Abstract
Background
The study aims at scientifically investigating the genetic effect of four polymorphisms (rs7975232, rs1544410, rs2228570, and rs731236) within the human Vitamin D Receptor (VDR) gene on the odds of psoriasis through an updated meta-analysis.
Methods
We searched eight databases and screened the studies for pooling. Finally, a total of eighteen eligible case-control studies were included. BH (Benjamini & Hochberg) adjusted P-values of association (Passociation) and odd ratios (ORs) with the corresponding 95% confidence intervals (CIs) were calculated under the allele, homozygote, heterozygote, dominant, recessive, and carrier models.
Results
Compared with the negative controls, no statistically significant difference in the odds of psoriasis was detected for the cases under any genetic models (BH adjusted Passociation > 0.05). We also performed subgroup meta-analyses by the source of controls, ethnicity, country, Hardy-Weinberg equilibrium, and genotyping method. Similar results were observed in most subgroup meta-analyses (BH adjusted Passociation > 0.05). Besides, data of Begg’s and Egger’s tests excluded the significant publication bias; while the sensitivity analysis data further indicated the statistical reliability of our pooling results.
Conclusion
The currently available data fails to support a robust association between VDR rs7975232, rs1544410, rs2228570 and rs731236 polymorphisms and psoriasis susceptibility, which still required the support of more case-control studies.
Similar content being viewed by others
Background
Vitamin D Receptor (VDR) protein, a member of the nuclear receptor superfamily of ligand-activated transcription factors, is thought to be implicated in several cell biological events (e.g., calcium and phosphate homeostasis, cell differentiation and apoptosis) [1, 2]. The human VDR gene is mapped on chromosome 12 and contains four common polymorphisms, namely rs7975232 A/C in intron eight (ApaI) rs1544410 G/A in intron eight (BsmI), rs2228570 T/C in exon two (FokI), and rs731236 T/C in exon nine (TaqI) [3,4,5]. In addition, linkage disequilibrium exists among the rs7975232, rs1544410, and rs731236 polymorphisms [6, 7]. Here, we investigated the possible role of VDR rs7975232, rs1544410, rs2228570, and rs731236 polymorphisms in the susceptibility to psoriasis disease.
Psoriasis is a type of chronic inflammatory immune-mediated disease with discrete, erythematous scaly plaques on the skin, and is characterized by the abnormal proliferation of keratinocytes and disordered maturation of the epidermis [8,9,10]. Genetic factors are potentially linked to the occurrence or pathogenesis of psoriasis [11, 12]. We observed the open questions of the association between the VDR polymorphisms and psoriasis susceptibility among different populations. For instance, the rs7975232 polymorphism of VDR was reportedly associated with the psoriasis risks in the Korean population [13, 14], Chinese population [15], or Turkish population [16, 17]. However, the VDR rs7975232 polymorphism was not considered a risk factor for psoriasis cases in Japan [18], Italy [19], Croatia [20], or Egypt [21]. Therefore, it is meaningful to conduct a meta-analysis to pool the relevant data for a comprehensive assessment of this issue. Even though a recent meta-analysis was conducted by searching three databases in February 2018 [3], the publication of possible new data, different database retrieval, data collection and analysis strategies led us to perform another updated comprehensive pooling analysis and a series of followed stratification analysis, of gene-disease association up to August 18, 2019.
Methods
Database retrieval
Referring to the HuGENet™ HuGE Review Handbook, version 1.0, we retrieved the relevant publications from eight online databases, including PubMed, Web of Science (WOS), Excerpta Medica Database (EMBASE), China National Knowledge Infrastructure (CNKI), WANFANG, OVID, Scopus and Cochrane, up to August 18, 2019, without any restrictions regarding geographical, language or publication time. We provided the searching terms in Additional file 1: Table S1.
Inclusion and exclusion criteria
Three investigators (J. Li, L. Sun, and J. Sun) designed the inclusion and exclusion criteria, independently screened the above articles, and evaluated the eligibility. Inclusion criteria: (1) comparing psoriasis cases versus negative controls; (2) detecting the VDR polymorphisms; (3) containing the major/minor allele frequency or completed genotype distribution. Exclusion criteria: (1) non-human studies; (2) reviews; (3) meeting or conference abstracts; (4) meta-analyses; (5) other diseases; (6) other genes; (7) expression or non-single nucleotide polymorphism (SNP); (8) duplicate or overlapped data.
