco, X., Vidal, G., Pizarro, G., Hernández, C., Tocornal, M. A., et al. (2010). “Spatial and temporal variability of Alexandrium catenella and PSP in southern Chile (438–558S) (May 2006–July 2010),” in Proceedings 14th International Conference on Harmful Algae Pagou, eds P. Hallegraeff and G. Crete (Greece), 69–71.
Hallegraeff, G. M. (1993). A review of harmful algal blooms and their apparent global increase. Phycologia 32, 79–99. doi: 10.2216/i0031-8884-32-2-79.1
Hallegraeff, G. M. (1995). “Harmful algal blooms: a global overview,” in Manual on Harmful Marine Microalgae, 2nd Edition, eds G. M. Hallegraeff, D. M. Anderson, A. D. Cembella (Paris: UNESCO), 25–50.
Hallegraeff, G. M., Blackburn, S., Doblin, M., and Bolch, C. (2012). Global toxicology, ecophysiology and population relationships of the chainforming PST dinoflagellate Gymnodinium catenatum. Harmful Algae 14, 130–143. doi: 10.1016/j.hal.2011.10.018
Halperin, I., Ma, B., Wolfson, H., and Nussinov, R. (2002). Principles of docking: an overview of search algorithms and a guide to scoring functions. Proteins 47, 409–443. doi: 10.1002/prot.10115
Häussermann, V., Gutstein, C. S., Bedington, M., Cassis, D., Olavarria, C., Dale, A. C., et al. (2017). Largest baleen whale mass mortality during strong El Niño event is likely related to harmful toxic algal bloom. PeerJ 5:e3123. doi: 10.7717/peerj.3123
Heileman, L., and Siung-Chang, A. (1990). An analysis of fish kills in coastal and inland waters of Trinidad and Tobago, West Indies, 1976-1990. Carib. Mar. Stud. 1, 126–136.
Hernández, C., Díaz, P. A., Molinet, C., and Seguel, M. (2016). Exceptional climate anomalies and northwards expansion of Paralytic Shellfish Poisoning outbreaks in Southern Chile. Harmful Algae News 54, 1–2.
Hernández-Becerril, D. U., Alonso-Rodríguez, R., Álvarez-Góngora, C., Baron-Campis, S. A., Ceballos-Corona, G., Herrera-Silveira, J., et al. (2007). Toxic and harmful marine phytoplankton and microalgae (HABs) in Mexican Coasts. J. Environ. Sci. Heal. A. 42, 1349–1363. doi: 10.1080/10934520701480219
Hernández-Becerril, D. U., Lau, W. L. S., Hii, K. S., Leaw, C. P., Varona-Cordero, F., and Lim, P. T. (2018). Abundance and distribution of the potentially toxic thecate dinoflagellate Alexandrium tamiyavanichii (Dinophyceae) in the Central Mexican Pacific, using the quantitative PCR method. Front. Mar. Sci. 5:366. doi: 10.3389/fmars.2018.00366
Hernández-Sandoval, F. E., López-Cortés, D. J., Band-Schmidt, C. J., Gárate-Lizárraga, I., Núñez-Vázquez, E. J., and Bustillos-Guzmán, J. J. (2009). Toxinas paralizantes en moluscos bivalvos durante una proliferación de Gymnodinium catenatum Graham en la Bahía de La Paz, México. Hidrobiologica 19, 245–256.
Hold, G. L., Smith, E. A., Rappë, M. S., Maas, E. W., Moore, E. R., Stroempl, C., et al. (2001). Characterisation of bacterial communities associated with toxic and non-toxic dinoflagellates: Alexandrium spp. and Scrippsiella trochoidea. FEMS Microbiol. Ecol. 37, 161–173. doi: 10.1111/j.1574-6941.2001.tb00864.x
IPCS (1984). “Environmental Health Criteria 37. Aquatic (Marine and Freshwater) Biotoxins”, in International Programme on Chemical Safety, World Health Organization (Geneva).
Jedlicki, A., Fernández, G., Astorga, M., Oyarzún, P., Toro, J. E., Navarro, J. M., et al. (2012). Molecular detection and species identification of Alexandrium (Dinophyceae) causing harmful algal blooms along the Chilean coastline. AoB Plants 2012:pls033. doi: 10.1093/aobpla/pls033
Kodama, M., Ogata, T., Sakamoto, S., Sato, S., Honda, T., and Miwatani, T. (1990). Production of paralytic shellfish toxins by a bacterium Moraxella sp. isolated from Protogonyaulax tamarensis. Toxicon 28, 707–714. doi: 10.1016/0041-0101(90)90259-A
Kremp, A., Tahvanainen, P., Litaker, W., Krock, B., Suikkanen, S., Leaw, C. P., et al. (2014). Phylogenetic relationships, morphological variation, and toxin patterns in the Alexandrium ostenfeldii (Dinophyceae) complex: implications for species boundaries and identities. J. Phycol. 50, 81–100. doi: 10.1111/jpy.12134
Krock, B., Borel, C. M., Barrera, F., Tillmann, U., Fabro, E., Almandoz, G. O., et al. (2015). Analysis of the hydrographic conditions and cyst beds in the San Jorge Gulf, Argentina, that favor dinoflagellate population development including toxigenic species and their toxins. J. Mar. Syst. 148, 86–100. doi: 10.1016/j.jmarsys.2015.01.006
Krock, B., Seguel, C. G., and Cembella, A. D. (2007). Toxin profile of Alexandrium catenella from the Chilean coast as determined by liquid chromatography with fluorescence detection and liquid chromatography coupled with tandem mass spectrometry. Harmful Algae 6, 734–744. doi: 10.1016/j.hal.2007.02.005
La Barbera-Sánchez, A., Soler, F. J., Rojas de Astudillo, L., and Chang-Yen, I. (2004). Paralytic shellfish poisoning (PSP) in Margarita Island, Venezuela. Rev. Biol. Trop. 52(Suppl. 1), 89–98.
Lagos, N. (1998). Microalgal blooms: a global issue with negative impact in Chile. Biol. Res. 31, 375–386.
