Abstract
Background
While the Glasgow coma scale (GCS) is one of the strongest outcome predictors, the current classification of traumatic brain injury (TBI) as ‘mild’, ‘moderate’ or ‘severe’ based on this fails to capture enormous heterogeneity in pathophysiology and treatment response. We hypothesized that data-driven characterization of TBI could identify distinct endotypes and give mechanistic insights.
Methods
We developed an unsupervised statistical clustering model based on a mixture of probabilistic graphs for presentation (< 24 h) demographic, clinical, physiological, laboratory and imaging data to identify subgroups of TBI patients admitted to the intensive care unit in the CENTER-TBI dataset (N = 1,728). A cluster similarity index was used for robust determination of optimal cluster number. Mutual information was used to quantify feature importance and for cluster interpretation.
Results
Six stable endotypes were identified with distinct GCS and composite systemic metabolic stress profiles, distinguished by GCS, blood lactate, oxygen saturation, serum creatinine, glucose, base excess, pH, arterial partial pressure of carbon dioxide, and body temperature. Notably, a cluster with ‘moderate’ TBI (by traditional classification) and deranged metabolic profile, had a worse outcome than a cluster with ‘severe’ GCS and a normal metabolic profile. Addition of cluster labels significantly improved the prognostic precision of the IMPACT (International Mission for Prognosis and Analysis of Clinical trials in TBI) extended model, for prediction of both unfavourable outcome and mortality (both p < 0.001).
Conclusions
Six stable and clinically distinct TBI endotypes were identified by probabilistic unsupervised clustering. In addition to presenting neurology, a profile of biochemical derangement was found to be an important distinguishing feature that was both biologically plausible and associated with outcome. Our work motivates refining current TBI classifications with factors describing metabolic stress. Such data-driven clusters suggest TBI endotypes that merit investigation to identify bespoke treatment strategies to improve care.
Trial registration
The core study was registered with ClinicalTrials.gov, number NCT02210221, registered on August 06, 2014, with Resource Identification Portal (RRID: SCR_015582).
Similar content being viewed by others
Background
Traumatic brain injury (TBI) is a heterogeneous disease with a wide variety of injury mechanisms and tissue pathologies, affecting people at all stages of life. It is one of the leading causes of mortality and morbidity in young individuals globally, with a leading global cause being road traffic incidents (RTI) [1]. Additionally, the incidence of TBI in older patients is increasing as this multi-morbid and fall-prone population increases in prevalence [2].
Although mortality from TBI has decreased over the last 30 years, the proportion of patients with favourable outcomes have remained relatively unchanged [2,3,4], despite developments such as intracranial pressure (ICP) monitoring [5]. A recent report identified large variation in TBI management in a European multi-centre cohort, without a corresponding variation in outcomes [6]. While it is possible that these management variations truly had no impact on outcome, this result could also be due to a substantial heterogeneity of the disease masking treatment effect in relevant subgroups. Due to lack of high-quality evidence, variations in treatment strategies are based largely on local strategies rather than mechanistically aligned to injury types [7,8,9]. A better characterization of patients could allow discrimination into more specific and biologically relevant sub-groups based on clinical, biomarker, pathoanatomic, and physiological features.
This approach could provide a basis for determining whether specific treatments and interventions might be more effective in some of these sub-groups [7, 9,10,11]. However, implementation of such individualized treatment strategies relies on the identification of robust and relevant endotypes. Endotypes are subtypes of a clinical condition or syndrome, which can be characterized by distinct pathophysiology, and have an implicit likelihood of variation in response to therapies. This approach was first used to describe subgroups in asthma [12], but has now been used in other conditions [13]. Recently, unsupervised machine learning methods have been successful in discovering subgroups and endotypes with specific treatment-responses in diseases such as acute respiratory distress syndrome (ARDS) and sepsis in the intensive care unit (ICU) [14, 15].
The current classification of TBI simply as ‘mild’, ‘moderate’ and ‘severe’ is based on the level of consciousness at presentation, assessed using the Glasgow coma scale (GCS). While this is well known to be an important predictor of outcome and easy to operationalize, it is also clearly an overly simplistic description of such a complex disease and unlikely to be aligned with underlying pathobiology. As such, this simple classification provides a poor substrate from which to individualise care. Furthermore, it limits research into personalised medicine as populations stratified in this way retain biological heterogeneity and therefore are likely to be diverse in terms of their treatment response.
Instead, we hypothesize the existence of distinct clinically and/or physiologically determinable endotypes in patients with TBI requiring ICU treatment and that these may be described not only by canonical measures of injury severity, such as Glasgow Coma Scale (GCS), but also by pathophysiology. We further hypothesize that these parameters might present complex or nonlinear relationships to disease course and outcome, so that unsupervised/machine learning methods may be required to reveal underlying relationships between parameters. The aim of this study is not primarily to describe endotypes associated with outcome, but to describe endotypes that could motivate tailored treatments in the future, and potentially lead to improved outcome in patients with TBI.
Methods
Patient and feature selection
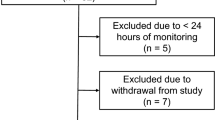
All patients over 18 years old enrolled in the multinational study Collaborative European Neuro Trauma Effectiveness Research in TBI (CENTER-TBI) ICU cohort (N = 2006) were included in the study, between 2014 and 2018 [4, 10], Additional file 1: Fig S1. All patients met the general inclusion criteria for CENTER-TBI (clinical diagnosis of TBI, presentation at hospital within 24 h from injury, and a clinical need for a CT scan) and were admitted to the ICU immediately after hospital admission. More than 2000 parameters were collected for each patient. Of these, a total of 35 early features were selected, including those in the core IMPACT prediction model for TBI [16] and features identified to be of clinical interest. The data and variables in the CENTER-TBI database were based on the synthesis of current knowledge of TBI in concert with much of European and Northern American expertise. The variables chosen for this analysis were early features with known or plausible relations with outcome, or deranged physiology (Table 1) as judged by clinicians with extensive neurointensive care experience. CT characteristics were extracted from a central imaging review. All selected features were recorded at presentation, i.e., either prehospital, during emergency room (ER) admission or early within the ICU, but no later than 24 h post-injury. Both GCS total and motor sub-score were included, as the total score is of clinical interest and the motor sub-score have shown prediction ability in the IMPACT model. Outcome was represented by the eight-point Glasgow outcome scale extended, (GOS-E) score, where GOS-E 1 = dead and GOS-E 8 = full recovery.
Version 3.0 of the CENTER-TBI dataset was used for this work. Models were created using proprietary low-level code in C ++ and all other analyses were performed using R version 1.1.453 [17].
The clustering model
We used a mixture of probabilistic graph models to construct an unsupervised classifier suitable for dealing with the mix of discrete and continuous features with missingness. The univariate probability distributions for all features were modelled as a product model, and compensating factors for each pair of strongly correlated features were included.
