Abstract
Background
Patients with immune-mediated inflammatory diseases (IMIDs) on immunosuppressants (ISPs) may have impaired long-term humoral immune responses and increased disease activity after SARS-CoV-2 infection. We aimed to investigate long-term humoral immune responses against SARS-CoV-2 and increased disease activity after a primary SARS-CoV-2 infection in unvaccinated IMID patients on ISPs.
Methods
IMID patients on active treatment with ISPs and controls (i.e. IMID patients not on ISP and healthy controls) with a confirmed SARS-CoV-2 infection before first vaccination were included from an ongoing prospective cohort study (T2B! study). Clinical data on infections and increased disease activity were registered using electronic surveys and health records. A serum sample was collected before first vaccination to measure SARS-CoV-2 anti-receptor-binding domain (RBD) antibodies.
Results
In total, 193 IMID patients on ISP and 113 controls were included. Serum samples from 185 participants were available, with a median time of 173 days between infection and sample collection. The rate of seropositive IMID patients on ISPs was 78% compared to 100% in controls (p < 0.001). Seropositivity rates were lowest in patients on anti-CD20 (40.0%) and anti-tumor necrosis factor (TNF) agents (60.5%), as compared to other ISPs (p < 0.001 and p < 0.001, respectively). Increased disease activity after infection was reported by 68 of 260 patients (26.2%; 95% CI 21.2–31.8%), leading to ISP intensification in 6 out of these 68 patients (8.8%).
Conclusion
IMID patients using ISPs showed reduced long-term humoral immune responses after primary SARS-CoV-2 infection, which was mainly attributed to treatment with anti-CD20 and anti-TNF agents. Increased disease activity after SARS-CoV-2 infection was reported commonly, but was mostly mild.
Trial registration
NL74974.018.20, Trial ID: NL8900. Registered on 9 September 2020.
Similar content being viewed by others
Background
Development of an adequate humoral immune response after an infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an important mediator of protection against future infections [1]. Hybrid immunity (i.e., immunity in individuals who have been both vaccinated and infected) is superior to humoral immunity elicited by vaccination only [2]. Patients with immune-mediated inflammatory diseases (IMIDs) can have impaired humoral immune responses after SARS-CoV-2 vaccination, depending on the type of immunosuppressant (ISP) used [3]. However, data on humoral immune responses following SARS-CoV-2 infection are scarce and limited to specific diseases. Patients with inflammatory bowel diseases (IBD) treated with anti-tumor necrosis factor (TNF) and patients with multiple sclerosis (MS) or rheumatic disease receiving treatment with anti-CD20 therapies showed impaired humoral immune responses directly after infection [4,5,6,7]. The long-term humoral immune response following SARS-CoV-2 infections of patients with IMIDs should be studied in order to better understand the development of protective immunity in these patients.
Another concern is the possible interplay between infection and underlying disease activity in IMID patients. Previous studies indicated that a SARS-CoV-2 infection might trigger increased disease activity of the underlying IMID [8,9,10,11].
In this study we aimed to investigate long-term humoral immune responses and changes in disease activity after a primary SARS-CoV-2 infection in unvaccinated IMID patients using different types of ISPs.
Methods
This is a substudy of an ongoing national multicenter observational cohort study in the Netherlands (Target-to-B! (T2B!) study), studying vaccination responses in IMID patients. For this substudy, we used baseline clinical and serological data at enrollment. A full description of the T2B! study with different types of IMIDs and ISPs that were included has been published previously [3].
We included IMID patients and healthy controls, with a SARS-CoV-2 infection any time before receiving the first SARS-CoV-2 vaccination (i.e. primary infection). A SARS-CoV-2 infection was defined as a positive PCR test or a positive antigen test. Suspected SARS-CoV-2 infections based solely on clinical symptoms were excluded. Participants who did not complete electronic questionnaires after enrollment were also excluded. Participants were recruited between February 2021 and July 2021. Data from this cohort has been used in previous studies [3, 7, 12,13,14].
Patients received electronic questionnaires at enrollment collecting clinical data on demographics, possible SARS-CoV-2 (re)infections before vaccination and whether an increase in disease activity occurred in the four weeks after infection. The investigators collected clinical data on IMID diagnosis, ISP use since January 2020, and coronavirus disease 2019 (COVID-19) severity (based on the WHO scale [15]) from patient files using an electronic case record form.