Lagos, N. (2003). Paralytic shellfish poisoning phycotoxins: occurrence in South America. Comm. Toxicol. 9, 1–19. doi: 10.1080/08865140302429
Lagos, N., Compagnon, D., Seguel, M., and Oshima, Y. (1996). “Paralytic shellfish toxin composition: a quantitative analysis in Chilean mussels and dinoflagellate,” in Harmful and Toxic Algal Blooms, eds T. Yasumoto, T. Oshima, and Y. Fukuyo (Paris: IOC-UNESCO), 121–124.
Landsberg, J. H., Hall, S., Johannessen, J. N., White, K. D., Conrad, S. M., Abbott, J. P., et al. (2006). Saxitoxin puffer fish poisoning in the United States, with the first report of Pyrodinium bahamense as the putative toxin source. Environ. Health Persp. 114:1502. doi: 10.1289/ehp.8998
Leal, S., Delgado, G., and Nodas, F. (2001). Distribución y abundancia del fitoplancton en un área de la zona nororiental de Cuba. Rev. Invest. Mar. 23, 45–51.
Li, R. A., Ennis, I. L., French, R. J., Dudley, S. C., Tomaselli, G. F., and Marbán, E. (2001). Clockwise domain arrangement of the sodium channel revealed by μ-conotoxin (GIIIA) docking orientation. J. Biol. Chem. 276, 11072–11077. doi: 10.1074/jbc.M010862200
Licea, S., Navarrete, A., Castañeda, V., and Bustillos-Guzmán, J. J. (2012). “Monitoring program for harmful algal blooms in Salvadoran waters: report of Pyrodinium bahamense from November 2009 to June 2010,” in Proceedings of the 14th International Conference on Harmful Algae. International Society for the Study of Harmful Algae and Intergovernmental Oceanographic Commission of UNESCO 2013. ISSHA, eds K. A. Pagou and G. M. Hallegraeff (Hersonissos-Crete: IOC-UNESCO).
Licea, S., Navarrete, A., Rodríguez, R., Bustillos-Guzmán, J., Martínez, B., and Ramírez, C. (2008). “Monitoring a bloom of Pyrodinium bahamense var. compressum in El Salvador and the southern coast of Mexico (November 2005–March 2006),” in Proceedings 12th International Conference on Harmful Algae, eds Ø. D. Moestrup, G. Doucette, H. Enevoldsen, A. Godhe, G. Hallegraeff and B. Luckas (Copenhague: IOC-UNESCO), 219–220.
Licea, S., Zamudio, M. E., Cortés-Altamirano, R., Luna, R., and Soto, P. J. (2013). “Distribution of known or presumed toxic dinoflagellates in the southern Gulf of Mexico, 1979-2008,” in Biological and Geological Perspectives of Dinoflagellates, eds J. M. Lewis, F. Marret, L. R. Bradley (London: The Micropalaeontological Society), 155–160.
Licea, S., Zamudio, M. E., Luna, R., and Soto, J. (2004). Free-living dinoflagellates in the southern Gulf of Mexico: report of data (1979–2002). Phycol. Res. 52, 419–428. doi: 10.1111/j.1440-1835.2004.tb00351.x
Licea-Durán, S., Gómez-Aguirre, S., Cortés-Altamirano, R., and Gómez, S. (1999). “Notas sobre algunos florecimientos algales y la presencia de especies tóxicas en cinco localidades del Pacífico Mexicano (1996-1999),” in VIII Congreso Latinoamericano Sobre Ciencias del Mar, octubre 1999, eds A. E. Tresierra-Aguilar and Z. G. Culquichicón-Malpica (Trujillo), 335–337.
Limoges, A., de Vernal, A., and Ruiz-Fernández, A. C. (2015). Investigating the impact of land use and the potential for harmful algal blooms in a tropical lagoon of the Gulf of Mexico. Estuar. Coast Shelf. S. 167, 549–559. doi: 10.1016/j.ecss.2015.11.005
Llewellyn, L., Negri, A., and Robertson, A. (2006). Paralytic shellfish toxins in tropical oceans. Toxin. Rev. 25, 159–196. doi: 10.1080/15569540600599217
Llewellyn, L. E. (2006). Saxitoxin, a toxic marine natural product that targets a multitude of receptors. Nat. Prod. Rep. 23, 200–222. doi: 10.1039/b501296c
Maas, E. W., Latter, R. M., Thiele, J., Waite, A. M., and Brooks, H. J. (2007). Effect of multiple antibiotic treatments on a paralytic shellfish toxin-producing culture of the dinoflagellate Alexandrium minutum. Aquat. Microb. Ecol. 48, 255–260. doi: 10.3354/ame048255
MacKenzie, L., de Salas, M., Adamson, J., and Beuzenberg, V. (2004). The dinoflagellate genus Alexandrium (Halim) in New Zealand coastal waters: comparative morphology, toxicity and molecular genetics. Harmful Algae 3, 71–92. doi: 10.1016/j.hal.2003.09.001
Mafra-Junior, L. L., Fernandes, L. F., and Proença, L. A. O. (2006). Harmful algae and toxis in Paranaguá Bay, Brazil: bases for monitoring. Braz. J. Oceanogr. 54, 107–121. doi: 10.1590/S1679-87592006000200002
Manrique, F. A., and Molina, R. E. (1997). Presencia de mareas rojas en la Bahía de Bacochibampo, Guaymas, Sonora, México. Hidrobiológica 7, 81–84.
Mardones, J., Clément, A., Rojas, X., and Aparicio, C. (2010). Alexandrium catenella during 2009 in Chilean waters, and recent expansion to coastal ocean. Harmful Algae News 41, 8–9.