Data collecting
Two investigators (J. Li and L. Sun) designed a form and independently collected the information, including the first author, publication year, ethnicity, source of controls, gender, age, calcipotriol response, family history, genotyping method and genotype frequency. Based on the genotype frequency distribution, we utilized the chi-square test to calculate the P-value of HWE. The summarized data were assessed together for errors. When the frequency data were missing, the investigator (M. Yan) sent an email to the corresponding author. In addition, two investigators (J. Li and L. Sun) assessed the study quality using the Newcastle-Ottawa quality assessment scale (NOS) where scores range between 1 and 9. When a disagreement was encountered, we discussed with the third investigator (M. Yan) to obtain consensus. We considered studies high quality when the NOS score ≥ 5.
Tests for association, heterogeneity
After data sorting via Microsoft Excel 2016, STATA 12.0 software (StataCorp, USA) was applied to obtain the P-value of association, ORs and 95% CI under the allele (allele C vs. A for VDR rs7975232 polymorphism; allele A vs. G for rs1544410 polymorphism; allele C vs. T for rs2228570 polymorphism; allele C vs. T for rs731236 polymorphism), homozygote (CC vs. AA; AA vs. GG; CC vs. TT; CC vs. TT), heterozygote (AC vs. AA; GA vs. GG; TC vs. CC; TC vs. TT), dominant (AC + CC vs AA; GA + AA vs. GG; TC + CC vs. TT; TC + CC vs. TT), recessive (CC vs. AA+AC; AA vs. GG + GA; CC vs. TT + TC; CC vs. TT + TC) and carrier (carrier C vs. A; carrier A vs. G; carrier C vs. T; carrier C vs. T) models. We utilized the BH (Benjamini & Hochberg) correction method to adjust the Passociation value through the p.adjust () function of R software version 3.4.4. BH-corrected Passociation < 0.05 from the association test was considered statistically significant.
>Based on the “meta-analysis of binary data” function of STATA 12.0 software, we obtained the I2 value (variation in ORs attributable to heterogeneity) and P-value of heterogeneity. When P-value < 0.05 or the I2 value > 50%, we utilized the random-effect pooling model (DerSimonian and Laird method); Otherwise, we used a fixed-effect model (Mantel-Haenszel method). To assess data stability and the source of potential heterogeneity, we conducted a series of subgroup analyses based on the factors of the control source, ethnicity, country, HWE, and genotyping method.
We performed the sensitivity analyses under all the genetic models, through the “influence analysis, metan-based (metaninf)” function of STATA 12.0 software. Upon the exclusion of each study one by one, the lack of largely affected meta-analysis estimates in figures suggested the statistical stability of data. If not, the omitted studies are deemed as the source of heterogeneity.
Tests for publication bias
We also performed the Begg’s test and Egger’s test to evaluate the potential publication bias through the “Publication Bias (metabias)” function of STATA 12.0 software. Begg’s funnel plot and Egger’s publication bias plot were generated, respectively. The basically symmetrical funnel plot, P-values for Begg’s test and Egger’s test greater than 0.05 indicate the absence of larger publication bias.
Results
Case-control study identification
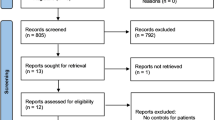
Figure 1 presents the flow chart of study identification. We first retrieved 1955 records from eight on-line databases [PubMed (n = 251), EMBASE (n = 342), WOS (n = 451), CNKI (n = 54), WANFANG (n = 6), OVID (n = 684), Scopus (n = 141) and Cochrane (n = 26)]. We then screened a total of 705 records after removing duplicate records from different databases. Next, we excluded an additional 620 records per the exclusion criteria. The detailed information was shown in Fig. 1. After assessing the eligibility of 85 full-text articles, we removed an additional 67 articles with “expression or non-SNP” data. Finally, we included a total of 18 case-control studies [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30] for our meta-analysis. We also summarized and listed the genotypic distribution (Table 1) and clinical characteristics, (Additional file 2: Table S2). No low-quality studies with a NOS quality score ≥ five were included in this analysis (Additional file 3: Table S3).