The date of SARS-CoV-2 infection was defined as the date of the PCR test or in case an infection was only proven by an antigen test, the date of symptom onset. PCR dates could be reliably retrieved by participants (for example from COVID-19 passports), whereas dates of antigen tests were not centrally registered and therefore usually not precisely known. Active treatment with ISPs was defined as receiving treatment during or in the three months before SARS-CoV-2 infection. ISPs with long-lasting biological effects (i.e. anti-CD20 therapy, cyclophosphamide, cladribine, and alemtuzumab) were also considered active treatment, if administered up to one year before infection.
Investigators extracted findings from patient files when a patient reported increased disease activity in the four weeks after infection, to assess whether the treating physician also reported an increased disease activity, and whether ISP treatment was intensified as a consequence of the increased disease activity.
A serum sample was collected by on-site venipuncture or home-based fingerprick, during enrolment before first vaccination against SARS-CoV-2, as described previously [3]. SARS-CoV-2 antibodies were measured using two different assays in a central laboratory. The primary assay to assess seropositivity was a semi-quantitative bridging enzyme-linked immuno sorbent assay (ELISA) measuring immunoglobulins of all isotypes against the receptor-binding domain (RBD) of the spike protein, which has a high sensitivity in very low antibody ranges (98·1% sensitivity and 99·5% specificity) [16]. In addition, we measured anti-RBD IgG titers (expressed in AU/ml) using a quantitative ELISA [17]. Results for anti-RBD antibodies were excluded if the sample arrived at the central laboratory more than 14 days after the first vaccination.
The primary outcome was the seropositivity rate for SARS-CoV-2 antibodies after primary SARS-CoV-2 infection. Seropositivity rates of specific groups of ISPs were considered a secondary outcome. Two specific ISP groups were formed a priori based on previous literature indicating impaired humoral immune responses after SARS-CoV-2 infection in IMID patients using ISPs (i.e., anti-CD20 therapy and anti-TNF therapy, both as mono- or combination therapy) [4,5,6,7]. IMID patients that were using ISPs other than anti-TNF or anti-CD20 agents were classified as ‘other ISPs’. Additional secondary outcomes were seropositivity rates and antibody titers in relation to time since infection. Due to a low number of observations, the anti-CD20 group was excluded from this analysis. Furthermore, the rate of patients with self-reported increased disease activity was documented, including the proportion of these patients in whom the physician reported increased disease activity and in whom treatment with ISPs was intensified.
Outcomes from patients with IMIDs on (any type of) ISP were compared to a combined control group of IMID patients not on ISPs and healthy controls (hereafter combined and named ‘controls’). The Wilcoxon rank-sum test and Kruskal–Wallis test were used to assess the difference in days between SARS-CoV-2 infection and sample collection between groups. For the difference in proportions between groups, Fisher’s exact test was used. Spearman’s rank correlation coefficient was used to test for correlation between time since infection and antibody titer. For the difference in antibody titers between two groups in time, Wilcoxon rank-sum test was used. Data analysis was performed by two authors (KPJvD and LW) using R version 4.2.1 (R foundation for Statistical Computing, Vienna, Austria).
Results
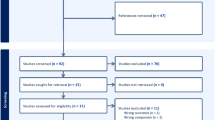
In total, 306 participants with a primary SARS-CoV-2 infection were analyzed, including 193 IMID patients on ISPs, 67 IMID patients not on ISPs, and 46 healthy controls (Fig. 1). Participant characteristics are shown in Table 1. The mean age was 44.6 years (SD 13.8) and 192/306 (62.7%) participants were women. Focusing on patients using ISPs, 14/193 (7.3%) patients were on anti-CD20 agents (mono- or combination therapy), 49/193 (25.4%) were on anti-TNF agents (mono- or combination therapy), and 130/193 (67.4%) used other ISPs (see Additional file 1, table S1 for detailed ISP treatments within groups). COVID-19 severity was classified as asymptomatic in 14/306 (4.6%) cases, mild in 279/306 (91.5%), moderate in 10/306 (3.3%) and severe (ICU admission) in 2/306 (0.7%). No participants died (Table 1).