Mardones, J. I., Bolch, C., Guzmán, L., Paredes, J., Varela, D., and Hallegraeff, G. M. (2016). Role of resting cysts in Chilean Alexandrium catenella dinoflagellate blooms revisited. Harmful Algae 55, 238–249. doi: 10.1016/j.hal.2016.03.020
Mardones, J. I., Dorantes-Aranda, J. J., Nichols, P. N., and Hallegraeff, G. M. (2015). Fish gill damage by the dinoflagellate Alexandrium catenella from Chilean fjords: synergistic action of ROS and PUFA. Harmful Algae 49, 40–49. doi: 10.1016/j.hal.2015.09.001
Martínez-López, A., Ulloa-Pérez, A. E., and Escobedo-Urías, D. C. (2007). First record of vegetative cells of Pyrodinium bahamense (Gonyaulacales: Goniodomataceae) in the Gulf of California. Pac. Sci. 61, 289–293. doi: 10.2984/1534-6188(2007)61[289:FROVCO]2.0.CO;2
Mata, L., Abarca, G., Marranghello, L., and Víquez, R. (1990). Paralytic shellfish poisoning by Spondylus calcifer contaminated with Pyrodinium bahamense, Costa Rica, 1989-1990. Rev. Biol. Trop. 38, 129–136.
McLeod, C., Dowsett, N., Hallegraeff, G., Harwood, D. T., Hay, B., Ibbott, S., et al. (2017). Accumulation and depuration of paralytic shellfish toxins by Australian abalone Haliotis rubra: Conclusive association with Gymnodinium catenatum dinoflagellate blooms. Food Control. 73, 971–980. doi: 10.1016/j.foodcont.2016.10.012
Meave del Castillo, E., Rodríguez, S., and Vargas, M. (2006). “Blooms of Pyrodinium bahamense var. compressa along the Central America Pacific coast and south of Mexico,” in Proceedings 12th International Conference of Harmful Algae, eds Ø. D. Moestrup, G. Doucette, H. Enevoldsen, A. Godhe, G. Hallegraeff, and B. Luckas (Copenhaguen: IOC–UNESCO), 239.
Medina-Elizalde, J., García-Mendoza, E., Turner, A. D., Sánchez-Bravo, Y. A., and Murillo-Martínez, R. (2018). Transformation and depuration of paralytic shellfish toxins in the geoduck clam Panopea globosa from the northern Gulf of California. Front. Mar. Sci. 5:335. doi: 10.3389/fmars.2018.00335
Medlin, L. K., Lange, M., Wellbrock, U., Donner, G., Elbrächter, M., Hummert, C., et al. (1998). Sequence comparisons link toxic European isolates of Alexandrium tamarense from the Orkney Islands to toxic North American stocks. Eur. J. Protistol. 34, 329–335. doi: 10.1016/S0932-4739(98)80060-6
Mee, L. D., Espinosa, M., and Díaz, G. (1986). Paralytic shellfish poisoning with a Gymnodinium catenatum red tide on the Pacific coast of Mexico. Mar. Environ. Res. 19, 77–92. doi: 10.1016/0141-1136(86)90040-1
Méndez, S., Anderson, D. M., and Kulis, D. M. (2001). “PSP toxin production of Uruguayan isolates of Gymnodinium catenatum and Alexandrium tamarense,” in Harmful Algal Blooms, eds G. M. Hallegraeff, S. Blackburn, R. Lewis, C. Bolch (Paris: IOC-UNESCO), 352–355.
Méndez, S., Severov, D., Ferrari, G., and Mesones, C. (1996). “Early spring Alexandrium tamarense toxic blooms in Uruguayan waters,” in Harmful and Toxic Algal Blooms, eds T. Yasumoto, Y. Oshima, and Y. Fukuyo (Paris: IOC-UNESCO), 113–116.
Méndez, S. M. (2006). “Impacto de las floraciones algales nocivas en Uruguay: origen, dispersión, monitoreo, control y mitigación,” in Bases Para la Conservación y el Manejo de la Costa Uruguaya. eds. R. Menafra, F. Scarabino and D. Conde, 57–69.
Mendoza-Flores, A., Leyva-Valencia, I., Band-Schmidt, C. J., Galindo-Sánchez, C. E., and Bustillos-Guzmán, J. J. (2018). Identification of the Gene sxtA (domains sxtA1 and sxtA4) in Mexican strains of Gymnodinium catenatum (Dinophyceae) and their evolution. Front. Mar. Sci. 5:289. doi: 10.3389/fmars.2018.00289
Menezes, M., Branco, S., Miotto, M. C., and Alves-de-Souza, C. (2018). The genus Alexandrium (Dinophyceae, Dinophyta) in Brazilian coastal waters. Front. Mar. Sci. 5:421. doi: 10.3389/fmars.2018.00421
Menezes, M., Proença, L. A. O., Branco, S., and Schramm, M. A. (2007). Bloom of Alexandrium minutum Halim on Rio de Janeiro coast: occurrence and toxicity. Harmful Algal News. 34, 7–9.
Menezes, M., Varela, D., de Oliveira Proença, L. A., da Silva Tamanaha, M., and Paredes, J. (2010). Identification of the toxic alga Alexandrium tamiyavanichi (Dinophyceae) from northeastern Brazil: a combined morphological and rDNA sequence (partial LSU and ITS) approach. J. Phycol. 46, 1239–1251. doi: 10.1111/j.1529-8817.2010.00918.x
Mertens, K. N., Wolny, J., Carbonell-Moore, C., Bogus, K., Ellegaard, M., Limoges, A., et al. (2015). Taxonomic re-examination of the toxic armored dinoflagellate Pyrodinium bahamense Plate 1906: can morphology or LSU sequencing separate P. bahamense var. compressum from var. bahamense? Harmful Algae 41, 1–24. doi: 10.1016/j.hal.2014.09.010
Molinet, C., Lafon, A., Lembeye, G., and Moreno, C. A. (2003). Patrones de distribución espacial y temporal de floraciones de Alexandrium catenella (Whedon & Kofoid) Balech 1985, en aguas interiores de la Patagonia noroccidental de Chile. Rev. Chil. Hist. Nat. 76, 681–698. doi: 10.4067/S0716-078X2003000400011
Montebruno, D. (1993). Paralytic shellfish poisoning in Chile. Med. Sci. Law. 33, 243–246. doi: 10.1177/002580249303300310
Montes, M. R., Rojas, X., Artacho, P., Tello, A., and Quiñones, R. A. (2018). Quantifying harmful algal bloom thresholds for farmed salmon in southern Chile. Harmful Algae 77, 55–65. doi: 10.1016/j.hal.2018.05.004
Montoya, N., Akselman, R., Carignan, M., and Carreto, J. (2006). Pigment profile and toxin composition during a red tide of Gymnodinium catenatum Graham and Myrionecta rubra (Lohman) Jankowski in coastal waters off Mar del Plata, Argentina. Afr. J. Mar. Sci. 28, 199–202. doi: 10.2989/18142320609504147
Montoya, N. G., and Carreto, J. I. (2007). Informe Sobre Mortandad de Aves Marinas Ocurrida en las Costas de Chubut (Noviembre de 2006), Asociada a la Presencia de Toxinas Paralizantes de Moluscos. Informe Técnico INIDEP No. 34/2017, Mar del Plata: INIDEP.