To first determine which features were correlated and therefore would need to be considered jointly, linear correlations between features were examined graphically using the R package corrplot (version 0.84), Fig. 1 [18]. Pairs of strongly correlated features (pH and base excess, pH and arterial partial pressure of carbon dioxide (PaCO2), GCS motor and total score, Rotterdam CT score and midline shift, Rotterdam CT score and Fisher classification, GCS motor score and pupil response, age and ASA PS-class (American Society of Anesthesiologists physical status classification), and age and anticoagulants at baseline) were modelled as bivariate joint Gaussian distributions. Completeness of data is presented in, Additional file 2: Table S1.
Linear correlation between all pairs of features. To visualize the strength of linear correlation between each pair of features, the value of the Pearson correlation coefficient is represented by the size and colour of the dots in the matrix. Strongly correlated features (pH and base excess, pH and arterial partial pressure of carbon dioxide (PaCO2), GCS motor and total score, Rotterdam CT score and midline shift, Rotterdam CT score and Fisher classification, GCS motor score and pupil response, age and ASA PS-class (American Society of Anesthesiologists physical status classification), and age and anticoagulants at baseline) were modelled as bivariate joint Gaussian distributions. GCS, Glasgow coma scale; ISS, injury severity score; SpO2, oxygen saturation; PaO2, arterial partial pressure of oxygen; PaCO2, arterial partial pressure of carbon dioxide; BMI, body mass index; TAI, traumatic axonal injury; EDH, epidural hematoma; aSDH, acute subdural hematoma; tSAH, traumatic subarachnoid haemorrhage; MAP, mean arterial pressure; ICP, intracranial pressure; TIL, therapy intensity level
To estimate the parameters and cluster membership probabilities in our graph mixture model, we used an expectation maximisation (EM) algorithm [19, 20]. This is a generalization of the maximum likelihood estimation of incomplete data and offers a principled, probabilistic approach to the unsupervised clustering of large multivariable datasets without the need for imputation when missingness is present [20]. Conceptually, the EM algorithm is a two-step iterative algorithm: in the expectation (E) step, the probability distribution over all clusters for each patient is calculated from the given parameters of the features in the cluster (i.e., the probability for cluster membership for each patient), and the maximization (M) step is the re-estimation of parameter distributions in all clusters. These steps are repeated until convergence, giving the most probable separation of clusters given the chosen number of clusters and predictor features. Further mathematical details are described in Additional file 3.
Determination of number of clusters
We used a cluster stability to robustly determine the most appropriate number of clusters to choose [21]. Numbers from three to fifteen clusters were evaluated for stability. This selection was a clinical trade off – too many clusters might not be clinically relevant, despite the risk that they may represent potentially important separation of phenotypes. However, within this range a methodologically principled optimum may be identified.
For each number of clusters considered, we created ten different models, using different random seeds. The log-likelihood for each model was calculated, and the model with the highest log-likelihood was selected. This process was repeated twenty times (Fig. 2) and cluster similarity was quantified using a cluster similarity index (CSI) defined as the fraction of patients who were assigned to the same cluster in two models [21]. CSI was calculated between all pairs of the models of the same number of clusters, and median and interquartile range (IQR) was calculated. As the CSI, when numbers of clusters < < number of patients, by nature is higher for lower number of clusters, a penalty for the number of clusters was added by subtracting 1/n clusters from all median CSI. The optimal clustering was defined as number of clusters with the highest median CSI (representing the most stable number of clusters). When describing the clusters, the model of the optimal number of clusters with the highest log-likelihood was chosen to represent our model.
Ten models of each number of clusters between three to fifteen were created. The model with the highest log likelihood was chosen as the best model. This was repeated twenty times. Median, minimum, and maximum cluster similarity index (CSI, defined as the fraction of patients assigned to the same cluster in two models), of the twenty models were calculated. The median CSI is presented in Fig. 3
Evaluation of the clusters
To investigate the importance of each feature for the model, the mutual information (MI) was calculated between each feature and the cluster labels. The MI represents how well the cluster label is determined by a feature, with respect to how the distributions differ between the clusters. Features were considered to be of value if the MI > 0.1. A descriptive analysis of the clusters using these features was undertaken. Univariable logistic regression analysis was performed to determine the pseudo-explained variance between cluster label and outcome, and a multivariable regression analysis was performed to investigate if the cluster label could improve predicted outcome over the “International Mission for Prognosis and Analysis of Clinical Trials in TBI” (IMPACT) variables which have been well characterised as predictors in TBI [16]. For the outcome prediction (but not the clustering), missing values were imputed using the multiple imputation with chained equations (MICE) algorithm in R [22]. The observed mortality and unfavourable outcome (defined as GOS-E < 5) frequencies in all clusters was compared to the IMPACT predicted outcome.
Results
Patient characteristics
278 patients were excluded due to missing Glasgow outcome scale extended (GOS-E) score at 6 months, leaving 1728 patients for the analysis. The mean age was 50.4 years (SD 19.3) and 1269 (73.4%) were male. The most common causes of injury were RTIs (46.5%) and falls (43.7%). The overall mortality in the cohort was 22%, and 45% had unfavourable outcomes (defined as Upper Severe Disability or worse according to the GOS-E outcome scale) 6 months post-injury. Based on the IMPACT core model, the overall analysis cohort had a predicted mortality of 31%, and a predicted unfavourable outcome of 51%.
Optimal number of clusters
Applying a penalty of 1/n from the median CSI of each number of clusters revealed a peak in median CSI, indicating the highest cluster stability, for 6 clusters, Fig. 3. Cluster assignments in twenty randomly generated models of 6 clusters are presented in Fig. 4, demonstrating the robust reproducibility of our model. The number of patients in the clusters was 48, 262, 360, 343, 218, and 497, respectively.
Importance of features included in the model
GCS motor score, GCS total score, lactate, oxygen saturation (SpO2), creatinine, glucose, base excess, pH, PaCO2 and body temperature were identified as the most important features in our model with respect to MI. Median values in all clusters are presented in Fig. 5, Table 2, and a full list of MI and cluster medians for all features is provided in, Additional file 4: Table S2. A description of cluster characteristics is given in Fig. 6 and Table 3. The results were interpreted by the authors with extensive academic and clinical neurointensive care experience. The six clusters may generally be described by combinations of GCS score and degrees or patterns of deranged metabolism. Outcome predictions and parameters, as well as injury severity and treatment features which were not used in the clustering, are presented in Table 4.