Serum samples before vaccination were available for 190/306 (62.1%) participants. For five participants, serology results were excluded because the sample arrived more than 14 days after the first vaccination at the central laboratory, resulting in 185 samples that were analyzed (Fig. 1). From the analyzed samples, 57/185 (31%) were received at the laboratory before or at the day of first vaccination, 103/185 (56%) were received within the first week after first vaccination and 25/185 (14%) in the second week. No effect of time between first vaccination to arrival at the laboratory on the antibody titer was observed (Additional file 1, figure S1). The median time from infection to serum sample collection was 173 days [IQR 94.0–227], and this did not differ between ISP (165 days [IQR 96.3–224]) and controls (191 days [IQR 85.5–232], p = 0.45). Specific ISP groups were comparable in median time from infection to sample collection, with 155 days [IQR 146–185] for anti-CD20, 186 days [IQR 128–235] for anti-TNF, and 153 days [IQR 77.5–221] for other ISPs (p = 0.45). The rate of seropositive participants in IMID patients on ISPs (92/118; 78.0%), was lower compared to the control group (67/67; 100%; p < 0.001). A sensitivity analysis, restricting time from first vaccination to arrival at the laboratory to 7 days, did not change the results of the primary outcome (Additional file 1). From the patients on ISPs, patients on anti-CD20 were less often seropositive (4/10; 40.0%) compared to IMID patients on other ISPs (65/70; 92.9%; p < 0.001) or the control group (67/67; 100%; p < 0.001) (Fig. 2). In addition, patients on anti-TNF agents were less often seropositive (23/38; 60.5%) compared to IMID patients using other ISPs (65/70; 92.9%; p < 0.001) or the control group (67/67; 100%; p < 0.001). Seropositivity rates of patients on anti-CD20 therapy did not differ significantly from patients on anti-TNF agents (p = 0.30). The seropositivity rate of patients using anti-TNF monotherapy (12/22; 54.5%) was similar to patients using anti-TNF combination therapy (11/16; 68.8%; p = 0.51). A trend was found towards less seropositivity, but no significant difference was seen between IMID patients on other ISPs and controls (p = 0.06).
Time between SARS-CoV-2 infection and sample collection showed a weak negative correlation with antibody titer for anti-TNF (rs = -0.27 p = 0.05), other ISP (rs = -0.29 p < 0.01), and controls (rs = -0.32 p < 0.01); Fig. 3). The rate of seropositivity was compared for patients with an in infection in the six months before serum sample collection and an infection more than six months before serum sample collection, for patients on anti-TNF, other ISPs and controls (Additional file 1, figure S2). There was a non-significant trend towards less seropositivity in the anti-TNF group for patients with an infection more than six months before sample collection (10/19 52.6%) compared to an infection in the six months before sample collection (13/19 68.4%; p = 0.51). In comparison, all controls were seropositive in both time groups and proportions were comparable between the two time groups for the other ISP group.
Effect of time since infection on anti-RBD antibody titer. Relation between SARS-CoV-2 RBD-specific antibody titer from all serum samples (dots) and time in days from SARS-CoV-2 infection to obtaining the serum sample. The colored lines are regression lines of titers, for patients with anti-TNF (rs = -0.27; p = 0.05), other ISP (rs = -0.29; p < 0.01), and controls (rs = -0.32; p < 0.01). Colored dots reflect samples of patients with anti-TNF, other ISP, and controls. AU Arbitrary units, ISP Immunosuppressant, RBD Receptor-binding domain, rs Spearman’s rho, TNF Tumor necrosis factor
Of the IMID patients, 68/260 (26.2%; 95% CI 21.2–31.8%) reported increased disease activity within four weeks after SARS-CoV-2 infection. Self-reported increased disease activity was most frequently reported in patients with spondyloarthritis (7/13; 53.8%) and myasthenia gravis (7/14; 50.0%; Fig. 4). From all reported increases in disease activity, 19/68 (27.9%; 95% CI 18.7–39.6%) were also reported by the treating physician. The self-reported increased disease activity led to ISP treatment intensification in 6/68 cases (8.8%; 95% CI 6.1–21.5).