Montoya, N. G., Fulco, V. K., Carignan, M. O., and Carreto, J. I. (2010). Toxin variability in cultured and natural populations of Alexandrium tamarense from southern South America–Evidences of diversity and environmental regulation. Toxicon 56, 1408–1418. doi: 10.1016/j.toxicon.2010.08.006
Montoya, N. G., Reyero, M. I., Akselman, R., Franco, J. M., and Carreto, J. J. (1998). “Paralytic shellfish toxins in the anchovy Engraulis anchoita from the Argentinian coast,” in Harmful Algae, Proceedings VIII International Conference on Harmful Algae (June 1999, Vigo, Spain), eds B. Reguera, J. Blanco, M. Fernández, and T. Wyatt (Vigo: Xunta de Galicia, IOC-UNESCO), 72–73.
Morales-Blake, A., Hernández-Becerril, D. U., and Cavazos-Guerra, C. (2000). “Registros de mareas rojas en las Bahías de Manzanillo, Colima, México,” in Estudios Sobre Plancton en Mexico y el Caribe, eds E. Ríos-Jara, E. Juárez-Carrillo, M. Pérez-Peña, E. López-Linares, E. G. Robles-Jarero, D. U. Hernández-Becerril, and M. Silva-Briano (Guadalajara: Laboratorio de Ecosistemas Marinos y Acuicultura, Departamento de Ecología, Centro Universitario de Ciencias Biológicas y Agropecuaria, Universidad de Guadalajara), 81–82.
Moreira-González, A. (2009). First record of Gymnodinium cf. catenatum and other potentially toxic plankton in southern Cuba. Harmful Algae News 40, 14–15.
Moreira-González, A., Betancourt, C., Toledo, L., Barcia, S., and Comas, A. (2013). Notas acerca del fitoplancton de la Laguna Guanaroca, Cienfuegos, Cuba. Rev. Invest. Mar. 33, 39–45.
Morey-Gaines, G. (1982). Gymnodinium catenatum Graham (Dinophyceae): morphology and affinities with armoured forms. Phycologia 21, 154–163. doi: 10.2216/i0031-8884-21-2-154.1
Morquecho, L. (2008). Morphology of Pyrodinium bahamense plate (Dinoflagellata) near Isla San José, Gulf of California, Mexico. Harmful Algae 7, 664–670. doi: 10.1016/j.hal.2008.02.003
Morquecho, L., and Lechuga-Devéze, C. H. (2004). Seasonal occurrence of planktonic dinoflagellates and cyst production in relationship to environmental variables in subtropical Bahía Concepción, Gulf of California. Bot. Mar. 47, 313–322. doi: 10.1515/BOT.2004.037
Muciño-Márquez, R. E. (2010). Variación Estacional de la Comunidad Fitoplanctónica en Granjas Atuneras en la Bahía de La Paz, Baja California Sur. Master thesis, Instituto Politécnico Nacional, Centro Interdisciplinario de Ciencias Marinas, 122.
Navarro, J., Munoz, M., and Contreras, A. (2006). Temperature as a factor regulating growth and toxin content in the dinoflagellate Alexandrium catenella. Harmful Algae 5, 762–769. doi: 10.1016/j.hal.2006.04.001
Negri, A., Bolch, C., Blackburn, S., Dickman, M., Llewellyn, L., and Méndez, S. (2001). “Paralytic shellfish toxins in Gymnodinium catenatum strains from six countries,” in Proceedings 9th International Conference-Harmful Algal Blooms 2000, eds G. M. Hallegraeff, S. I. Blackburn, C. J. Bolch, and R. J. Lewis (Paris: IOC-UNESCO), 210–213.
Negri, A., Llewellyn, L., Doyle, J., Webster, N., Frampton, D., and Blackburn, S. (2003a). Paralytic shellfish toxins are restricted to few species among Australia's taxonomic diversity of cultured microalgae. J. Phycol. 39, 663–667. doi: 10.1046/j.1529-8817.2003.02131.x
Negri, A., Stirling, D., Quilliam, M., Blackburn, S., Bolch, C., Burton, I., et al. (2003b). Three novel hydroxybenzoate saxitoxin analogues isolated from the dinoflagellate Gymnodinium catenatum. Chem. Res. Toxicol. 16, 1029–1033. doi: 10.1021/tx034037j
Negri, A. P., Bolch, C. J., Geier, S., Green, D. H., Park, T.-G., and Blackburn, S. I. (2007). Widespread presence of hydrophobic paralytic shellfish toxins in Gymnodinium catenatum. Harmful Algae 6, 774–780. doi: 10.1016/j.hal.2007.04.001
Núñez-Vázquez, E. J., Band-Schmidt, C. J., Hernández-Sandoval, F. E., Bustillos-Guzmán, J. J., López-Cortés, D. J., Medina-Elizalde, J., et al. (2016). “Impactos de los FAN en la salud pública y animal (silvestres y de cultivo) en el Golfo de California,” in Florecimientos Algales Nocivos en México (Ensenada: CICESE), 196–212.