Description of the 6 clusters. The six identified clusters can, in general, be seen as distinguished by GCS and degree of metabolic derangement. The percentage of patients in each cluster with unfavourable outcome and cluster mortality is indicated as well. RTIs, road traffic incidents; DC, decompressive craniectomy; TAI, traumatic axonal injury
Relation of clusters to outcome
Outcome information was not included in the clustering process. In all clusters except Clusters B and C, the IMPACT model overestimated mortality with over-estimation ranging from 4 to 7%, but underestimated functional outcome in four of the six clusters, with an underestimation ranging from − 2 to − 15%. By adding the cluster label to the IMPACT extended model variables (age, GCS motor score, pupil reactivity, Rotterdam CT score, presence of traumatic subarachnoid haemorrhage, intraventricular haemorrhage, epidural hematoma, hypoxia, and hypotension), predictions for mortality as well as unfavourable outcome were improved with a small but statistically significant increase of Nagelkerke pseudo-R2 from 0.42 to 0.44 and from 0.36 to 0.38, respectively (p = 0.001 and p = 2.9 × 10–5, respectively). The improvement in explained variance was comparable to that achieved by the addition of laboratory values within the original IMPACT model for mortality prediction (Nagelkerke pseudo-R2 0.42 to 0.44, p = 3.6 × 10–5), and prediction of unfavourable outcome (Nagelkerke pseudo-R2 of 0.36 to 0.37, p = 2.1 × 10–4). These clusters, therefore, appear to represent groups with outcomes that differ in both directions from current prediction models. The relationships of clusters to outcomes and IMPACT predicted outcomes are seen in Table 4.
Discussion
We have used an EM clustering approach, based on early clinical and laboratory data, that identified six distinct potential clusters of TBI patients admitted to the ICU. These clusters exhibited distinct systemic metabolic profiles defined by combinations of plasma lactate, SpO2, creatinine, glucose, base excess, pH, PaCO2, and body temperature, which in combination with GCS, characterizes 6 clinically distinct patient endotypes.
Profiles of metabolic derangement may be readily recognized clinically, and arguably contribute to our impression of severity state in TBI patients in the ICU. However, except for blood glucose, the identified features are not incorporated into current formal definitions of TBI severity or outcome prediction models, although earlier publications have reported improved accuracy adding physiology-based prediction scores, such as APACHE II score (Acute Physiology and Chronic Health Evaluation II) [23, 24].
We hypothesize there to be several diverging mechanisms leading to deranged metabolism in TBI patients that are not fully captured by conventional ICU disease severity metrics, such as APACHE II scores. These may include interplay of secondary and extracranial injury, and concurrent comorbidities. This is the rationale for defining metabolic profiles using several features highly correlated to pH– base excess, PaCO2, and lactate that may reflect several intrinsic mechanisms. In Cluster C, a deranged metabolic picture appears to reflect a general stress response, with high lactate and high blood glucose in more elderly patients prone to insulin resistance. In contrast, Cluster E is representative of younger patients displaying tachycardia and relative hypotension, in whom the cause of metabolic compromise is more likely to reflect a state of systemic shock, which is more likely to be related to extracranial injury. It must be noted that the endotype with a general stress response is a relatively small subset of patients (N = 48) but may nevertheless be of clinical importance as it seems likely to result from a distinct pathology. These two metabolic pictures may easily be distinguished clinically and likely benefit from different treatment approaches allowing for articulation of broad strategies of care and overall management in endotypic groups.
Although GCS has been shown to be one of the most principal factors in classification of TBI [16], the weakness of GCS alone as a classifier of TBI severity becomes apparent in this study. In Cluster A, comprising 28% (N = 497) of the total number of patients TBI severity would be classified as ‘mild’ based on GCS. This group was in general characterized by patients who were older with comorbidities and receiving anticoagulant or antiplatelet treatments pre-injury and the cause of ICU admission did not seem to be explained by extracranial injuries, Table 4, but may rather have been motivated by a need for clinical observation, something which was highlighted in a previous CENTER-TBI sub-study [6]. However, the morbidity and treatment burden in this group is substantial: 45% of patients in this cluster were intubated, 15% had ICP monitoring, and 7% died within 6 months post-injury. In addition, Cluster C, the cluster with deranged metabolism, had the largest deviation in outcome prediction in comparison with the IMPACT model. When compared to Cluster D (which comprises patients with severe TBI but without such metabolic derangement), Cluster C had a worse outcome, which further supports the impact of assessing the metabolic profile in TBI patients, beyond derangements that are simply explained by extracranial injuries. It may also reflect an increased vulnerability of the brain in older patients which is not captured in other factors associated with severity of brain damage, such as GCS. Although they did not have as complete a description of biochemical derangements in their dataset, Folweiler et al. elegantly showed TBI clustering that did not relate well to ‘mild’, ‘moderate’ or ‘severe’ descriptions of TBI [25]. In our study again, although GCS is here shown to be an important component of endotypes in an ICU cohort, metabolic profiles may add additional, clinically important, information as descriptors of TBI severity, and perhaps identify patient groups in which treatment should be individualized.
Surprisingly, neither our model nor earlier endotypic multidimensional descriptions of TBI patients generated by unsupervised machine learning methods have identified the type of intracranial injuries and CT characteristics as relevant for describing endotypes [25, 26]. However, a recent study could identify clusters based solely on CT characteristics [27], supporting that these factors may play an essential role in understanding the type of injury and determining the need for intracranial surgery, and prediction models using CT findings such as the Marshall, Rotterdam, Helsinki and Stockholm CT scores do discriminate outcome [28,29,30,31]. These findings are less evident in multivariable analyses when including GCS and other IMPACT variables as covariates, then contributing approximately only 5% additional pseudo-variance toward outcome [31, 32]. This covariance may be a possible explanation as to why we could not identify CT characteristics as one of the most important discriminative factors between the clusters.
Unsupervised learning is appealing from the point of view of objectivity, but cannot be performed entirely without making certain choices, and requires subsequent interpretation. The number of clusters is a trade-off between not being overwhelmed by multitudes of clusters with small sizes that cannot be interpreted, and very few clusters inherently containing little discriminant information. The identification of six clusters of TBI patients was supported by both the maximal and stable reproducibility represented by a CSI of 80%, as well as a suggestion of clinical relevance. Most clusters were relatively stable across different random initializations of the clustering, with the exception of cluster B and C, both representing patients with intermediate GCS, Fig. 4. By nature, more extreme patient characteristics and their corresponding clusters tend to be more stable while the intermediate level characteristics and clusters are less stable. Most patients were clearly assigned to a stable cluster, as seen in, Additional file 1: Fig. S2. It is unrealistic to expect perfectly stable cluster assignment in heterogenous real world data with any method, particularly with random assignment to initial clusters. We believe our evaluation of model robustness to be an important and generalisable strength of our work.