Disease activity after primary SARS-CoV-2 infection per IMID. Bar chart showing proportions of increase in IMID activity after SARS-CoV-2 primo infection per IMID. Different categories of severity of the increase in IMID activity are shown in colors. Proportions are all derived from the IMID group size, shown on the Y-axis. IBD Inflammatory bowel disease, IMID Immune-mediated inflammatory disorder, MS Multiple sclerosis, NMO Neuromyelitis optica, SLE Systemic lupus erythematosus
Discussion
In this study, we found lower long-term seropositivity rates after SARS-CoV-2 infection in IMID patients on active treatment with ISPs compared to IMID patients who did not receive treatment with ISPs and healthy controls. This lower seropositivity rate was primarily explained by treatment with anti-CD20 or anti-TNF therapy. Finally, a quarter of patients reported increased disease activity after SARS-CoV-2 infection, leading to treatment intensification in a minority of patients.
The lower rate of seropositivity after a SARS-CoV-2 infection in patients using anti-CD20 therapy is in line with previous studies on the humoral immune response after SARS-CoV-2 infection, and our previous study on the humoral immune response after SARS-CoV-2 vaccination in patients on anti-CD20 [3, 7, 18]. Likewise, the decreased seropositivity rate in patients on anti-TNF therapy confirms previous studies that reported reduced humoral immune responses after SARS-CoV-2 infection in IBD patients on anti-TNF agents [5, 19]. However, these studies assessed antibodies against the nucleocapsid protein, which are unlikely to neutralize SARS-CoV-2 [20]. Studies assessing neutralizing antibodies against the RBD after SARS-CoV-2 infection in patients on anti-TNF are scarce. Boekel et al. reported no significant effect of anti-TNF on either seropositivity rate or anti-RBD antibody titers after infection compared to healthy controls, although the lack of effect may have resulted from selection bias towards seropositive participants [4]. To our knowledge, this is the first study demonstrating reduced anti-RBD antibody responses after PCR- or antigen-proven SARS-CoV-2 infection in patients using anti-TNF agents. Interestingly, early seropositivity rates after completed SARS-CoV-2 vaccination in our cohort and in other cohorts of IMID patients were not affected by anti-TNF, although titers were lower compared to healthy controls [3, 21]. Yet, multiple studies have shown a reduction in seropositivity rate, antibody titer and neutralizing capacity at six months after second vaccination in patients on anti-TNF [22,23,24]. Together, these results suggest that IMID patients on anti-TNF seem to be able to mount a relatively adequate humoral immune response, but also that this humoral response is less well maintained. In one study, it was hypothesized that anti-TNF leads to a reduced capacity to form de-novo long-lived SARS-CoV-2 specific plasma cells, which could explain the more rapid decay of antibodies over time [22]. For patients who used one of the other common prescribed ISPs, the seropositivity rate after SARS-CoV-2 infection was comparable to the control group of patients not using ISPs and healthy controls.
In line with previous work including different patient populations, the present study demonstrates that the antibody titer after SARS-CoV-2 infection decreases over time [2, 25, 26]. However, a significant number of IMID patients remained seropositive, even with a follow-up period exceeding six months. This indicates that there is considerable heterogeneity in long-term humoral immune responses among IMID patients on ISPs, and ISPs do not necessarily prevent long-term humoral immune responses.