Núñez-Vázquez, E. J., Bustillos-Guzmán, J. J., Ramírez-Camarena, C., and Hernández-Sandoval, F. (2007a). “Perfiles cromatográficos de toxinas paralizantes en moluscos bivalvos asociados a Pyrodinium bahamense var. compressum en el Pacífico Sur Mexicano,” in Proceedings of II Taller sobre Florecimientos Algales Nocivos (CICESE-CETMAR) (Ensenada), 18.
Núñez-Vázquez, E. J., Cordero-Tapia, A., and Arnaud, G. (2007b). “Origen e impacto de las Biotoxinas Marinas en la Salud de Tortugas Marinas Salud Pública,” in 1er Encuentro Internacional de Medicina de la Conservación, 18-21 July, (Vitoria).
Núñez-Vázquez, E. J., Gárate-Lizárraga, I., Band-Schmidt, C. J., Cordero-Tapia, A., López-Cortés, D. J., Sandoval, F. E. H., et al. (2011). Impact of harmful algal blooms on wild and cultured animals in the Gulf of California. J. Environ. Biol. 32, 413–423.
Ochoa, J. L., Sierra-Beltrán, A., Alonso-Colmenares, G., Barrada-Sánchez, H., Cruz-Villacorta, A., Núñez-Vázquez, E., et al. (1998). “Biotoxins in the Pacific Coast of Mexico,” in Mycotoxins and Phycotoxins Developments in Chemistry, Toxicology and Food Safety, International Union Purity Analytical Chemistry (IUPAC). IX IUPAC International Symposium on Mycotoxins and Phycotoxins, eds M. Miraglia, H. Van Egmond, C. Brera and J. Gilbert (Rome: Alaken, Inc. Fort Collins Co. U.S.A), 441–448.
Odebrecht, C., Azevedo, S. M. F. O., Garcia, V. M. T., Huszar, V. L. M., Magalhaes, V. F., Menezes, M., et al. (2002). “Floraciones de microalgas nocivas en Brasil: estado del arte y proyectos en curso,” in Floraciones Algales Nocivas en el Cono Sur Americano, eds E. A. Sar, M. E. Ferrairo and B. Reguera (Vigo: Instituto Español de Oceanografía, IOC Harmful Algal Bloom Programme), 219–233.
Ogata, T., Kodama, M., and Ishimaru, T. (1987). Toxin production in the dinoflagellate Protogonyaulax tamarensis. Toxicon 25, 923–928. doi: 10.1016/0041-0101(87)90154-1
Ordás, M. C., Fraga, S., Franco, J. M., Ordás, A., and Figueras, A. (2004). Toxin and molecular analysis of Gymnodinium catenatum (Dinophyceae) strains from Galicia (NW Spain) and Andalucia (S Spain). J. Plankton Res. 26, 341–349. doi: 10.1093/plankt/fbh037
Orellana-Cepeda, E., Martínez-Romero, E., Muñoz-Cabrera, L., López-Ramírez, P., Cabrera-Mancilla, E., and Ramírez-Camarena, C. (1998). “Toxicity associated with blooms of Pyrodinium bahamense var. compressum in southwestern Mexico,” in Proceedings of the Harmful algae, VIII International Conference on Harmful Algae, eds B. Reguera, J. Blanco, M. L. Fernández, and T. Wyatt (Vigo: Xunta de Galicia and IOC-UNESCO), 60.
Orlova, T. Y., Selina, M. S., Lilly, E. L., Kulis, D. M., and Anderson, D. M. (2007). Morphogenetic and toxin composition variability of Alexandrium tamarense (Dinophyceae) from the east coast of Russia. Phycologia 46, 534–548. doi: 10.2216/06-17.1
Orr, R. J., Stüken, A., Murray, S. A., and Jakobsen, K. S. (2013). Evolutionary acquisition and loss of saxitoxin biosynthesis in dinoflagellates: the second “core” gene-sxtG. Appl. Environ. Microbiol. 03279–03212. doi: 10.1128/AEM.03279-12
Oshima, Y. (1995). “Chemical and enzymatic transformation of paralytic shellfish toxins in marine organisms,” in Harmful Algal Blooms, eds P. Lassus, G. Arzul, P. Gentien and C. Marcaillou (Paris: Technique et documentation-Lavoisier), 475–480.
Osorio-Tafall, B. (1943). El Mar de Cortés y la productividad fitoplanctónica de sus aguas. An. Esc. Nal. Cienc. Biol. 3, 73–118.
Oyaneder-Terrazas, J., Contreras, H. R., and García, C. (2017). Prevalence, variability and bioconcentration of saxitoxin-group in different marine species present in the food chain. Toxins 9:190. doi: 10.3390/toxins9060190
Palomares-García, R., Bustillos-Guzmán, J., Band-Schmidt, C., López-Cortés, D., and Luckas, B. (2006). Effect of the toxic dinoflagellate Gymnodinium catenatum on the grazing, egg production, and hatching success of the copepod Acartia clausi. Cienc. Mar. 32, 97–109. doi: 10.7773/cm.v32i12.1041
Paredes-Banda, P. E., Cruz-López, R., García-Mendoza, E., Santiago-Morales, I., Ruiz-de la Torre, M. C., Murillo-Martínez, R., et al. (2016). “Estudios de ecofisiología sobre especies fitoplanctónicas nocivas en Baja California,” in Florecimientos Algales Nocivos en México, eds E. García-Mendoza, S. I. Quijano-Scheggia, A. Olivos-Ortíz and E. J. Núñez-Vázquez (Ensenada: CICESE), 78–91.
Parrilla-Cerrillo, M. C., Vázquez-Castellanos, J. L., Sáldate-Castañeda, O., and Nava-Fernández, L. M. (1993). Brotes de toxiinfecciones alimentarias de origen microbiano y parasitario. Sal. Pub. Mex. 35, 456–463.