VDR rs7975232 polymorphism
There are a total of thirteen case-control studies with 1654 cases and 1991 controls for the meta-analysis of the VDR rs7975232 polymorphism and psoriasis susceptibility. The heterogeneity under the carrier C vs. A model (Table 2, I2 = 42.3%, Pheterogeneity = 0.053) led to the utilization of a random-effects pooling model, and a fixed-effects pooling model was utilized for the other genetic models. Pooling results of Table 2 showed no statistically significant difference in the odds of psoriasis between cases and controls under the following six genetic models: allele C vs. A [Passociation (P-value of association) =0.640, BH-adjusted Passociation = 0.960], homozygote CC vs. AA (Passociation = 0.585, BH-adjusted Passociation = 0.960), heterozygote AC vs. AA (Passociation = 0.370, BH-adjusted Passociation = 0.960), dominant AC + CC vs. AA (Passociation = 0.356, BH-adjusted Passociation = 0.960), recessive CC vs. AA+AC (Passociation = 0.928, BH-adjusted Passociation = 0.977), and carrier C vs. A (Passociation = 0.977, BH-adjusted Passociation = 0.977). Figure 2 presents the forest plot under the allele model.
We also performed subgroup meta-analyses based on the factors of control source, ethnicity, country, HWE, and genotyping method. We observed no significant differences between cases and controls in any subgroup (Table 2, all Passociation > 0.05, BH-adjusted Passociation > 0.05) except the subgroup of “China” under the carrier model (Passociation = 0.020, BH-adjusted Passociation = 0.120, OR = 1.23). Additional file 4: Figure S1 and Additional file 5: Figure S2 show the forest plots in the subgroup analysis by the factors of ethnicity and the source of controls (allele model). These results suggested that the VDR rs7975232 polymorphism has no significant influence on the susceptibility to psoriasis.
VDR rs1544410 polymorphism
For VDR rs1544410, thirteen studies containing 1620 cases/2001 controls were included. A random-effects pooling model was used for the allele A vs. G (Table 3, I2 = 54.9%, Pheterogeneity = 0.009), whereas a fixed-effects pooling model was utilized for the others (all I2 < 50.0%, Pheterogeneity > 0.05). We did not observe the statistical differences between cases and controls under any genetic model during the overall meta-analysis and subsequent subgroup analysis (Table 3, all Passociation > 0.05, BH-adjusted Passociation > 0.05) with the exception of the “PHWE > 0.05” subgroup under the AA vs. GG + GA model (Passociation = 0.018, BH-adjusted Passociation = 0.108, OR = 0.99) and “PCR-RFLP” subgroup under the GG + GA vs. GG model (Passociation = 0.035, BH-adjusted Passociation = 0.144, OR = 1.46). Figure 3 presents a forest plot of the allele model in the overall meta-analysis, and Additional file 6: Figure S3 and Additional file 7: Figure S4 show the forest plots in the subgroup analysis by the factors of ethnicity and source of controls (allele model). These data suggested that the VDR rs1544410 polymorphism seems not to be linked to the psoriasis susceptibility.
VDR rs2228570 polymorphism
A total of eight studies involving 1308 cases/1253 controls were enrolled for meta-analysis of VDR rs2228570. A fixed-effect pooling model was utilized for the TC vs. TT (Table 4, I2 = 46.2%, Pheterogeneity = 0.84), whereas a random-effects pooling model was used for the others (all I2 > 50.0%, Pheterogeneity < 0.05). As shown in Table 4, no statistically significant association was detected in the overall meta-analysis and subsequent subgroup analysis (Passociation > 0.05, BH-adjusted Passociation > 0.05). Figure 4 shows the forest plot under the allele model, and Additional file 8: Figure S5 and Additional file 9: Figure S6 show the forest plots in the subgroup analysis by the factors of ethnicity and source of controls (allele model). These findings indicated that VDR rs2228570 might not be associated with the risk of psoriasis.