In this study we are naturally limited by the variables collected. These represent nevertheless the compound experience and knowledge of a large cohort of leading TBI researchers and clinicians during the planning of the study. However, additional variables such as future biomarkers and genetic profiles may be needed to sufficiently describe patient heterogeneity in TBI. Furthermore, despite that the object of clustering is to identify reproducible compound and complex patterns, it does not weigh variables toward severity as would for example an experienced clinician and represents a general limitation of unsupervised leaning.
The aim of this study was not primarily to create clusters of TBI patients toward outcome prediction, but to identify clinically relevant and distinct endotypes of patients, which could potentially infer personalization of future treatment strategies. Current TBI therapy is based on limited high-level evidence, leading to between-centre treatment variability beyond that of case mix [7, 9, 33]. Further discrimination of patient heterogeneity has been identified as necessary to further the field [7, 9]. Prediction of both mortality and functional outcome using the IMPACT extended model was significantly improved by adding cluster labels. That the metabolic cluster profiles identified in this study are significantly associated with outcome, despite an unsupervised clustering method (not including outcome), supports a biological underpinning and motivates further investigation. A natural progression will be to investigate if the clusters described in this study exhibit a different temporal trajectories in the ICU or, in analogy with work within the field of ARDS [14] respond differently to treatments in earlier RCTs.
Conclusions
While GCS is a strong predictor of TBI outcome, an admission metabolic profile incorporating hypothermia, lactatemia, blood glucose, SpO2, PaCO2, pH, base excess and creatinine allows for a more holistic description of patients with TBI who require ICU care. Synthesis of these data using an unsupervised clustering method reveals six distinct and stable subgroups of TBI patients. Although not a key objective of the analysis, we found that clusters contain information that can provide a significantly better explanation of outcome beyond that provided by variables used in current outcome prediction models. The addition of biomarkers and genetics may improve this endotypic classification further. Future studies should address replication and validation of this approach, but our work provides an important starting point from which to devise and prospectively investigate therapeutic strategies individualised to more biologically relevant groups or TBI patients.
Availability of data and materials
CENTER-TBI encourages data sharing, and there is a data sharing statement published. Data will be made available to researchers who provide a study proposal that is approved by the management committee to achieve the aims in the approved proposal. Proposals can be submitted online at https://www.center-tbi.eu/data/sharing. A data access agreement is required, and all access must comply with regulatory restrictions imposed on the original study.
Abbreviations
- APACHE II score:
-
Acute physiology and chronic health evaluation II score
- ARDS:
-
Acute respiratory distress syndrome
- ASA-PS class:
-
American society of anesthesiologists physical status classification
- aSDH:
-
Acute subdural hematoma
- BMI:
-
Body mass index
- CENTER-TBI:
-
Collaborative European neuro trauma effectiveness research in TBI
- CSI:
-
Cluster similarity index
- DC:
-
Decompressive craniectomy
- EDH:
-
Epidural hematoma
- EM:
-
Expectation maximization
- GCS:
-
Glasgow coma scale
- GOS-E:
-
Glasgow outcome scale extended
- ICU:
-
Intensive care unit
- ICP:
-
Intracranial pressure
- IMPACT:
-
International mission for prognosis and analysis of clinical trials in TBI
- IQR:
-
Interquartile range
- ISS:
-
Injury severity score
- MAP:
-
Mean arterial pressure
- MI:
-
Mutual Information
- MICE:
-
Multiple imputation with chained equations
- PaCO2 :
-
Arterial partial pressure of carbon dioxide
- PaO2 :
-
Arterial partial pressure of oxygen
- RTC:
-
Road traffic collision
- RTI:
-
Road traffic incident
- SpO2 :
-
Oxygen saturation
- TAI:
-
Traumatic axonal injury
- TBI:
-
Traumatic brain injury
- TIL:
-
Therapy intensity level
- tSAH:
-
Traumatic subarachnoid haemorrhage
References
Rubiano AM, Carney N, Chesnut R, Puyana JC. Global neurotrauma research challenges and opportunities. Nature. 2015;527(7578):S193–7.
Roozenbeek B, Maas AIR, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013;9(4):231–6.
Bragge P, Synnot A, Maas AI, Menon DK, Cooper DJ, Rosenfeld JV, et al. A state-of-the-science overview of randomized controlled trials evaluating acute management of moderate-to-severe traumatic brain injury. J Neurotrauma. 2016;33(16):1461–78.
Steyerberg EW, Wiegers E, Sewalt C, Buki A, Citerio G, De Keyser V, et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicentre, longitudinal, cohort study. Lancet Neurol. 2019;18(10):923–34.
Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;36726367(27):2471–81.
Huijben JA, Wiegers EJA, Lingsma HF, Citerio G, Maas AIR, Menon DK, et al. Changing care pathways and between-center practice variations in intensive care for traumatic brain injury across Europe: a CENTER-TBI analysis. Intensive Care Med. 2020;46(5):995–1004.
Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GWJ, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery [Internet]. 2016 [cited 2017 Oct 26]; Available from: www.neurosurgery-online.com
Newcombe VFJ, Chow A. The features of the typical traumatic brain injury patient in the ICU are changing: what will this mean for the intensivist ? Curr Opin Crit Care. 2021;27:80.
Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987–1048.
Maas AIR, Menon DK, Steyerberg EW, Citerio G, Lecky F, Manley GT, et al. Collaborative European neurotrauma effectiveness research in traumatic brain injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery. 2015;76:67–80.
Tenovuo O, Diaz-Arrastia R, Goldstein LE, Sharp DJ, van der Naalt J, Zasler ND. Assessing the severity of traumatic brain injury—time for a change? J Clin Med. 2021;10(1):148.
Kuruvilla ME, Lee FEH, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56(2):219–33.
Prescott HC, Calfee CS, Taylor Thompson B, Angus DC, Liu VX. Toward smarter lumping and smarter splitting: rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am J Respir Crit Care Med. 2016;194(2):147–55.
Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA, et al. Latent class analysis of ARDS subphenotypes: analysis of data from two randomized controlled trials. Lancet Respir Med. 2014;2:611–20.
Antcliffe DB, Burnham KL, Al-Beidh F, Santhakumaran S, Brett SJ, Hinds CJ, et al. Transcriptomic signatures in sepsis and a differential response to steroids. From the VANISH randomized trial. Am J Respir Crit Care Med. 2019;199(8):980–6.
Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, Mchugh GS, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. 2008 [cited 2018 May 24]; Available from: http://journals.plos.org/plosmedicine/article/file?id=https://doi.org/10.1371/journal.pmed.0050165&type=printable
R Core Team. R: A language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2018. Available from: https://www.r-project.org/
Wei T, Simko V, Levy M, Xie Y, Jin Y, Zemla J. R package “corrplot”: visualization of a correlation matrix. Statistician. 2017;56:316–24.
Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. J Roy Stat Soc Ser B (Methodol). 1977;39(1):1–22.