Previous literature on increased underlying IMID activity after SARS-CoV-2 infection is largely limited to case reports and a few cohort studies [8, 11, 27]. Increased disease activity after a SARS-CoV-2 infection was reported in 41% of adult rheumatoid artritis patients. In a pediatric rheumatic cohort, a disease flare was reported in 16% of patients and another study found that 6% of patients suffering from systemic lupus erythematosus had a disease flare that was thought to be triggered by COVID-19. However, these studies used patient-reported disease activity only, without confirming severity of the increased disease activity based on reports of the physician and changes in treatment. In our cohort, increased disease activity in four weeks after SARS-CoV-2 infection was reported in more than a quarter of patients. In these patients, increased disease activity was also reported by physicians in one quarter, and resulted in ISP treatment intensification in a relatively small proportion of patients. This suggests that increased disease activity after infection is mild and mostly self-limiting, since treatment intensification was not often required. However, it is important to note that the COVID-19 severity in our study was generally classified as mild. In line with that notion, a mild infection may have a smaller chance of provoking an increase in disease activity compared to a severe infection due to less activation of the immune system. Interestingly, patients with spondyloarthritis and myasthenia gravis reported increased disease activity much more often than patients with other IMIDs, as nearly half of these patients reported increased disease activity. Infections are a well-known trigger of exacerbations in myasthenia gravis, whereas infections have not been found to be associated with increased disease activity in spondyloarthritis [28, 29]. Alternatively, COVID-19 symptoms such as myalgia and fatigue might mimic certain IMID symptoms, and might have contributed to the high proportion of patients with myasthenia and spondyloarthritis reporting increased disease activity. Moreover, reporting bias could have overestimated the incidence of increased disease activity after infection. Alltogether, it remains difficult to provide a true estimate of increased disease activity after infection nor to proof a causal relationship between COVID-19 and increased disease activity, especially in diseases where fluctuating symptoms are commonly seen.
The strengths of this study are the relatively large sample size of IMID patients with previous SARS-CoV-2 infections before vaccination, the disease overarching study-design and the control group. Compared to previous work, this study describes long-term humoral immune responses after SARS-CoV-2 infection, and therefore adds to the understanding of long-term humoral immunity in IMID patients on different types of ISP. Since participants were recruited up to August 2021, patients were either infected with the Wuhan, Alpha, Beta, Gamma or Delta SARS-CoV-2 strain. Meanwhile, measuring and interpreting long-term humoral responses is becoming increasingly difficult because of the interplay of different intervals between booster vaccinations, breakthrough infections with different SARS-CoV-2 variants and the resulting changing affinity of the humoral repertoire over time. Nonetheless, this study has some limitations. The samples arrived at the central laboratory up to 14 days after the first vaccination due to logistical reasons. Although patients were specifically instructed to perform a finger prick before vaccination, we cannot rule out that some samples were obtained after first vaccination, therefore possibly representing an early recall response. However, we did not observe an effect of time from first vaccination to arrival of the sample on the antibody titer, and a sensitivity analysis with a more restricted interval did not change the results of the primary outcome. Furthermore, the size of this cohort was not large enough to assess other specific groups of ISPs, which were shown to decrease seropositivity rates after SARS-CoV-2 vaccination, such as mycophenolate mofetil and S1P-modulators [3]. Also, SARS-CoV-2 antibodies were not measured longitudinally. Hence, we did not observe changes of the humoral immune response within individual participants. As we studied long-term antibody responses with low to very low antibody titers we relied on a semiquantitaive bridging ELISA to detect seropositivity and could therefore not reliably compare differences in antibody titers quantitatively between groups. Lastly, due to a small number of cases of increased disease activity in the different IMID groups, we could not study determinants of increased disease activity after infection or use disease-specific endpoints for increased disease activity.
Conclusions
IMID patients on ISPs showed reduced long-term humoral immune responses after primary SARS-CoV-2 infection compared to participants not on ISPs. This difference could be mainly attributed to IMID patients treated with anti-CD20 and anti-TNF therapy. Overall, antibody titers decreased slightly over time, although most IMID patients on ISPs remained seropositive for more than six months following infection. Approximately a quarter of patients reported an increase in disease activity after SARS-CoV-2 infection, leading to ISP treatment intensification in only a minority of patients.
Availability of data and materials
After publication, anonymized individual participant data and a data dictionary will be made available upon request to the corresponding author to researchers who provide a methodologically sound proposal. Data will be shared through a secure online platform.
Abbreviations
- AU:
-
Arbitrary units
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- COVID-19:
-
Coronavirus disease 2019
- IBD:
-
Inflammatory bowel diseases
- ICU:
-
Intensive-care unit
- IMIDs:
-
Immune-mediated inflammatory diseases
- IQR:
-
Interquartile range
- ISPs:
-
Immunosuppressants
- MS:
-
Multiple sclerosis
- NMO:
-
Neuromyelitis optica
- PCR:
-
Polymerase chain reaction
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SLE:
-
Systemic lupus erythematosus
- RBD:
-
Receptor-binding domain
- TNF:
-
Tumor necrosis factor
References
Cromer D, Juno JA, Khoury D, et al. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol. 2021;21(6):395–404.