VDR rs731236 polymorphism
During the meta-analysis of VDR rs731236 containing 1690 cases/1857 controls, a random-effect model was used for the allele C vs. T (Pheterogeneity = 0.034), TC vs. TT (Pheterogeneity = 0.043) and TC + CC vs. TT (I2 = 50.7%, Pheterogeneity = 0.027), and a fix-effect model was applied for others (all I2 < 50.0%, Pheterogeneity > 0.05). As shown in Table 5, no differences between cases and controls were detected in all analyses (Table 5, all Passociation > 0.05, BH-adjusted Passociation > 0.05). Figure 5 presents the forest plot of the allele model, and Additional file 10: Figure S7 and Additional file 11: Figure S8 show the forest plot in the subgroup analysis by the factors of ethnicity and source of controls (allele model). As a result, VDR rs731236 polymorphism is not significantly associated with the odds of psoriasis disease.
Sensitivity analysis and publication bias
We did not observe largely altered meta-analysis estimates in the results of our sensitivity analysis (Fig. 6 for the allele model; and other data not shown), suggesting the statistical reliability of pooling results. We also conducted the Begg’s and Egger’s tests to assess the potential publication bias. As shown in Table 6, the P-value of Begg’s and Egger’s test was greater than 0.05 under all the above genetic models. Additional file 12: Figure S9 and Additional file 13: Figure S10 show the Begg’s funnel plots and Egger’s publication bias plots under the allele model. We observed basically symmetrical funnel plots. Therefore, there is no large publication bias in our study.
Discussion
In the current study, we searched eight online electronic databases, including PubMed, EMBASE, WOS, CNKI, WANFANG, OVID, Scopus and Cochrane (up to August 18, 2019), to enroll a total of 18 case-control studies. Based on the currently available data, we conducted a series of overall meta-analysis and subgroup analysis to evaluate the genetic relationship regarding VDR rs7975232, rs1544410, rs2228570, and rs731236 polymorphisms and psoriasis susceptibility. Here, we used the “RS” naming, the most common polymorphism nomenclature in the single nucleotide polymorphism database (dbSNP), rather than the name of restriction enzymes in polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay, namely ApaI, BsmI, FokI, and TaqI. Moreover, six genetic models, including allele, homozygote, heterozygote, dominant, recessive, and carrier models, were employed. BH correction method was also utilized to adjust the P-values obtained from the multiple comparisons.
In our updated meta-analysis of VDR rs7975232, we enrolled thirteen case-control studies for pooling and did not detect any significant statistical association between the VDR rs7975232 polymorphism and the odds of psoriasis. In 2012, Lee, YH et al. included six case-control studies [14, 16,17,18, 21, 24] for a meta-analysis regarding the association between the VDR rs7975232 polymorphism and psoriasis susceptibility [31]. Data from the “Turkish” subgroup containing two case-control studies [16, 17] indicated a potential genetic correlation between the VDR rs7975232 polymorphism and psoriasis susceptibility [31]. In 2013, Liu, J. L. et al. included eight case-control studies [14, 16,17,18, 20, 21, 24, 25] for an updated meta-analysis and only found a positive result under the dominant model (Passociation = 0.043) but not other genetic models [5]. In 2013, Stefanic, M. et al. performed another meta-analysis, which did not include one study [14] but added another study [13], and reported no robust correlation between the VDR rs7975232 polymorphism and psoriasis risk [4]. In the present meta-analysis, we added four new studies [15, 19, 29, 30] in the overall population and subgroup meta-analyses based on the factors of the control source, ethnicity, country, HWE and genotyping method under six genetic models. Our data failed to support the essential role of the VDR rs7975232 polymorphism in the odds of psoriasis, which is in line with the data of Lee, YH [3]..
For rs1544410, rs2228570, and rs731236 polymorphisms, compared with three previous meta-analyses [4, 5, 31], we added four new eligible studies [15, 19, 29, 30] in our updated meta-analysis. Nevertheless, no statistically significant conclusions between VDR rs1544410, rs2228570 and VDR rs731236 polymorphisms and psoriasis susceptibility were observed. The conclusions regarding the genetic effect of VDR rs1544410, rs2228570, but not VDR rs731236 polymorphisms on the odds of psoriasis disease were consistent with the pooling results of Lee, YH [3]., which contains sixteen studies [13, 14, 16,17,18,19,20,21,22, 24,25,26,27,28,29,30]. Subgroup analysis of “Caucasian” suggested that the VDR rs731236 polymorphism is linked to the risk of psoriasis in the Caucasian population under the recessive model, but not the allele, homozygote and dominant models [3]. In our updated study, we added another two new studies [15, 23], and applied two more models, including heterozygote and carrier models. Apart from ethnicity, we also considered the factors of control source, country, and HWE in the subgroup analyses. However, no positive conclusion was observed in any comparison of VDR rs731236. The potential slight genetic effect of VDR rs731236 polymorphism in the high susceptibility to psoriasis in the Caucasian population was masked by the adding of more sample size, and the utilization of BH correction of P-value. Despite of this, we cannot exclude the VDR rs731236 polymorphism in the odds of psoriasis in the Caucasian population, the support of more case-control studies is required.