Holst A. The use of a bayesian neural network model for classification tasks. [Internet]. 1997 p. 172. Available from: oai:DiVA.org:su-61748
Lange T, Roth V, Braun ML, Buhmann JM. Stability-based validation of clustering solutions. Neural Comput. 2004;16(6):1299–323.
van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67.
Hyam JA, Welch CA, Harrison DA, Menon DK. Case mix, outcomes and comparison of risk prediction models for admissions to adult, general and specialist critical care units for head injury: a secondary analysis of the ICNARC case mix programme database. Crit Care. 2006;10(Suppl 2):S2.
Raj R, Skrifvars MB, Bendel S, Selander T, Kivisaari R, Siironen J, et al. Predicting six-month mortality of patients with traumatic brain injury: usefulness of common intensive care severity scores. Crit Care. 2014;18(2):1–9.
Folweiler KA, Sandsmark DK, Diaz-Arrastia R, Cohen AS, Masino AJ. Unsupervised machine learning reveals novel traumatic brain injury patient phenotypes with distinct acute injury profiles and long-term outcomes. J Neurotrauma. 2020;1444:1431–44.
Gravesteijn BY, Sewalt CA, Ercole A, Akerlund C, Nelson D, Maas AIR, et al. Toward a new multi-dimensional classification of traumatic brain injury: a collaborative european neurotrauma effectiveness research for traumatic brain injury study. J Neurotrauma. 2020;37(7):1002–10.
Yuh EL, Jain S, Sun X, Pisică D, Harris MH, Taylor SR, et al. Pathological computed tomography features associated with adverse outcomes after mild traumatic brain injury: A TRACK-TBI study with external validation in CENTER-TBI. JAMA Neurol. 2021;78(9):1137–48.
Maas AIR, Hukkelhoven CWPM, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57(6):1173–81.
Thelin EP, Nelson DW, Vehviläinen J, Nyström H, Kivisaari R, Siironen J, et al. Evaluation of novel computerized tomography scoring systems in human traumatic brain injury: an observational, multicenter study. PLoS Med. 2017;14(8):1–19.
Raj R, Siironen J, Skrifvars MB, Hernesniemi J, Kivisaari R. Predicting outcome in traumatic brain injury: development of a novel computerized tomography classification system (Helsinki Computerized Tomography Score). Neurosurgery. 2014;75(6):632–46.
Nelson DW, Nyström H, MacCallum RM, Thornquist B, Lilja A, Bellander BM, et al. Extended analysis of early computed tomography scans of traumatic brain injured patients and relations to outcome. J Neurotrauma. 2010;27(1):51–64.
Lingsma HF, Roozenbeek B, Steyerberg EW, Murray GD, Maas AI. Early prognosis in traumatic brain injury: from prophecies to predictions. Lancet Neurol. 2010;9(5):543–54.
Hawryluk GWJ, Aguilera S, Buki A, Bulger E, Citerio G, Cooper DJ, et al. A management algorithm for patients with intracranial pressure monitoring: the seattle international severe traumatic brain injury consensus conference (SIBICC). Intensive Care Med. 2019;45(12):1783–94.
CENTER-TBI. CENTER-TBI Ethical approval [Internet]. Available from: https://www.center-tbi.eu/project/ethical-approval
Acknowledgements
Data for the CENTER-TBI study was collected through Quesgen e-CRF (Quesgen Systems Inc, USA), hosted on the INCF platform and extracted via the INCF Neurobot tool (INCF, Sweden). Version 3.0 of the CENTER-TBI dataset was used in this manuscript.
The authors would like to thank the patients for their participation in the CENTER-TBI study, and all CENTER-TBI Participants and Investigators for taking part in the collection and sharing their valuable expertise: Cecilia Åkerlund1, Krisztina Amrein7, Nada Andelic8, Lasse Andreassen9, Audny Anke10, Anna Antoni11, Gérard Audibert12, Philippe Azouvi13, Maria Luisa Azzolini14, Ronald Bartels15, Pál Barzó16, Romuald Beauvais17, Ronny Beer18, Bo-Michael Bellander19, Antonio Belli20, Habib Benali21, Maurizio Berardino22, Luigi Beretta14, Morten Blaabjerg23, Peter Bragge24, Alexandra Brazinova25, Vibeke Brinck26, Joanne Brooker27, Camilla Brorsson28, Andras Buki29,30, Monika Bullinger31, Manuel Cabeleira32, Alessio Caccioppola33, Emiliana Calappi33, Maria Rosa Calvi14, Peter Cameron34, Guillermo Carbayo Lozano35, Marco Carbonara33, Simona Cavallo22, Giorgio Chevallard36, Arturo Chieregato36, Giuseppe Citerio37,38, Hans Clusmann39, Mark Coburn40, Jonathan Coles41, Jamie D. Cooper42, Marta Correia43, Amra Čović44, Nicola Curry45, Endre Czeiter29,30, Marek Czosnyka32, Claire DahyotFizelier46, Paul Dark47, Helen Dawes48, Véronique DeKeyser49, Vincent Degos21, Francesco Della Corte50, Hugo den Boogert15, Bart Depreitere51, Đula Đilvesi52, Abhishek Dixit53, Emma Donoghue27, Jens Dreier54, GuyLoup Dulière55, Ari Ercole53, Patrick Esser53, Erzsébet Ezer61, Martin Fabricius62, Valery L. Feigin63, Kelly Foks59, Shirin Frisvold60, Alex Furmanov61, Pablo Gagliardo62, Damien Galanaud21, Dashiell Gantner34, Guoyi Gao63, Pradeep George64, Alexandre Ghuysen65, Lelde Giga66, Ben Glocker67, Jagoš Golubovic52, Pedro A. Gomez68, Johannes Gratz69, Benjamin Gravesteijn70, Francesca Grossi50, Russell L. Gruen71, Deepak Gupta72, Juanita A. Haagsma70, Iain Haitsma73, Raimund Helbok18, Eirik Helseth74, Lindsay Horton75, Jilske Huijben70, Peter J. Hutchinson76, Bram Jacobs77, Stefan Jankowski78, Mike Jarrett26, Jiyao Jiang64, Faye Johnson79, Kelly Jones58, Mladen Karan52, Angelos G. Kolias74, Erwin Kompanje80, Daniel Kondziella57, Evgenios Kornaropoulos53, LarsOwe Koskinen81, Noémi Kovács82, Ana Kowark83, Alfonso Lagares68, Linda Lanyon64, Steven Laureys84, Fiona Lecky85,86, Didier Ledoux84, Rolf Lefering87, Valerie Legrand88, Aurelie Lejeune89, Leon Levi90, Roger Lightfoot91, Hester Lingsma70, Andrew I. R. Maas49, Ana M. CastañoLeón68, Marc Maegele92, Marek Majdan25, Alex Manara91, Geoffrey Manley94, Costanza Martino95, Hugues Maréchal55, Julia Mattern96, Catherine McMahon97, Béla Melegh98, David Menon53, Tomas Menovsky49, Ana Mikolic70, Benoit Misset84, Visakh Muraleedharan64, Lynnette Murray34, Ancuta Negru99, David Nelson1, Virginia Newcombe53, Daan Nieboer70, József Nyirádi7, Otesile Olubukola85, Matej Oresic100, Fabrizio Ortolano33, Aarno Palotie101,102,103, Paul M. Parizel104, JeanFrançois Payen105, Natascha Perera17, Vincent Perlbarg21, Paolo Persona106, Wilco Peul107,108, Anna Piippo-Karjalainen109, Matti Pirinen101, Dana Pisica70, Horia Ples99, Suzanne Polinder70, Inigo Pomposo35, Jussi P. Posti110, Louis Puybasset111, Andreea Radoi112, Arminas Ragauskas113, Rahul Raj109, Malinka Rambadagalla114, Isabel Retel