Abstract
Purpose
Colorectal cancer is a common malignant tumor worldwide. In China, the ratio of rectal cancer to colon cancer in terms of incidence is close to 1: 1. Low rectal cancer accounts for more than half of all cases of rectal cancer. In recent years, the proportion of rectal cancer has trended downward, however the incidence of rectal cancer in younger adults is increasing. The CACA Guidelines for Holistic Integrative Management of Rectal Cancer were edited to help improve the diagnosis and comprehensive treatment in China.
Methods
This guideline has been prepared by consensuses reached by the CACA Committee of Colorectal Cancer Society, based on a careful review of the latest evidence including China’s studies, and referred to domestic and international relative guidelines, also considered China’s specific national conditions and clinical practice.
Results
The CACA Guidelines for Holistic Integrative Management of Rectal Cancer include the epidemiology of rectal cancer, prevention and screening, diagnosis, treatment of nonmetastatic and metastatic rectal cancer, follow-up, and whole-course rehabilitation management.
Conclusion
Committee of Colorectal Cancer Society, Chinese Anti-Cancer Association, standardizes the diagnosis and treatment of rectal cancer in China through the formulation of the CACA Guidelines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Epidemiology
Colorectal cancer (CRC) is a common malignant tumor, and its incidence and mortality are continually increasing. According to global cancer statistics [1, 2], in 2020, there were 555, 000 new cases of CRC in China, ranking CRC as the third-most common cancer among all malignant tumors. The incidence of CRC was 23.9/100,000, and it was higher in males than in females, with 319, 000 males and 236, 000 females affected. The mortality rate was 12.0/100, 000, which ranks fifth among all cancers. Of the deaths due to CRC, there were 165, 000 in males and 121, 000 in females, and the mortality rates were 14.8/100,000 and 9.4/100,000, respectively. According to the latest statistical data from the National Cancer Center [3,4,5], the new cases of CRC in China account for 9.9% of all new malignant tumors. The incidence varies in different regions and is much higher in urban areas (33.5 / 100, 000) than in rural areas (21.4 / 100, 000). In addition, the incidence differs significantly among the eastern, central, and western regions, with the 24.8/100, 000 in the eastern region being significantly higher than the 19.1/100,000 in the central region and 19.8/100, 000 in the western region. The number of deaths due to CRC also varies across different regions, and the mortality rate is significantly higher in urban areas (16.1/100, 000) than in rural areas (10.5/100, 000). In addition, the mortality rate is significantly higher in the eastern region (15.7/100, 000) than in the central region (12.5/100,000) and western region (12.2/100,000). In China, the ratio rectal cancer (RC) to colon cancer (CC) in terms of incidence is close to 1: 1. Low RC accounts for a high proportion, up to 60% to 75%, of all cases of RC. In recent years, the proportion of RC has trended downward, but the proportion of RC in young patients is high, ranging from 10% to 15% [6].
2 Prevention and screening
2.1 Preventive actions
The exact etiology of RC is unclear and may be associated with various factors, such as diet, environment, genetics, and mental factors. Studies have shown that maintaining a healthy lifestyle and measures [7] such as health examination, tumor screening, and management of precancerous lesions can effectively reduce the incidence and mortality of RC for people of different sexes, ages and genetic factors.
2.1.1 Recommended level I preventive measures
-
(1)
People are recommended to maintain healthy eating habits, have an appropriate and balanced diet plan and reduce their intake of red meat and marinated products. The intake of plant-based foods should be emphasized, along with eating more coarse grains, vegetables and fruits, and the diet plan should be adjusted according to their stool patterns. The consumption of alcoholic beverages should be limited.
-
(2)
People are advised to maintain a healthy lifestyle and a healthy weight by exercising actively. A consistent, healthy sleep schedule and smoking cessation are recommended.
-
(3)
Exposure to environmental carcinogens, including those considered chemical, physical, biological, etc., should be reduced.
-
(4)
The management of individuals’ health should be considered. The tumor-promoting effects of heredity, immune and endocrinological factors should be understood.
-
(5)
It is also suggested to be mentally healthy and optimistic and to have good social connections.
2.1.2 Recommended level II preventive measures
The early detection of precancerous RC lesions, early diagnosis, and early treatment can reduce the incidence of RC and improve the cure rate.