In this study, three investigators tried the best to reduce the potential bias during database retrieval, study selection, data extraction, and statistical analysis. However, some limitations should be addressed. First, less than ten case-control studies were included in the meta-analysis of the VDR rs2228570 in the overall population. In addition, only one case-control study of the African population [21] is included in the subgroup analysis of VDR rs7975232 and rs731236 by the factor of ethnicity. Given the lack of sufficient genotype data, we did not detect the potential genetic influence of the other VDR variants (such as rs4516035) or the combined variants of VDR and other relevant genes. Second, high heterogeneity between studies was detected in some analyses of VDR polymorphisms and psoriasis susceptibility. We observed a decreased level of between-study heterogeneity in some subgroups of “Asian” or “Caucasian”, indicating that the factor of ethnicity may be implicated in the source of heterogeneity. Third, conflicting conclusions regarding the potential role of VDR polymorphisms in the partial resistance of psoriasis patients to calcipotriol therapy were reported [15, 16, 23, 26, 27]. We extracted the basic information regarding the gender, age, calcipotriol response, and family history within the included case-control studies; nevertheless, the lack of sufficient data did not support the preformation of the relevant stratification analysis or adjusted effect estimates. Increased sample sizes are still needed to investigate the genetic relationship between different VDR polymorphisms and the response of psoriasis patients to drug treatments.
Conclusions
Above all, based on the presently available case-control studies, our pooling analysis data and previous reports do not provide the robust statistical evidence linking VDR rs7975232, rs1544410, and rs2228570 polymorphisms with the odds of psoriasis. More case-control studies will be of assistance to us to further confirm the effect of the VDR polymorphisms on the psoriasis susceptibility in the Caucasian population.
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Abbreviations
- BH:
-
Benjamini & Hochberg
- CNKI:
-
China National Knowledge Infrastructure
- dbSNP:
-
single nucleotide polymorphism database
- EMBASE:
-
Excerpta Medica Database
- HWE:
-
Hardy-Weinberg equilibrium
- LDR:
-
Ligase detection reactions
- NOS:
-
Newcastle-Ottawa quality assessment scale
- ORs:
-
Odd ratios
- PCR-RFLP:
-
Polymerase chain reaction-restriction fragment length polymorphism
- SNP:
-
Single nucleotide polymorphism
- VDR:
-
Vitamin D Receptor
- WOS:
-
Web of Science
References
Zenata O, Vrzal R. Fine tuning of vitamin D receptor (VDR) activity by post-transcriptional and post-translational modifications. Oncotarget. 2017;8(21):35390–402.
Saccone D, Asani F, Bornman L. Regulation of the vitamin D receptor gene by environment, genetics and epigenetics. Gene. 2015;561(2):171–80.
Lee YH. Vitamin D receptor ApaI, TaqI, BsmI, and FokI polymorphisms and psoriasis susceptibility: an updated meta-analysis. Clin Exp Dermatol. 2019;44(5):498–505.
Stefanic M, Rucevic I, Barisic-Drusko V. Meta-analysis of vitamin D receptor polymorphisms and psoriasis risk. Int J Dermatol. 2013;52(6):705–10.
Liu JL, Zhang SQ, Zeng HM. ApaI, BsmI, FokI and TaqI polymorphisms in the vitamin D receptor (VDR) gene and the risk of psoriasis: a meta-analysis. J Eur Acad Dermatol Venereol. 2013;27(6):739–46.
Kostner K, Denzer N, Muller CS, Klein R, Tilgen W, Reichrath J. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: a review of the literature. Anticancer Res. 2009;29(9):3511–36.