Helmrich70, Jonathan Rhodes115, Sylvia Richardson116, Sophie Richter53, Samuli Ripatti101, Saulius Rocka113, Cecilie Roe117, Olav Roise118,119, Jonathan Rosand120, Jeffrey V. Rosenfeld121, Christina Rosenlund122, Guy Rosenthal61, Rolf Rossaint83, Sandra Rossi106, Daniel Rueckert67, Martin Rusnák123, Juan Sahuquillo112, Oliver Sakowitz96,124, Renan SanchezPorras124, Janos Sandor125, Nadine Schäfer87, Silke Schmidt126, Herbert Schoechl127, Guus Schoonman128, Rico Frederik Schou129, Elisabeth Schwendenwein11, Charlie Sewalt70, Ranjit D. Singh107,108, Toril Skandsen130,131, Peter Smielewski32, Abayomi Sorinola132, Emmanuel Stamatakis53, Simon Stanworth45, Robert Stevens133, William Stewart134, Ewout W. Steyerberg4,70,135, Nino Stocchetti3,136, Nina Sundström137, Riikka Takala138, Viktória Tamás132, Tomas Tamosuitis139, Mark Steven Taylor25, Braden Te Ao58, Olli Tenovuo110, Alice Theadom58, Matt Thomas93, Dick Tibboel140, Marjolein Timmers80, Christos Tolias141, Tony Trapani34, Cristina Maria Tudora99, Andreas Unterberg96, Peter Vajkoczy142, Shirley Vallance34, Egils Valeinis66, Zoltán Vámos56, Mathieu van der Jagt143, Gregory Van der Steen49, Joukje van der Naalt77, Jeroen T. J. M. van Dijck107,108, Inge A. van Erp107,108, Thomas A. van Essen107,108, Wim Van Hecke144, Caroline van Heugten48, Dominique Van Praag145, Ernest van Veen70, Thijs Vande Vyvere144, Roel P. J. van Wijk107,108, Alessia Vargiolu38, Emmanuel Vega89, Kimberley Velt70, Jan Verheyden144, Paul M. Vespa146, Anne Vik130,147, Rimantas Vilcinis139, Victor Volovici73, Nicole von Steinbüchel44, Daphne Voormolen70, Petar Vulekovic52, Kevin K. W. Wang148, Daniel Whitehouse53, Eveline Wiegers70, Guy Williams53, Lindsay Wilson75, Stefan Winzeck53, Stefan Wolf149, Zhihui Yang120, Peter Ylén150, Alexander Younsi96, Frederick A. Zeiler53,151, Veronika Zelinkova25, Agate Ziverte66, Tommaso Zoerle33
1Section of Perioperative Medicine and Intensive Care, Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden 2,*School of Electrical Engineering and Computer Science, KTH Royal Institute of Technology, Stockholm, Sweden 3Neuroscience Intensive Care Unit, Department of Pathophysiology and Transplants, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, University of Milan, Milan, Italy 4Department of Biomedical Data Sciences, Leiden University Medical Center, Leiden, The Netherlands 5Division of Anaesthesia, Department of Medicine, University of Cambridge, Cambridge, UK 6Centre for Artificial Intelligence in Medicine, University of Cambridge, Cambridge, UK 7János Szentágothai Research Centre, University of Pécs, Pécs, Hungary 8Division of Surgery and Clinical Neuroscience, Department of Physical Medicine and Rehabilitation, Oslo University Hospital and University of Oslo, Oslo, Norway 9Department of Neurosurgery, University Hospital Northern Norway, Tromso, Norway 10Department of Physical Medicine and Rehabilitation, University Hospital Northern Norway, Tromso, Norway 11Trauma Surgery, Medical University Vienna, Vienna, Austria 12Department of Anesthesiology and Intensive Care, University Hospital Nancy, Nancy, France 13Raymond Poincare Hospital, Assistance Publique – Hopitaux de Paris, Paris, France 14Department of Anesthesiology and Intensive Care, S Raffaele University Hospital, Milan, Italy 15Department of Neurosurgery, Radboud University Medical Center, Nijmegen, The Netherlands 16Department of Neurosurgery, University of Szeged, Szeged, Hungary 17International Projects Management, ARTTIC, Munchen, Germany 18Neurological Intensive Care Unit, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria 19Department of Neurosurgery and Anesthesia and Intensive Care Medicine, Karolinska University Hospital, Stockholm, Sweden 20NIHR Surgical Reconstruction and Microbiology Research Centre, Birmingham, UK 21Anesthesie-Réanimation, Assistance Publique – Hopitaux de Paris, Paris, France 22Department of Anesthesia and ICU, AOU Città della Salute e della Scienza di Turin – Orthopedic and Trauma Center, Turin, Italy 23Department of Neurology, Odense University Hospital, Odense, Denmark 24BehaviourWorks Australia, Monash Sustainability Institute, Monash University, Victoria, Australia 25Department of Public Health, Faculty of Health Sciences and Social Work, Trnava University, Trnava, Slovakia 26Quesgen Systems Inc., Burlingame, CA, USA 27Department of Epidemiology and Preventive Medicine, Australian and New Zealand Intensive Care Research Centre, School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia 28Department of Surgery and Perioperative Science, Umeå University, Umeå, Sweden 29Department of Neurosurgery, Medical School, University of Pécs, Pécs, Hungary 30Neurotrauma Research Group, János Szentágothai Research Centre, University of Pécs, Pécs, Hungary 31Department of Medical Psychology, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany 32Brain Physics Lab, Division of Neurosurgery, Department of Clinical Neurosciences, Addenbrooke’s Hospital, University of Cambridge, Cambridge, UK 33Neuro ICU, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy 34Department of Epidemiology and Preventive Medicine, ANZIC Research Centre, Monash University, Melbourne, VIC, Australia 35Department of Neurosurgery, Hospital of Cruces, Bilbao, Spain 36NeuroIntensive Care, Niguarda Hospital, Milan, Italy 37School of Medicine and Surgery, Università Milano Bicocca, Milan, Italy 38NeuroIntensive Care, ASST di Monza, Monza, Italy 39Department of Neurosurgery, Medical Faculty RWTH Aachen University, Aachen, Germany 40Department of Anesthesiology and Intensive Care Medicine, University Hospital Bonn, Bonn, Germany 41Department of Anesthesia and Neurointensive Care, Cambridge University Hospital NHS Foundation Trust, Cambridge, UK 42School of Public Health and PM, The Alfred Hospital, Monash University, Melbourne, VIC, Australia 43Radiology/MRI Department, MRC Cognition and Brain Sciences Unit, Cambridge, UK 44Institute of Medical Psychology and Medical Sociology, Universitätsmedizin Göttingen, Göttingen, Germany 45Oxford University Hospitals NHS Trust, Oxford, UK 46Intensive Care Unit, CHU Poitiers, Potiers, France 47Critical Care Directorate, University of Manchester NIHR Biomedical Research Centre, Salford Royal Hospital NHS Foundation Trust, Salford, UK 48Movement Science Group, Faculty of Health and Life Sciences, Oxford Brookes University, Oxford, UK 49Department