Pérez-Linares, J., Ochoa, J., and Gago-Martínez, A. (2008). Effect of PSP toxins in white leg shrimp Litopenaeus vannamei Boone (1931). J. Food. Sci. 73, T69–T73. doi: 10.1111/j.1750-3841.2008.00710.x
Persich, G. R., Kulis, D. M., Lilly, E. L., Anderson, D. M., and García, V. M. (2006). Probable origin and toxin profile of Alexandrium tamarense (Lebour) Balech from southern Brazil. Harmful Algae 5, 36–44. doi: 10.1016/j.hal.2005.04.002
Pizarro, G., Paz, B., Alarcón, C., Toro, C., Frangópulos, M., Salgado, P., et al. (2018). Winter distribution of toxic, potentially toxic phytoplankton, and shellfish toxins in fjords and channels of the Aysén region, Chile. Lat. Am. J. Aquat. Res. 46, 120–139. doi: 10.3856/vol46-issue1-fulltext-13
Plate, L. (1906). Pyrodinium bahamense ng, n. sp., die Leucht-Peridinee des“ Feuersees” von Nassau, Bahamas. Arch. Protistenk. 7, 411–428.
Poot-Delgado, C. A., Okolodkov, Y. B., Castillo, J. A. A., and Rendón-Von Osten, J. (2015). Annual cycle of phytoplankton with emphasis on potentially harmful species in oyster beds of Términos Lagoon, southeastern Gulf of Mexico. Rev. Biol. Mar. Oceanog. 50, 465–477. doi: 10.4067/S0718-19572015000400006
Proença, L. A. O., Tamanaha, M. S., and de Souza, N. P. (2001). The toxic dinoflagellate Gymnodinium catenatum Graham in southern Brazilian waters: occurrence, pigments and toxins. Atlântica 23, 59–65.
Quijano-Scheggia, S., Olivos-Ortíz, A., Bustillos-Guzmán, J. J., Garcés, E., Gaviño-Rodríguez, J. H., Galicia-Pérez, M. A., et al. (2012). Bloom of Gymnodinium catenatum in Bahía Santiago and Bahía Manzanillo, Colima, Mexico. Rev. Biol. Trop. 60, 173–186. doi: 10.15517/rbt.v60i1.2750
Quijano-Scheggia, S., Olivos-Ortíz, A., Pérez-Morales, A., Álvarez-García, C., Gaviño-Rodríguez, J. H., and Sosa-Avalos, R. (2016). “Registros de microalgas nocivas o toxicas formadoras de florecimientos algales en la Bahía de Manzanillo, Colima, México,” in Florecimientos Algales Nocivos en México, eds E. García-Mendoza, S. I. Quijano-Scheggia, A. Olivos-Ortíz, E. J. Núñez-Vázquez (Ensenada: CICESE), 218–227.
Ramírez-Camarena, C., Muñoz-Cabrera, L., Cabrera-Mancilla, E., Castro-Ramos, R., López-Ramírez, P., and Orellana-Cepeda, E. (1996). “Identificación de la marea roja frente a la costa suroeste de México en Oct.-Dic. 1995,” in I Reunión Internacional de Planctología y VIII Reunión Nacional de la Sociedad Mexicana de Planctología (Pátzcuaro), 47.
Ramírez-Camarena, C., Rojas-Crisóstomo, R., Muñoz-Cabrera, L., Sarniento-Nafate, S., and Juárez-Ruíz, N. O. (2002). “Mortandad de peces e intoxicaciones humanas en la Costa de Chiapas en el 2001,” in IX Congreso Nacional de Ciencia y Tecnología del Mar. Nuevo Vallarta (Nayarit), 1–2.
Ramírez-Camarena, C. Cortés-Altamirano, R., and Muñoz-Cabrera, L. (1999). Mareas rojas provocadas por el dinoflagelado Gymnodinium catenatum (Gymnodinales: Gymnodiniaceae) en la Bahía de Mazatlán, Sin., México, en 1997. Rev. Biol. Trop. 47, 77–80.
Rodríguez, D. C., Etzel, R. A., Hall, S., de Porras, E., Velasquez, O. H., Tauxe, R. V., et al. (1990). Lethal paralytic shellfish poisoning in Guatemala. Am. J. Trop. Med. Hyg. 42, 267–271. doi: 10.4269/ajtmh.1990.42.267
Rodríguez-Salvador, R., and Meave del Castillo, M. E. (2007). “Monitoreo del dinoflagelado tóxico Pyrodinium bahamense var. compressum en las costas de Chiapas,” in II Taller sobre Florecimientos Algales Nocivos (Ensenada), 6.
Rojas-Crisóstomo, R., Ramírez-García, H., Barón-Campis, S., and Ojeda-Salinas, H. (2007). “Presencia de Pyrodinium bahamense var. compressum, niveles de toxina en moluscos bivalvos e identificación del fitoplancton marino en las costas de La Colorada y La Ventosa, Oaxaca durante 2006,” in Resúmenes del II Taller sobre Florecimientos Algales Nocivos Ensenada, México, Noviembre 2007 (Ensenada: CICESE-CETMAR), 2007.
Rosales-Loessener, F. (1989). “The guatemala experience with red tides and paralytic shellfish poisoning,” in Biology, Epidemiology and Management of Pyrodinium Red Tides, ICLARM Conference Proceedings, eds G. M. Hallegraeff and J. Maclean (Manila: Fisheries Department, Ministry of Development, Brunei Darussalam, and International Center for Living Aquatic Resources Management), 49–51.
Rosales-Loessener, F., Porras, E. D., and Dix, M. V. (1989). “Toxic shellfish poisoning in Guatemala,” in Red Tides, Biology, Environmental Science and Toxicology, eds T. Okaichi, D. M. Anderson, T. Nemoto (New York, NY: Elsevier), 113–116.
Saldate-Castañeda, O., Vázquez-Castellanos, J., Galván, J., Sánchez-Anguiano, A., and Nazar, A. (1991). Poisoning from paralytic shellfish toxins in Oaxaca, Mexico. Sal. Pub. Mex. 33, 240–247.