Labuda M, Fujiwara TM, Ross MV, Morgan K, Garcia-Heras J, Ledbetter DH, Hughes MR, Glorieux FH. Two hereditary defects related to vitamin D metabolism map to the same region of human chromosome 12q13-14. J Bone Miner Res. 1992;7(12):1447–53.
Schadler ED, Ortel B, Mehlis SL. Biologics for the primary care physician: review and treatment of psoriasis. Dis Mon. 2019;65(3):51–90.
Yadav K, Singh D, Singh MR. Protein biomarker for psoriasis: A systematic review on their role in the pathomechanism, diagnosis, potential targets and treatment of psoriasis. Int J Biol Macromol. 2018;118(Pt B):1796–810.
Grozdev I, Korman N, Tsankov N. Psoriasis as a systemic disease. Clin Dermatol. 2014;32(3):343–50.
Chandra A, Ray A, Senapati S, Chatterjee R. Genetic and epigenetic basis of psoriasis pathogenesis. Mol Immunol. 2015;64(2):313–23.
O'Rielly DD, Rahman P. Genetic, epigenetic and Pharmacogenetic aspects of psoriasis and psoriatic arthritis. Rheum Dis Clin N Am. 2015;41(4):623–42.
Lee DY, Park BS, Choi KH, Jeon JH, Cho KH, Song KY, Kim IG, Youn JI. Vitamin D receptor genotypes are not associated with clinical response to calcipotriol in Korean psoriasis patients. Arch Dermatol Res. 2002;294(1–2):1–5.
Park BS, Park JS, Lee DY, Youn JI, Kim IG. Vitamin D receptor polymorphism is associated with psoriasis. J Invest Dermatol. 1999;112(1):113–6.
Liu JL, Zeng HM, Lin MG, Ju M, Wu ZM, Li MJ, Li MJ, Liu YY, Yin M. Association of vitamin D receptor polymorphisms with susceptibility to psoriasis vulgaris and clinical response to calcipotriol in patients with psoriasis vulgaris. Chin J Dermatol. 2017;50(12):889–93.
Dayangac-Erden D, Karaduman A, Erdem-Yurter H. Polymorphisms of vitamin D receptor gene in Turkish familial psoriasis patients. Arch Dermatol Res. 2007;299(10):487–91.
Kaya TI, Erdal ME, Tursen U, Camdeviren H, Gunduz O, Soylemez F, Ikizoglu G. Association between vitamin D receptor gene polymorphism and psoriasis among the Turkish population. Arch Dermatol Res. 2002;294(6):286–9.
Okita H, Ohtsuka T, Yamakage A, Yamazaki S. Polymorphism of the vitamin D(3) receptor in patients with psoriasis. Arch Dermatol Res. 2002;294(4):159–62.
Richetta AG, Silvestri V, Giancristoforo S, Rizzolo P, D'Epiro S, Graziano V, Mattozzi C, Navazio AS, Campoli M, D'Amico C, et al. A-1012G promoter polymorphism of vitamin D receptor gene is associated with psoriasis risk and lower allele-specific expression. DNA Cell Biol. 2014;33(2):102–9.
Rucevic I, Stefanic M, Tokic S, Vuksic M, Glavas-Obrovac L, Barisic-Drusko V. Lack of association of vitamin D receptor gene 3′-haplotypes with psoriasis in Croatian patients. J Dermatol. 2012;39(1):58–62.
Zuel-Fakkar NM, Kamel MM, Asaad MK, Mahran MZ, Shehab AA. A study of ApaI and TaqI genotypes of the vitamin D receptor in Egyptian patients with psoriasis. Clin Exp Dermatol. 2011;36(4):355–9.
Acikbas I, Sanli B, Tepeli E, Ergin S, Aktan S, Bagci H. Vitamin D receptor gene polymorphisms and haplotypes (Apa I, Bsm I, Fok I, Taq I) in Turkish psoriasis patients. Med Sci Monit. 2012;18(11):Cr661–6.
Kontula K, Valimaki S, Kainulainen K, Viitanen AM, Keski-Oja J. Vitamin D receptor polymorphism and treatment of psoriasis with calcipotriol. Br J Dermatol. 1997;136(6):977–8.