Precancerous lesions
Precancerous lesions include traditional adenomas (tubular adenoma, villous adenoma, tubulovillous adenoma), serrated adenomas (traditional serrated adenoma, sessile serrated lesions, sessile serrated lesions with atypical hyperplasia, etc.), hereditary syndromes (polyposis and nonpolyposis), atypical hyperplasia associated with inflammatory bowel disease (intraepithelial neoplasia), and aberrant crypt foci. In particular, all lesions with atypical hyperplasia are considered precancerous lesions.
Principle of treatment: the resection of adenomas and follow-up examinations can significantly reduce the occurrence of RC. There is no clear evidence on the canceration rate and prognosis of lesions ≤5 mm in diameter. Aggressive treatment may not be required for elevated and superficial elevated adenomas ≤5 mm. However, for superficial depressed lesions ≤5 mm, there is still a possibility of canceration and submucosal infiltration, and these lesions should be resected. Most benign rectal tumors are adenomas that can be cured by endoscopic resection [8].
Endoscopic classification of precancerous lesions (developmental morphological classification)
-
(1)
Elevated type: The lesion is visibly elevated from the intestinal lumen, with the diameter of the base being significantly smaller than the maximum diameter of the lesion (pedunculated or subpedunculated); or the lesion is hemispherical, with the diameter of the base being significantly larger than that of its head. There are three subtypes:
-
1)
Ip: this type is pedunculated and refers to a lesion with an obvious pedicle at the base that is connected to the intestinal wall;
-
2)
Isp: the subpedunculated type, which refers to a lesion with an obvious subpedicle at the base that is connected with the intestinal wall;
-
3)
Is: a lesion visibly elevated on the mucosal surface, without any obvious pedicle structures at the base, for which the diameter at the base is significantly smaller or larger than the maximum diameter at the head.
-
1)
-
(2)
Flat type: Highly flat or flat and elevated lesions are collectively referred to as the flat type, which can be divided into 5 subtypes:
-
1)
IIa: a flat lesion or slightly higher lesion relative to the surrounding mucosa, with a diameter < 10 mm;
-
2)
IIb: a lesion that has almost no differences from the surrounding mucosa in terms of height;
-
3)
IIa + dep: a lesion with a shallow depression on type IIa lesions;
-
4)
LST-NG: nongranular laterally spreading adenomas, which are divided into the flat type (type IIa) and pseudodepressed type (type IIa + IIc, type IIc + IIa);
-
5)
LST-G: granular laterally spreading adenomas, which are divided into the granular homogeneous type (type IIa) and nodular mixed type (type IIa, type Is + IIa, type IIa + Is).
-
1)
-
(3)
Superficial depressed type: the lesion is visibly depressed relative to the surrounding mucosa and can be divided into the following 4 types:
-
1)
IIc: The lesion is slightly depressed relative to the surrounding normal mucosa;
-
2)
IIc + IIa: a depressed lesion with elevated areas;
-
3)
IIa + IIc: an elevated lesion with depressed areas, but the elevated areas are relatively flat;
-
4)
Is + IIc: an elevated lesion with depressed areas, but the elevated areas are relatively raised. This type of lesion extensively infiltrates the submucosa; thus, endoscopic treatment is not indicated [9].
-
1)
Treatment methods
-
(1)
Rectal lesions less than 5 mm can be removed by polypectomy with hot biopsy forceps.
-
(2)
Type Ip, Isp, and Is elevated lesions can be resected using endoloop-assisted polyp electrotomy.
-
(3)
Type IIa and IIc lesions and some Is lesions can be completely resected and treated with endoscopic mucosal resection (EMR).
-
(4)
For lesions with a maximum diameter > 20 mm should be resected immediately under endoscopy, adenomas with a false-negative lifting sign, residual lesions after EMR measuring more than 10 mm, recurrent lesions for which retreatment with EMR is difficult, and low rectal lesions that cannot be confirmed as carcinoma by repeated biopsy, endoscopic submucosal dissection (ESD) is recommended.
-
(5)
The endoscopic procedure should be selected based on subtype for laterally spreading tumors: pseudodepressed LST-NG and nodular mixed LST-G lesions are prone to submucosal infiltration and should be resected en bloc through ESD; flat LST-NG and granular homogeneous LST-G lesions can be resected piecemeal through EMR or ESD, depending on lesion size.
2.2 Screening
2.2.1 RC screening in the natural population
General population
People aged 50 to 74 years are recommended to undergo screening for RC [10, 11]. Colonoscopy should be performed every 5 ~ 10 years. If the subject refuses to undergo a screening colonoscopy, a high-risk factor questionnaire and fecal immunochemical test (FIT) are recommended. Further colonoscopy is required for those showing positive results in any test. If a colonoscopy is not possible, multitarget fecal FIT-DNA testing may be considered. A digital rectal exam (DRE) can also be used for RC screening. It remains unclear whether people over 74 years should continue to be screened [12, 13].