Salgado, P., Riobó, P., Rodríguez, F., Franco, J. M., and Bravo, I. (2015). Differences in the toxin profiles of Alexandrium ostenfeldii (Dinophyceae) strains isolated from different geographic origins: evidence of paralytic toxin, spirolide, and gymnodimine. Toxicon 103, 85–98. doi: 10.1016/j.toxicon.2015.06.015
Sánchez-Cabeza, J. A., Ruiz-Fernandez, A. C., de Vernal, A., and Machain-Castillo, M. L. (2012). Reconstruction of Pyrodinium blooms in the tropical East Pacific (Mexico): are they related to ENSO? Environ. Sci. Technol. 46, 6830–6834. doi: 10.1021/es204376e
Sánchez-Flores, H. E. (2011). Envenenamiento paralizante severo por consume de moluscos. Reporte de un caso. Arch. Med. Urgen. Mex. 3, 30–33.
Sánchez-Suárez, I., and Troncone-Osorio, F. (1994). Diversidad y equitabilidad del fitoplancton del Golfo de Paria (Venezuela, junio 1984). Acta Cient Venez. 45, 296–306.
Scholin, C., Hallegraeff, G., and Anderson, D. (1995). Molecular evolution of the Alexandrium tamarense ‘species complex’(Dinophyceae): dispersal in the North American and West Pacific regions. Phycologia 34, 472–485.
Scholin, C. A., Herzog, M., Sogin, M., and Anderson, D. M. (1994). Identification of group and strain specific genetic markers for globally distributed Alexandrium (Dinophyceae). II Sequence analyisis of a fragment of the LSU rDNA gene. J. Phycol. 30, 999–1011. doi: 10.1111/j.0022-3646.1994.00999.x
Schut, F., de Vries, E. J., Gottschal, J. C., Robertson, B. R., Harder, W., Prins, R. A., et al. (1993). Isolation of typical marine bacteria by dilution culture: growth, maintenance, and characteristics of isolates under laboratory conditions. Appl. Environ. Microbiol. 59, 2150–2160.
Seok Jin, O., Matsuyama, Y., Yoon, Y. H., Miyamura, K., Choi, C. G., Yang, H. S., et al. (2010). Comparative analysis of paralytic shellfish toxin content and profile produced by dinoflagellate Gymnodinium catenatum isolated from Inokushi Bay, Japan. J. Fac. Agr. Kyushu 55, 47–54.
Shen, H., Zhou, Q., Pan, X., Li, Z., Wu, J., and Yan, N. (2017). Structure of a eukaryotic voltage-gated sodium channel at near-atomic resolution. Science 355:eaal4326. doi: 10.1126/science.aal4326
Sierra-Beltrán, A., Cruz, A., Núñez-Vázquez, E., Del Villar, L. M., Cerecero, J., and Ochoa, J. L. (1998). An overview of the marine food poisoning in Mexico. Toxicon 36, 1493–1502. doi: 10.1016/S0041-0101(98)00139-1
Silva, E. S. (1982). Relationship between dinoflagellates and intracellular bacteria. Mar. Al. Pharmacol. Sci. 2, 269–288.
Silva, N. J., Tang, K. W., and Lopes, R. M. (2013). Effects of microalgal exudates and intact cells on subtropical marine zooplankton. J. Plankton. Res. 35, 855–865. doi: 10.1093/plankt/fbt026
Siu, G. K., Young, M. L., and Chan, D. (1997). “Environmental and nutritional factors which regulate population dynamics and toxin production in the dinoflagellate Alexandrium catenella,” in Asia-Pacific Conference on Science and Management of Coastal Environment Developments in Hydrobiology, eds Y.-S. Wong and N. F. Y. Tam (Dordrecht: Springer), 117–140.
Sotomayor-Navarro, O., and Domínguez-Cuellar, E. (1993). “Toxic red tide of Pyrodinium bahamense var. compressum, in the Tehuantepec Gulf, and the Central American Pacific System,” in 6th International Conference on Toxic Marine Phytoplankton (Nantes), 185.
Steidinger, K., Tester, L., and Taylor, F. J. R. (1980). A redescription of Pyrodinium bahamense var. compressa (Böhm) stat. nov. from Pacific red tides. Phycologia 19, 329–334. doi: 10.2216/i0031-8884-19-4-329.1
Su, Z., Sheets, M., Ishida, H., Li, F., and Barry, W. H. (2004). Saxitoxin blocks L-type ICa. J. Pharmac. Exp. Therap. 308, 324–329. doi: 10.1124/jpet.103.056564
Suárez-Isla, B., and Guzmán-Méndez, L. (1998). Floraciones Algales Nocivas. Mareas Rojas y Toxinas Marinas. Available online at http://www.ifop.cl/marearoja/wp-content/uploads/sites/2/2016/01/8_-FLORACIONES-DE-ALGAS-NOCIVAS-Mareas-Rojas-y-Toxinas-Marinas-Guzman-y-Suarez-1998.pdf
Sunesen, I., Lavigne, A., Goya, A., and Sar, E. A. (2014). Episodios de toxicidad en moluscos de aguas marinas costeras de la Provincia de Buenos Aires (Argentina) asociados a algas toxígenas (marzo de 2008-marzo de 2013). Bol. Soc. Argent. Bot. 49, 327–339.
Tillmann, U., Alpermann, T. L., da Purificação, R. C., Krock, B., and Cembella, A. (2009). Intra-population clonal variability in allelochemical potency of the toxigenic dinoflagellate Alexandrium tamarense. Harmful Algae 8, 759–769. doi: 10.1016/j.hal.2009.03.005
Torres, G. (2000). Mareas rojas durante 1989-1999, en aguas ecuatorianas. Acta Ocean Pac. 10, 127–136.
Torres-Chuquimarca, G. M. (2011). Eventos de Mareas Rojas: Estrategias de Manejo Preventivas en Ecuador. Master thesis: Universidad de Guayaquil. Available online at http://repositorio.ug.edu.ec/handle/redug/11942
Uribe, J. C., Oyarzún, S., and Latorre, V. (2010). Alexandrium catenella (Whedon & Kofoid) Balech, 1985, en aguas magallánicas, Chile. An. Inst. Patagonia 38, 103–110.