Goldberg Y, Mandel M, Bar-On YM, et al. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N Engl J Med. 2022;386(23):2201–12.
Wieske L, van Dam KPJ, Steenhuis M, et al. Humoral responses after second and third SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders on immunosuppressants: a cohort study. The Lancet Rheumatology. 2022;4(5):e338–50.
Boekel L, Hooijberg F, Vogelzang EH, et al. Antibody development and disease severity of COVID-19 in non-immunised patients with rheumatic immune-mediated inflammatory diseases: data from a prospective cohort study. RMD Open. 2022;8(1):e002035.
Kennedy NA, Goodhand JR, Bewshea C, et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut. 2021;70(5):865–75.
Klineova S, Harel A, Straus Farber R, et al. Outcomes of COVID-19 infection in multiple sclerosis and related conditions: One-year pandemic experience of the multicenter New York COVID-19 Neuroimmunology Consortium (NYCNIC). Mult Scler Relat Disord. 2021;55: 103153.
van Kempen ZLE, Strijbis EMM, Al MMCT, et al. SARS-CoV-2 Antibodies in Adult Patients With Multiple Sclerosis in the Amsterdam MS Cohort. JAMA Neurol. 2021;78(7):880–2.
Di Iorio M, Cook CE, Vanni KMM, et al. DMARD disruption, rheumatic disease flare, and prolonged COVID-19 symptom duration after acute COVID-19 among patients with rheumatic disease: A prospective study. Semin Arthritis Rheum. 2022;55: 152025.
Mobasheri L, Nasirpour MH, Masoumi E, Azarnaminy AF, Jafari M, Esmaeili S-A. SARS-CoV-2 triggering autoimmune diseases. Cytokine. 2022;154: 155873.
Sarmiento-Monroy JC, Espinosa G, Londoño M-C, et al. A multidisciplinary registry of patients with autoimmune and immune-mediated diseases with symptomatic COVID-19 from a single center. J Autoimmun. 2021;117:102580.
Schioppo T, Argolini LM, Sciascia S, et al. Clinical and peculiar immunological manifestations of SARS-CoV-2 infection in systemic lupus erythematosus patients. Rheumatology (Oxford). 2022;61(5):1928–35.
Boekel L, Stalman EW, Wieske L, et al. Breakthrough SARS-CoV-2 infections with the delta (B.1.617.2) variant in vaccinated patients with immune-mediated inflammatory diseases using immunosuppressants: a substudy of two prospective cohort studies. The Lancet Rheumatology. 2022;4(6):e417–29.
Verstegen NJM, Hagen RR, van den Dijssel J, et al. Immune dynamics in SARS-CoV-2 experienced immunosuppressed rheumatoid arthritis or multiple sclerosis patients vaccinated with mRNA-1273. eLife. 2022;11:e77969.
Wieske L, Kummer LYL, van Dam KPJ, et al. Risk factors associated with short-term adverse events after SARS-CoV-2 vaccination in patients with immune-mediated inflammatory diseases. BMC Med. 2022;20(1):100.
WHO, Novel Coronavirus. "COVID-19 therapeutic trial synopsis." R&D Blue Print. 2020. https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis.
Vogelzang EH, Loeff FC, Derksen NIL, et al. Development of a SARS-CoV-2 Total Antibody Assay and the Dynamics of Antibody Response over Time in Hospitalized and Nonhospitalized Patients with COVID-19. J Immunol. 2020;205(12):3491–9.
Steenhuis M, van Mierlo G, Derksen NI, et al. Dynamics of antibodies to SARS-CoV-2 in convalescent plasma donors. Clin Transl Immunology. 2021;10(5):e1285.
Conte WL. Attenuation of antibody response to SARS-CoV-2 infection in patients with multiple sclerosis on ocrelizumab: A case-control study. Multiple Sclerosis and Related Disorders. 2021;52:103014.