Saeki H, Asano N, Tsunemi Y, Takekoshi T, Kishimoto M, Mitsui H, Tada Y, Torii H, Komine M, Asahina A, et al. Polymorphisms of vitamin D receptor gene in Japanese patients with psoriasis vulgaris. J Dermatol Sci. 2002;30(2):167–71.
Zhu HQ, Xie KC, Chen LD, Zhu GD. The association between vitamin D receptor polymorphism and psoriasis. Chin J Dermatol. 2002;35(5):386–8.
Mee JB, Cork MJ. Vitamin D receptor polymorphism and calcipotriol response in patients with psoriasis. J Invest Dermatol. 1998;110(3):301–2.
Halsall JA, Osborne JE, Pringle JH, Hutchinson PE. Vitamin D receptor gene polymorphisms, particularly the novel A-1012G promoter polymorphism, are associated with vitamin D3 responsiveness and non-familial susceptibility in psoriasis. Pharmacogenet Genomics. 2005;15(5):349–55.
Ruggiero M, Gulisano M, Peruzzi B, Giomi B, Caproni M, Fabbri P, Pacini S. Vitamin D receptor gene polymorphism is not associated with psoriasis in the Italian Caucasian population. J Dermatol Sci. 2004;35(1):68–70.
Zhou X, Xu LD, Li YZ. The association of polymorphisms of the vitamin D receptor gene with psoriasis in the Han population of northeastern China. J Dermatol Sci. 2014;73(1):63–6.
Zhao Y, Chen X, Li J, He Y, Su J, Chen M, Zhang W, Chen W, Zhu W. VDR gene polymorphisms are associated with the clinical response to calcipotriol in psoriatic patients. J Dermatol Sci. 2015;79(3):305–7.
Lee YH, Choi SJ, Ji JD, Song GG. Vitamin D receptor ApaI, TaqI, BsmI, and FokI polymorphisms and psoriasis susceptibility: a meta-analysis. Mol Biol Rep. 2012;39(6):6471–8.
Acknowledgments
We appreciate American Journal Experts for help with English usage during the preparation of this manuscript.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
JL and MY designed the study. JL, LS, and JS extracted, analyzed, and interpreted the data. JL and MY drafted the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Searching terms for our meta-analysis (up to August 18, 2019).
Additional file 2: Table S2.
The clinical characteristics of included case-control studies.
Additional file 3: Table S3.
Quality assessment of included case-control studies.
Additional file 4: Figure S1.
The forest plot for VDR rs7975232 polymorphism in the subgroup analysis by ethnicity under the allele model.
Additional file 5: Figure S2.
The forest plot for VDR rs7975232 polymorphism in the subgroup analysis by the source of controls under the allele model.
Additional file 6: Figure S3.
The forest plot for VDR rs1544410 polymorphism in the subgroup analysis by ethnicity under the allele model.
Additional file 7: Figure S4.
The forest plot for VDR rs1544410 polymorphism in the subgroup analysis by the source of controls under the allele model.
Additional file 8: Figure S5.
The forest plot for VDR rs2228570 polymorphism in the subgroup analysis by ethnicity under the allele model.
Additional file 9: Figure S6.
The forest plot for VDR rs2228570 polymorphism in the subgroup analysis by the source of controls under the allele model.
Additional file 10: Figure S7.
The forest plot for VDR rs731236 polymorphism in the subgroup analysis by ethnicity under the allele model.
Additional file 11: Figure S8.
The forest plot for VDR rs731236 polymorphism in the subgroup analysis by the source of controls under the allele model.
Additional file 12: Figure S9.
Publication bias of VDR rs7975232 and rs1544410 polymorphism under the allele model. a-b rs7975232 polymorphism; c-d rs1544410 polymorphism.
Additional file 13: Figure S10.
Publication bias of VDR rs2228570 and rs731236 polymorphism under the allele model. a-b rs2228570 polymorphism; c-d rs731236 polymorphism.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, J., Sun, L., Sun, J. et al. Pooling analysis regarding the impact of human vitamin D receptor variants on the odds of psoriasis. BMC Med Genet 20, 161 (2019). https://doi.org/10.1186/s12881-019-0896-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12881-019-0896-6