High-risk population
The high-risk population refers to the population with a history of colorectal adenoma, family history of CRC, and diagnosis of inflammatory bowel disease. For the high-risk population, if CRC or advanced adenoma (diameter ≥ 1 cm, with villous structures or high-grade intraepithelial neoplasia) has been diagnosed in more than 2 relatives, a colonoscopy is recommended every 5 years from the age of 40 years or 10 years earlier than the youngest age at which CRC was first diagnosed in the family. Annual colonoscopy is recommended for patients with adenomatous polyposis syndrome or carriers with pathogenic mutations. For those carrying pathogenic mutations and a family history of Lynch syndrome, colonoscopy once every 2 years is recommended starting at the age of 20 to 25 years until the age of 40 years, and then annually thereafter.
Screening methods (1) questionnaire; (2) FIT; (3) multitarget fecal FIT-DNA testing; (4) DRE; and (5) rectoscopy/total colonoscopy.
2.2.2 Hereditary CRC screening
Approximately 1/3 of CRC patients have a genetic predisposition, and 5% to 6% of these cases are hereditary CRC caused by clearly inheritable germline gene mutations. Hereditary CRC can be generally divided into the following two types according to the presence of polyps: nonpolyposis CRC, including Lynch syndrome and Familial Colorectal Cancer Type X; and CRC characterized by polyposis, including familial adenomatous polyposis (FAP), MUTYH-associated polyposis, Peutz – Jeghers syndrome, juvenile polyposis syndrome, etc.
Clinical screening and genetic diagnosis of lynch syndrome
Lynch syndrome accounts for 2% to 4% of all CRC cases and is the most common hereditary CRC syndrome [14]; this condition develops through autosomal dominant inheritance and may lead to the development of colorectal tumors and tumors at other sites (e. g., endometrium, ovaries, and stomach). It is now clear that Lynch syndrome-associated pathogenetic genes include MLH1, MSH2, MSH6, and PMS2 genes in the mismatch repair (MMR) gene family, as well as EPCAM gene.
-
(1)
Clinical screening: Amsterdam diagnostic criteria I and II and other criteria are commonly used as screening criteria. Based on the current trends in family downsizing in China, the National Collaborative Group on Hereditary Colorectal Cancer proposed a family-related criteria for Lynch syndrome in China in 2003: at least 2 cases of histopathologically confirmed CRC in the family, of which at least 2 are in first-degree relatives, and any of the following conditions:
-
1)
At least 1 case of multiple CRC (including adenoma) in the family;
-
2)
At least 1 case of CRC in a family member < 50 years old at the initial diagnosis;
-
3)
At least one family member suffering from a Lynch syndrome-related parenteral malignancy (including gastric, endometrial, intestinal, ureteral, renal pelvis, ovarian, and hepatobiliary cancers) [15].
-
1)
-
(2)
Molecular screening: MMR gene mutations should be detected in Lynch syndrome patients [16]. Immunohistochemistry can be conducted to detect the presence or absence of MMR proteins, and polymerase chain reaction (PCR) can be conducted to detect the status of microsatellite instability (MSI). Clinical screening and molecular screening are recommended. Lynch syndrome is highly suspected when immunohistochemistry suggests deficiency mismatch repair (dMMR) or microsatellite instability-high (MSI -H), with germline gene mutations detected. The diagnosis of Lynch syndrome can be confirmed if a germline pathogenetic mutation is detected in any of the following genes: MLH1, MSH2, MSH6, PMS2, or EPCAM.
Familial adenomatous polyposis
FAP is an autosomal-dominant tumor syndrome that is mainly clinically characterized by multiple colorectal polyps. The most critical pathogenetic gene of FAP is the APC gene, and patients with classical FAP (more than 100 polyps) may concurrently develop gastric polyps, duodenal polyps, and extraintestinal symptoms such as congenital retinal pigment epithelial cell hypertrophy, desmoid fibroma, and osteoma. The clinical phenotype of attenuated FAP [17] is relatively mild (10 ~ 99 polyps). Gene detection can identify pathogenic genes and mutation sites. If no pathogenic mutation of the APC gene germline is found, further detection of MUTYH gene germline mutations should be performed. For classical FAP, if no pathogenetic mutation in the APC or MUTYH germline is found by conventional genetic testing, high-throughput sequencing for multiple genes or whole-exome sequencing should be performed to identify the pathogenetic gene [18].