Chanchlani N, Lin S, Chee D, et al. Adalimumab and infliximab impair SARS-CoV-2 antibody responses: results from a therapeutic drug monitoring study in 11422 biologic-treated patients. J Crohns Colitis. 2022;16(3):389–97.
Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–30.
Jena A, Mishra S, Deepak P, et al. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: Systematic review and meta-analysis. Autoimmun Rev. 2022;21(1): 102927.
Geisen UM, Rose R, Neumann F, et al. The long term vaccine-induced anti-SARS-CoV-2 immune response is impaired in quantity and quality under TNFα blockade. J Med Virol. 2022;94(12):5780–9.
Haberman RH, Um S, Axelrad JE, et al. Methotrexate and TNF inhibitors affect long-term immunogenicity to COVID-19 vaccination in patients with immune-mediated inflammatory disease. Lancet Rheumatol. 2022;4(6):e384–7.
Simon D, Tascilar K, Fagni F, et al. Intensity and longevity of SARS-CoV-2 vaccination response in patients with immune-mediated inflammatory disease: a prospective cohort study. Lancet Rheumatol. 2022;4(9):e614–25.
Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid Decay of Anti–SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N Engl J Med. 2020;383(11):1085–7.
Sakhi H, Dahmane D, Attias P, et al. Kinetics of Anti–SARS-CoV-2 IgG Antibodies in Hemodialysis Patients Six Months after Infection. J Am Soc Nephrol. 2021;32(5):1033–6.
Sengler C, Eulert S, Minden K, et al. Clinical manifestations and outcome of SARS-CoV-2 infections in children and adolescents with rheumatic musculoskeletal diseases: data from the National Paediatric Rheumatology Database in Germany. RMD Open. 2021;7(2): e001687.
Gilhus NE, Romi F, Hong Y, Skeie GO. Myasthenia gravis and infectious disease. J Neurol. 2018;265(6):1251–8.
Zeboulon-Ktorza N, Boelle PY, Nahal RS, et al. Influence of Environmental Factors on Disease Activity in Spondyloarthritis: A Prospective Cohort Study. J Rheumatol. 2013;40(4):469–75.
Acknowledgements
We would like to thank ZonMw (The Netherlands Organization for Health Research and Development, grant 10430072010007) for the funding of the study and the T2B partners, including the patient groups and Health Holland for the support in this study. This collaboration project is financed by the PPP Allowance made available by Top Sector Life Sciences & Health to Samenwerkende Gezondheidsfondsen (SGF) under project number LSHM18055-SGF to stimulate public-private partnerships and co-financing by health foundations that are part of the SGF. Also, we would like to thank E.P. Moll van Charante, J.A Bogaards and R.A. Scholte for their guidance in the data safety monitoring board.
Funding
This study was supported by ZonMw (The Netherlands Organization for Health Research and Development; project number 10430072010007). The sponsor had no role in the design, analysis or reporting of the study.
Author information
Authors and Affiliations
Consortia
Contributions
All authors met the criteria for authorship set by the International Committee of Medical Journal Editors. TR, MS, SK, JKe, AB, and OC performed serological assays; all other authors contributed to data acquisition. AV, PD, and LW wrote the first draft of the manuscript. PD and LW performed the analyses. LW, PD, MS, ES and LK had full access to and verified the underlying data. All authors helped to revise the manuscript for important intellectual content and had final responsibility for the decision to submit for publication. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the medical ethical committee, and all participants provided signed informed consent (NL74974.018.20, EudraCT 2021–001102-30, Dutch Trial Register Trial ID: NL8900).
Consent for publication
Not applicable.