Uribe, P., and Espejo, R. T. (2003). Effects of associated bacteria on the growth and toxicity of Alexandrium catenella. Appl. Environ. Microbiol. 69, 659–662. doi: 10.1128/AEM.69.1.659-662.2003
Uribe, P., Fuentes, D., Valdés, J., Shmaryahu, A., Zúñiga, A., Holmes, D., et al. (2008). Preparation and analysis of an expressed sequence tag library from the toxic dinoflagellate Alexandrium catenella. Mar. Biotechnol. 10, 692–700. doi: 10.1007/s10126-008-9107-8
Usup, G., Ahmad, A., Matsuoka, K., Lim, P. T., and Leaw, C. P. (2012). Biology, ecology and bloom dynamics of the toxic marine dinoflagellate Pyrodinium bahamense. Harmful Algae 14, 301–312. doi: 10.1016/j.hal.2011.10.026
Vale, P. (2008). Complex profiles of hydrophobic paralytic shellfish poisoning compounds in Gymnodinium catenatum identified by liquid chromatography with fluorescence detection and mass spectrometry. J. Chromatogr. A. 1195, 85–93. doi: 10.1016/j.chroma.2008.04.073
Vale, P. (2010). New saxitoxin analogues in the marine environment: developments in toxin chemistry, detection and biotransformation during the 2000s. Phytochem. Rev. 9, 525–535. doi: 10.1007/s11101-010-9196-7
Varela, D., Paredes, J., Alves-de-Souza, C., Seguel, M., Sfeir, A., and Frangópulos, M. (2012). Intraregional variation among Alexandrium catenella (Dinophyceae) strains from southern Chile: morphological, toxicological and genetic diversity. Harmful Algae 15, 8–18. doi: 10.1016/j.hal.2011.10.029
Vargas-Montero, M., and Freer, E. (2004). “Paralytic Shellfish poisoning outbreaks in Costa Rica,” in Harmful Algae 2002, eds. K. A. Steidinger, J. H. Landsberg, C. R. Tomas and G. A. Vargo (St. Petersburg Beach, FL: Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography. IOC-UNESCO), 482–484.
Vasquez, M., Gruttner, C., Gallacher, S., and Moore, E. (2001). Detection and characterization of toxigenic bacteria associated with Alexandrium catenella and Aulacomya ater contaminated with PSP. J. Shellfish. Res. 20, 1245–1249.
Vinuesa, J. H. (1993). “Marea Roja en el Canal de Beagle,” in Contribución Científico (Ushuaia Tierra del Fuego: Centro Austral de Investigaciones Científicas), 1–34.
Vinuesa, J. H., and Labal, M. L. (1996). Marea roja y Mortalidad masiva en el canal de Beagle. Nat. Patagón Cienc. Biol. 4, 167–171.
Wang, J., Salata, J. J., and Bennett, P. B. (2003). Saxitoxin is a gating modifier of HERG K+ channels. J. Gen. Physiol. 121, 583–598. doi: 10.1085/jgp.200308812
Warren, G. L., Andrews, C. W., Capelli, A.-M., Clarke, B., LaLonde, J., Lambert, M. H., et al. (2006). A critical assessment of docking programs and scoring functions. J. Med. Chem. 49, 5912–5931. doi: 10.1021/jm050362n
Wiese, M., D'Agostino, P. M., Mihali, T. K., Moffitt, M. C., and Neilan, B. A. (2010). Neurotoxic alkaloids: saxitoxin and its analogs. Mar. Drugs. 8, 2185–2211. doi: 10.3390/md8072185
Yen, I. C., De Astudillo, L. R., Soler, J. F., and La Barbera-Sánchez, A. (2004). Paralytic shellfish poisoning toxins in green mussels (Perna viridis) from the Gulf of Paria, Trinidad. Toxicon 44, 743–747. doi: 10.1016/j.toxicon.2004.07.030
Zamudio, M. E., Licea, S., and Luna, R. (2013). “Relative abundance and distribution of unarmoured dinoflagellate species in the South Gulf of Mexico (2005-2010),” in Biological and Geological Perspectives of Dinoflagellates, eds J. M. Lewis, F. Marret, L. Bradley (London: The Micropalaeontological Society, Special Publications. Geological Society), 233–238.
Keywords: Alexandrium, dinoflagellates, Gymnodinium catenatum, Latin America, paralytic toxins, Pyrodinium bahamense, review, strains
Citation: Band-Schmidt CJ, Durán-Riveroll LM, Bustillos-Guzmán JJ, Leyva-Valencia I, López-Cortés DJ, Núñez-Vázquez EJ, Hernández-Sandoval FE and Ramírez-Rodríguez DV (2019) Paralytic Toxin Producing Dinoflagellates in Latin America: Ecology and Physiology. Front. Mar. Sci. 6:42. doi: 10.3389/fmars.2019.00042
Received: 31 July 2018; Accepted: 28 January 2019;
Published: 21 February 2019.
Edited by:
Jorge I. Mardones, Instituto de Fomento Pesquero (IFOP), ChileReviewed by:
Patricio A. Díaz, University of Los Lagos, ChileClaudio Fuentes-Grünewald, Swansea University, United Kingdom
Copyright © 2019 Band-Schmidt, Durán-Riveroll, Bustillos-Guzmán, Leyva-Valencia, López-Cortés, Núñez-Vázquez, Hernández-Sandoval and Ramírez-Rodríguez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christine J. Band-Schmidt, cjband@yahoo.com; cbands@ipn.mx
†Deceased
 Christine J. Band-Schmidt
Christine J. Band-Schmidt Lorena M. Durán-Riveroll
Lorena M. Durán-Riveroll José J. Bustillos-Guzmán
José J. Bustillos-Guzmán Ignacio Leyva-Valencia4
Ignacio Leyva-Valencia4  David J. López-Cortés
David J. López-Cortés Erick J. Núñez-Vázquez
Erick J. Núñez-Vázquez Francisco E. Hernández-Sandoval
Francisco E. Hernández-Sandoval