of Neurosurgery, Antwerp University Hospital and University of Antwerp, Edegem, Belgium 50Department of Anesthesia and Intensive Care, Maggiore Della Carità Hospital, Novara, Italy 51Department of Neurosurgery, University Hospitals Leuven, Louvain, Belgium 52Department of Neurosurgery, Faculty of Medicine, Clinical Centre of Vojvodina, University of Novi Sad, Novi Sad, Serbia 53Division of Anaesthesia, Addenbrooke’s Hospital, University of Cambridge, Cambridge, UK 54Center for Stroke Research Berlin, Berlin Institute of Health, Charité – Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt-Universität Zu Berlin, Berlin, Germany 55Intensive Care Unit, CHR Citadelle, Liège, Belgium 56Department of Anaesthesiology and Intensive Therapy, University of Pécs, Pécs, Hungary 57Departments of Neurology, Clinical Neurophysiology and Neuroanesthesiology, Region Hovedstaden Rigshospitalet, Copenhagen, Denmark 58National Institute for Stroke and Applied Neurosciences, Faculty of Health and Environmental Studies, Auckland University of Technology, Auckland, New Zealand 59Department of Neurology, Erasmus MC, Rotterdam, The Netherlands 60Department of Anesthesiology and Intensive Care, University Hospital Northern Norway, Tromso, Norway 61Department of Neurosurgery, Hadassah-Hebrew University Medical Center, Jerusalem, Israel 62Fundación Instituto Valenciano de Neurorrehabilitación (FIVAN), Valencia, Spain 63Department of Neurosurgery, Shanghai Renji Hospital, Shanghai Jiaotong University/School of Medicine, Shanghai, China 64Karolinska Institutet, INCF International Neuroinformatics Coordinating Facility, Stockholm, Sweden 65Emergency Department, CHU, Liège, Belgium 66Neurosurgery Clinic, Pauls Stradins Clinical University Hospital, Riga, Latvia 67Department of Computing, Imperial College London, London, UK 68Department of Neurosurgery, Hospital Universitario 12 de Octubre, Madrid, Spain 69Department of Anesthesia, Critical Care and Pain Medicine, Medical University of Vienna, Vienna, Austria 70Department of Public Health, Erasmus Medical Center-University Medical Center, Rotterdam, The Netherlands 71College of Health and Medicine, Australian National University, Canberra, Australia 72Department of Neurosurgery, Neurosciences Centre and JPN Apex Trauma Centre, All India Institute of Medical Sciences, New Delhi, 110029, India 73Department of Neurosurgery, Erasmus MC, Rotterdam, The Netherlands 74Department of Neurosurgery, Oslo University Hospital, Oslo, Norway 75Division of Psychology, University of Stirling, Stirling, UK 76Division of Neurosurgery, Department of Clinical Neurosciences, Addenbrooke’s Hospital, University of Cambridge, Cambridge, UK 77Department of Neurology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands 78Neurointensive Care, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK 79Salford Royal Hospital NHS Foundation Trust Acute Research Delivery Team, Salford, UK 80Department of Intensive Care and Department of Ethics and Philosophy of Medicine, Erasmus Medical Center, Rotterdam, The Netherlands 81Department of Clinical Neuroscience, Neurosurgery, Umeå University, Umeå, Sweden 82Hungarian Brain Research Program – Grant No. KTIA_13_NAP-A-II/8, University of Pécs, Pécs, Hungary 83Department of Anaesthesiology, University Hospital of Aachen, Aachen, Germany 84Cyclotron Research Center, University of Liège, Liège, Belgium 85Centre for Urgent and Emergency Care Research (CURE), Health Services Research Section, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK 86Emergency Department, Salford Royal Hospital, Salford UK 87Institute of Research in Operative Medicine (IFOM), Witten/Herdecke University, Cologne, Germany 88VP Global Project Management CNS, ICON, Paris, France 89Department of Anesthesiology-Intensive Care, Lille University Hospital, Lille, France 90Department of Neurosurgery, Rambam Medical Center, Haifa, Israel 91Department of Anesthesiology and Intensive Care, University Hospitals Southhampton NHS Trust, Southhampton, UK 92Department of Traumatology, Cologne-Merheim Medical Center (CMMC), Orthopedic Surgery and Sportmedicine, Witten/Herdecke University, Cologne, Germany 93Intensive Care Unit, Southmead Hospital, Bristol, Bristol, UK 94Department of Neurological Surgery, University of California, San Francisco, CA, USA 95Department of Anesthesia and Intensive Care, M. Bufalini Hospital, Cesena, Italy 96Department of Neurosurgery, University Hospital Heidelberg, Heidelberg, Germany 97Department of Neurosurgery, The Walton centre NHS Foundation Trust, Liverpool, UK 98Department of Medical Genetics, University of Pécs, Pécs, Hungary 99Department of Neurosurgery, Emergency County Hospital Timisoara, Timisoara, Romania 100School of Medical Sciences, Örebro University, Örebro, Sweden 101Institute for Molecular Medicine Finland, University of Helsinki, Helsinki, Finland 102Analytic and Translational Genetics Unit, Department of Medicine; Psychiatric and Neurodevelopmental Genetics Unit, Department of Psychiatry, Department of Neurology, Massachusetts General Hospital, Boston, MA, USA 103Program in Medical and Population Genetics; The Stanley Center for Psychiatric Research, The Broad Institute of MIT and Harvard, Cambridge, MA, USA 104Department of Radiology, University of Antwerp, Edegem, Belgium 105Department of Anesthesiology and Intensive Care, University Hospital of Grenoble, Grenoble, France 106Department of Anesthesia and Intensive Care, Azienda Ospedaliera Università di Padova, Padua, Italy 107Department of Neurosurgery, Leiden University Medical Center, Leiden, The Netherlands 108Department of Neurosurgery, Medical Center Haaglanden, The Hague, The Netherlands 109Department of Neurosurgery, Helsinki University Central Hospital, Helsinki, Finland 110Division of Clinical Neurosciences, Department of Neurosurgery and Turku Brain Injury Centre, Turku University Hospital, University of Turku, Turku, Finland 111Department of Anesthesiology and Critical Care, Pitié -Salpêtrière Teaching Hospital, Assistance Publique, Hôpitaux de Paris and University Pierre et Marie Curie, Paris, France 112Neurotraumatology and Neurosurgery Research Unit (UNINN), Vall d’Hebron Research Institute, Barcelona, Spain 113Department of Neurosurgery, Kaunas University of Technology and Vilnius University, Vilnius, Lithuania 114Department of Neurosurgery, Rezekne Hospital, Rēzekne, Latvia 