Competing interests
Joep Killestein, has speaking relationships with Merck, Biogen, TEVA, Sanofi, Genzyme, Roche and Novartis. AmsterdamUMC, location VUmc, MS Center Amsterdam has received financial support for research activities from Merck, Celgene, Biogen, GlaxoSmithKline, Immunic, Roche, Teva, Sanofi, Genzyme, and Novartis. Geert D’Haens has been a consultant for AbbVie, Agomab, AstraZeneca, AM Pharma, AMT, Arena Pharmaceuticals, Bristol Meiers Squibb, Boehringer Ingelheim, Celltrion, Eli Lilly, Exeliom Biosciences, Exo Biologics, Galapagos, Index Pharmaceuticals, Kaleido, Roche, Gilead, GlaxoSmithKline, Gossamerbio, Pfizer, Immunic, Johnson & Johnson, Origo, Polpharma, Procise Diagnostics, Prometheus Laboratories, Prometheus Biosciences, Progenity, and Protagonist; and has received speaker’s bureau fees from AbbVie, Arena Pharmaceuticals, Galapagos, Gilead, Pfizer, BMS, and Takeda. Phyllis Spuls is involved in performing clinical trials with many pharmaceutical industries that manufacture drugs used for the treatment of e.g. psoriasis and atopic dermatitis, for which financial compensation is paid to the department/hospital and is one of the main investigator of the TREAT registry taskforce and SECURE-AD registry. Barbara Horváth reports fees from Janssen-Cilag (Advisory Boards, Educational grants, Consultations, Investigator Initiative Studies), AbbVie (Advisory Boards, Educational grants, Consultations, Investigator Initiative Studies), Novartis Pharma (Advisory Boards, Consultations, Investigator Initiative Studies), UCB Pharma (Advisory Boards, Consultations), Leo Pharma (Consultations), Solenne B.V. (Investigator Initiative Studies), Celgene (Consultations, Investigator Initiative Studies), Akari therapeutics (Consultations, Investigator Initiative Studies), Philips (Consultation), Roche (Consultation), Regeneron (Consultation) and Sanofi (Consultation), Argenx (Advisory Boards, Consultations) which fees were paid to the institution. Jan Verschuuren receives financial support from Target to B consortium, Prinses Beatrix Spierfonds, and has been involved in trials or consultancies for Argenx, Alexion, Rapharma. He is coinventor on patent applications based on MuSK-related research. The LUMC received royalties from IBL and Argenx for MG research. All reimbursements were received by the LUMC. The authors are members of the European Reference Network for Rare Neuromuscular Diseases [ERN EURO-NMD]. DirkJan Hijnen is investigator for AbbVie, LEO pharma, MedImmune/AstraZeneca, Novartis, Sanofi/Regeneron; consultancies for Regeneron/Sanofi, LEO pharma, MedImmune/AstraZeneca, Novartis, Incyte, Janssen, Pfizer. Mark Löwenberg has served as speaker and/or principal investigator for: Abbvie, Alimentiv, Bristol Myers Squibb, Celgene, Covidien, Dr. Falk, Ferring Pharmaceuticals, Galapagos, Gilead, GlaxoSmithKline, Janssen-Cilag, Merck Sharp & Dohme, Pfizer, Protagonist therapeutics, Receptos, Takeda, Tillotts, Tramedico. He has received research grants from AbbVie, Merck Sharp & Dohme, Dr Falk, Achmea healthcare, Galapagos and ZonMW. Filip Eftimov reports (governmental) grants from ZonMw (the Netherlands Organization for Health Research and Development) to study immune responses after SARS-CoV-2 vaccination in autoimmune diseases. He also received grants from Prinses Beatrix Spierfonds, CSL Behring, Kedrion, Terumo BCT, Grifols, Takeda Pharmaceutical Company, and Guillain-Barré Syndrome-Chronic Inflammatory Demyelinating Polyneuropathy (GBS-CIDP) Foundation; consulting fees from UCB Pharma and CSl Behring; and honoraria from Grifols. Diane van der Woude has received research grants from Inova diagnostics, FOREUM (Foundation for Research in Rheumatology) and ZonMw (the Netherlands Organization for Health Research and Development), as well as consulting fees from Galapagos. All other authors had no conflicts of interest. The authors/Several authors of this publication are members of the Netherlands Neuromuscular Center (NL-NMD) and the European Reference Network for rare neuromuscular diseases EURO-NMD.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mark Löwenberg and Filip Eftimov contributed equally as last authors
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
van Dam, K.P.J., Volkers, A.G., Wieske, L. et al. Primary SARS-CoV-2 infection in patients with immune-mediated inflammatory diseases: long-term humoral immune responses and effects on disease activity. BMC Infect Dis 23, 332 (2023). https://doi.org/10.1186/s12879-023-08298-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08298-6