3 Diagnosis
3.1 Clinical manifestations
Early RC may not have obvious symptoms. As the disease progresses to a certain extent, the following symptoms may occur: (1) changes in bowel movement habits and stool characteristics; (2) gradual thinning of stools; (3) rectal irritation; and (4) symptoms corresponding to tumor invasion into the bladder, urethra, vagina, or other surrounding organs.
3.2 Medical history and family history
The onset of RC may be associated with diseases such as rectal polyps, rectal adenomas, Crohn’s disease, ulcerative colitis, schistosomiasis, and other related diseases; a detailed medical history and family history should be obtained.
3.3 Physical examinations
The general condition of the systemic superficial lymph nodes, especially the inguinal and supraclavicular lymph nodes, should be evaluated. An abdominal inspection and palpation should be conducted to identify the presence of any intestinal-type lesions and intestinal peristaltic waves; abdominal percussion and auscultation can identify whether there is shifting dullness and abnormal bowel sounds.
DRE can be conducted to identify the size, shape, and texture of the rectal neoplasm; extent of invasion into the intestinal wall circumference; range of motion at the base; distance from the lower edge of the tumor to the anal verge; extraintestinal, surrounding organ, or pelvic floor invasion; and other conditions. In addition, the presence of blood on the glove surface can be noted. DRE can also help assess the function of the patient’s anal sphincter. For female RC patients, a vagino-recto-abdominal examination is recommended to identify the relationship between the mass and the posterior vaginal wall.
3.4 Laboratory tests
The laboratory tests include the following: (1) hematology; (2) urinalysis; (3) stool analysis; (4) fecal occult blood test; (5) biochemical profile; and (6) tumor markers. For RC patients, CEA and CA19–9 can be detected in peripheral blood at diagnosis, before treatment, during evaluations of treatment efficacy, and during follow-ups. Evaluations of AFP level are recommended for patients with suspected liver metastasis; evaluations of CA125 level are recommended for patients with suspected peritoneal and ovarian metastases.
3.5 Universal colonoscopy
Rectoscopy is indicated for low rectal lesions. Total colonoscopy is recommended for all patients with suspected RC. The observation indicators include tumor size, distance from the anal verge, tumor shape, and extent of local infiltration. For suspected lesions, a pathological biopsy must be performed. The location of the lesion should be identified in combination with computed tomography (CT) or magnetic resonance imaging (MRI) since the distance from the distal side of the tumor to the anal verge may not be accurately measured by endoscopy due to possible wrinkles in the intestinal wall during the examination. For patients with small lesions that are difficult to locate during surgery, an endoscopic injection of carbon nanoparticles, methylene blue, and other stains can be used for lesion localization. If the patient’s condition allows, intraoperative colonoscopy can be performed to assist in localization.
3.6 Imaging examinations
3.6.1 CT
Contrast-enhanced chest/abdominal/pelvic CT is recommended to exclude distant metastases for initial tumor staging, follow-up, and evaluations of treatment efficacy. The examination should include the following: (1) the location, invasion range, and infiltration depth of the primary tumor; (2) the presence of accompanying regional or distant lymph node metastasis; (3) the presence of accompanying distant organ metastasis; (4) screening for anastomotic recurrence and distant metastasis during follow-ups; (5) efficacy evaluation; and (6) the presence of any complication, such as intestinal obstruction, intestinal intussusception, intestinal perforation or other concomitant diseases that may affect the treatment decision.
3.6.2 MRI
MRI is recommended as a routine examination for RC. For patients with locally advanced RC, baseline and preoperative MRI examinations should be performed before and after neoadjuvant therapy to evaluate the effect of the therapy regimen. Structural MRI reports is recommended. For patients with MRI contraindications, contrast-enhanced pelvic CT can be an alternative. The specific evaluation should include the following: (1) tumor size and location; (2) distance from the lower edge to anal verge (or dentate line); (3) extent of tumor invasion to the intestinal wall; (4) depth of tumor invasion to the intestinal wall; (5) presence of extramural venous invasion; (6) status of the mesorectal fascia; and (7) presence of metastases in the regional and distant lymph nodes. For liver metastases that cannot be identified clinically, by ultrasound, or by CT, or when the treatment decision is affected by the number of liver metastases, contrast-enhanced MRI is recommended for further evaluations, and liver-specific contrast-enhanced scanning is feasible in qualified hospitals.