115Department of Anaesthesia, Critical Care and Pain Medicine NHS Lothian, University of Edinburg, Edinburgh, UK 116MRC Biostatistics Unit, Cambridge Institute of Public Health, Cambridge, UK 117Department of Physical Medicine and Rehabilitation, Oslo University Hospital, University of Oslo, Oslo, Norway 118Division of Orthopedics, Oslo University Hospital, Oslo, Norway 119Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway 120Broad Institute, Massachusetts General Hospital, Cambridge MA Harvard Medical School, Boston MA, USA 121National Trauma Research Institute, The Alfred Hospital, Monash University, Melbourne, VIC, Australia 122Department of Neurosurgery, Odense University Hospital, Odense, Denmark 123International Neurotrauma Research Organisation, Vienna, Austria 124Klinik Für Neurochirurgie, Klinikum Ludwigsburg, Ludwigsburg, Germany 125Division of Biostatistics and Epidemiology, Department of Preventive Medicine, University of Debrecen, Debrecen, Hungary 126Department Health and Prevention, University Greifswald, Greifswald, Germany 127Department of Anaesthesiology and Intensive Care, AUVA Trauma Hospital, Salzburg, Austria 128Department of Neurology, Elisabeth-TweeSteden Ziekenhuis, Tilburg, The Netherlands 129Department of Neuroanesthesia and Neurointensive Care, Odense University Hospital, Odense, Denmark 130Department of Neuromedicine and Movement Science, Norwegian University of Science and Technology, NTNU, Trondheim, Norway 131Department of Physical Medicine and Rehabilitation, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway 132Department of Neurosurgery, University of Pécs, Pécs, Hungary 133Division of Neuroscience Critical Care, John Hopkins University School of Medicine, Baltimore, USA 134Department of Neuropathology, Queen Elizabeth University Hospital and University of Glasgow, Glasgow, UK 135Deptartment of Department of Biomedical Data Sciences, Leiden University Medical Center, Leiden, The Netherlands 136Department of Pathophysiology and Transplantation, Neuroscience ICU, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan University, Milan, Italy 137Department of Radiation Sciences, Biomedical Engineering, Umeå University, Umeå, Sweden 138Intensive Care Medicine and Pain Management, Perioperative Services, Turku University Hospital, University of Turku, Turku, Finland 139Department of Neurosurgery, Kaunas University of Health Sciences, Kaunas, Lithuania 140Intensive Care and Department of Pediatric Surgery, Erasmus Medical Center, Sophia Children’s Hospital, Rotterdam, The Netherlands 141Department of Neurosurgery, Kings College London, London, UK 142Neurologie, Neurochirurgie und Psychiatrie, Charité – Universitätsmedizin Berlin, Berlin, Germany 143Department of Intensive Care Adults, Erasmus MC– University Medical Center Rotterdam, Rotterdam, The Netherlands 144Icometrix NV, Louvain, Belgium 145Psychology Department, Antwerp University Hospital, Edegem, Belgium 146Director of Neurocritical Care, University of California, Los Angeles, USA 147Department of Neurosurgery, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway 148Department of Emergency Medicine, University of Florida, Gainesville, FL, USA 149Department of Neurosurgery, Berlin Institute of Health, Charité – Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt-Universität Zu Berlin, Berlin, Germany 150VTT Technical Research Centre, Tampere, Finland 151Section of Neurosurgery, Department of Surgery, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada
Funding
Open access funding provided by Karolinska Institute. CENTER-TBI was supported by the European Union 7th Framework program (EC grant 602150). Additional funding was obtained from the Hannelore Kohl Stiftung (Germany), from OneMind (USA) and from Integra LifeSciences Corporation (USA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
AH, AE and DN undertook the primary supervision of the project. NS, ES, DM, DN took part in funding acquisition. CÅ, AE and DN were involved in data curation. CÅ, AH, AE and DN prepared the draft manuscript. CÅ and AH did the analyses and prepared the script used for the analyses. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This sub-study was approved by the CENTER-TBI management committee. The CENTER-TBI study was conducted in accordance with all relevant laws of the European Union if directly applicable or of direct effect and all relevant laws of the country where the Recruiting sites were located, including but not limited to, the relevant privacy and data protection laws and regulations (the “Privacy Law”), the relevant laws and regulations on the use of human materials, and all relevant guidance relating to clinical studies from time to time in force including, but not limited to, the ICH Harmonised Tripartite Guideline for Good Clinical Practice (CPMP/ICH/135/95) (“ICH GCP”) and the World Medical Association Declaration of Helsinki. Informed Consent by the patients or next of kin was obtained, according to the local legislations, for all patients recruited in the Core Dataset of CENTER-TBI and documented in the electronic case report form. Ethical approval for CENTER-TBI was obtained at each recruiting site. The list of sites, Ethical Committees, approval numbers and approval dates are available online [34].
Consent for publication
Not applicable.
Competing interests
DM reports grants, personal fees and non-financial support from GlaxoSmithKline, grants and personal fees from NeuroTrauma Sciences, personal fees from Pfizer Ltd, personal fees from PressuraNeuro, grants and personal fees from Lantmannen AB, grants and personal fees from Integra, outside the submitted work. All other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Figures of flowchart of patient selection and cluster assignment probabilities.
Additional file 2
: Table of completeness of all features in the dataset.
Additional file 3
: Additional details of the clustering method.
Additional file 4
: Table of Cluster means and overall means, min, max and standard deviation of all features.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Åkerlund, C.A.I., Holst, A., Stocchetti, N. et al. Clustering identifies endotypes of traumatic brain injury in an intensive care cohort: a CENTER-TBI study. Crit Care 26, 228 (2022). https://doi.org/10.1186/s13054-022-04079-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-04079-w