3.6.3 Ultrasound
Transrectal ultrasonography can be performed in RC patients to clarify the early RC stage, which is also valuable for the diagnosis of lymph node metastasis. For suspicious liver lesions that cannot be diagnosed by imaging examinations, ultrasound-guided paracentesis can be carried out to obtain a pathological diagnosis. Intraoperative ultrasound should be used to assess liver metastases and prepare for radiofrequency ablation (RFA).
3.6.4 Excretory urography
Excretory urography is not recommended as a routine examination, as it is only suitable for patients with large tumors that may invade the urinary system.
3.6.5 PET-CT
Positron emission tomography-computed tomography (PET-CT) is not recommended as a routine examination, but it can be used in patients who cannot be definitively diagnosed by conventional imaging examinations. PET-CT can also be used as an auxiliary examination for those with complex conditions that cannot be diagnosed or staged by conventional examinations and those with suspected recurrence. For patients with stage IV disease with a treatment goal of no evidence of disease (NED), PET-CT assessments are needed.
3.6.6 Laparotomy or laparoscopic exploration
Laparotomy or laparoscopic exploration is recommended in the following cases for definite diagnosis and treatment: (1) RC cannot be confirmed and is highly suspected after various diagnostic means; (2) an intestinal obstruction develops, and conservative treatment is ineffective; (3) intestinal perforation is suspected; and (4) there is major bleeding in the lower gastrointestinal tract for which conservative treatment is ineffective.
3.6.7 Pathological diagnosis
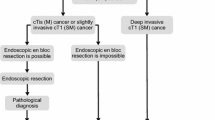
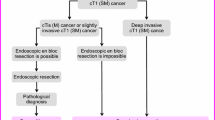
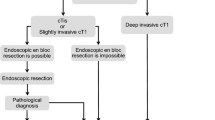
Pathological examination is the gold standard for the diagnosis of RC and is the basis for its treatment. Every attempt should be made to obtain a pathological diagnosis before treatment. For tumors that are palpable by digital examination, if the pathology cannot be identified by multiple biopsies, transanal surgery can be performed to obtain specimens to confirm the pathological diagnosis. Patients diagnosed with infiltrative carcinoma by biopsy should be treated with normative RC therapy; for patients diagnosed with high-grade intraepithelial neoplasia or intramucosal carcinoma by biopsy, clinicians should be aware that submucosal or deeper infiltration may not be identified by pathological biopsy due to the limited depth of biopsy and sampling. Detection of MMR protein expression or MSI from pathological specimens is needed to clarify microsatellite status. In addition, RAS and BRAF gene status should be investigated in the pathological examination of metastatic RC. The tumor regression grade (TRG) description should be specified for specimens obtained by radical resection treated with neoadjuvant therapy. The overall diagnostic flow of RC: See Fig. 1.
4 Treatment
4.1 MDT to HIM principles
The treatment mode for RC is surgery-based integrative treatment. The mode of multidisciplinary diagnosis and treatment to holistic integrative medicine (MDT to HIM) can effectively improve the diagnosis and treatment of RC. For conditional institutions, RC patients should be included in the MDT to HIM diagnosis and treatment mode. A patient-centered, integrative diagnosis and treatment team composed of qualified physicians from the departments of colorectal surgery/gastrointestinal surgery, liver surgery, medical oncology, radiotherapy, radiology, ultrasound imaging, and other related specialties should be formed to regularly conduct comprehensive assessments of the patient’s general condition, diagnosis, staging, disease progression, and prognosis at a fixed site and to develop and implement an individualized integrative diagnosis and treatment plan that is best suited for the patient according to the current domestic and foreign treatment specifications and guidelines.
4.2 Treatment of nonmetastatic RC
4.2.1 Endoscopic treatment
-
(1)
Treatment principles: Early RC lesions should be removed en bloc by endoscopic techniques [19]. Endoscopic ultrasonography, CT, and MRI should be performed for clinical staging before endoscopic treatment to exclude patients with muscularis or deeper invasion, regional lymph node metastasis, or distant metastasis. The depth of invasion of rectal lesions should be comprehensively determined with the pit-pattern classification, Sano’s classification, narrow-band imaging (NBI) international colorectal endoscopic (NICE) classification, presence of lifting sign after submucosal injection, and endoscopic ultrasonography to guide the selection of treatment options.
-
(2)
Indications: Early RC of Tis and T1 (submucosal invasion depth < 1000 μm).
-
(3)
Methods: ESD is the most suitable method for en bloc excision [20], especially for large lesions. Piecemeal EMR makes it difficult to pathologically determine the depth of invasion and resection borders. The number of excised tumor fragments should be minimized, and the area of suspected carcinoma (which can be viewed by magnifying endoscopy prior to treatment) should not be excised piecemeal.
-
(4)
For specimens that are resected endoscopically, a standard pathological analysis should be performed [21]. Additional surgery is required in the following cases: (1) positive basal resection margin; (2) poorly differentiated carcinoma on histology (poorly differentiated adenocarcinoma, undifferentiated carcinoma, signet-ring cell carcinoma, mucinous adenocarcinoma, etc.); (3) depth of submucosal invasion ≥1000 μm; (4) positive blood vessel and lymphatic vessel invasion; and (5) G2/G3 tumor budding.
4.2.2 Surgical treatment
Principles of Surgical Treatment
The principles of functional surgery, harm-to-benefit ratio, and likelihood of a tumor-free status should be considered [22]. Radical surgery is recommended following the principle of total mesorectal excision (TME) for removal of the lymph nodes in the lesion site and associated regions to achieve both radical resection and protection of organ function. The surgical team should have extensive experience in pelvic surgery or operate under the direction of a rectal specialist. If the scope of surgery needs to be expanded, it should be coordinated by surgical teams from the urology, gynecology, and orthopedics departments.
Choice of surgical technology platform
The surgical technology platform should be selected according to the actual situation of the medical unit performing the surgery. Laparotomy is the basic choice and the cornerstone of surgical treatment for RC. Laparoscopic surgery is a safe and minimally invasive option for most patients and should be performed in institutions equipped with 2D high-definition (HD) or 3D laparoscopes and other devices. “Robotic” surgery is an advanced option for laparoscopic surgery and is currently limited to regional medical centers with a platform for such procedures. The transanal surgical platform includes conventional transanal endoscopic microsurgery (TEM) and the single-port laparoscopic surgical platform-based transanal minimally invasive surgery (TAMIS) for the local resection of early rectal tumors or radical surgery of difficult RC; these techniques require a high skill level and hardware support from the surgical team.
Selection of surgical method
-
(1)
Local resection includes transanal RC resection under direct vision and transanal surgery using the TAMIS platform and TEM equipment. The following indications should all be met: maximum tumor diameter < 3 cm; tumor invasion < 30% of the intestinal circumference; tumor is movable and not immobilized; T1 stage by clinical imaging assessment, without signs of regional lymph node metastasis; and well or moderately differentiated. For patients who meet any of the following conditions after local resection, as confirmed by pathological examination, RC radical resection should be added: poor histological differentiation of the tumor, vascular invasion, positive resection margin, depth of submucosal invasion ≥1000 μm, and T2 staging.
-
(2)
Rectal anterior resection (Dixon procedure), the most commonly used radical resection method for RC, is performed for patients with advanced RC classified as clinical T2 stage or higher and / or with positive lymph nodes; the distal rectal resection margin should be 1 to 2 cm from the tumor or wherever a negative pathological result is obtained from the intraoperative frozen specimen, and the anus should be preserved. The total mesorectum should be completely resected following the principle of TME, and the pelvic autonomic nerves should be preserved. If the tumor is found to exceed the TME level during surgery, combined organ resection should be considered to achieve negative margins. A routine protective ileostomy is not recommended after low rectal anterior resection. In cases of preoperative obstruction, proximal intestinal edema, preoperative radiotherapy, very low anastomosis, or high-risk factors for anastomotic leakage, protective ileostomy should be performed with caution according to the patient’s condition.
-
(3)
Abdominoperineal resection (Miles’ operation) is used for patients with low RC for whom normal anal function cannot be reserved; in this procedure, the anus is removed, and a permanent proximal colostomy is then created. The operation is carried out following the TME principle, and the extent of resection needs to be appropriately increased according to the location of the tumor to ensure a negative circumferential resection margin in the lower rectum. If the perineal tissue defect is large, pelvic floor repair or reconstruction can be performed.
-
(4)
Hartmann’s procedure, that is, transabdominal rectal tumor resection and distal rectal closure in combination with proximal colostomy, is used for patients with significant edema of the proximal colon due to RC obstruction, perforation, and other factors that prevent safe anastomosis and Dixon’s procedure, as well as weak elderly patients in a very poor general condition who cannot tolerate the Miles operation.
-
(5)
The modified Bacon procedure is used in patients who cannot safely undergo anorectal anastomosis and are unwilling to undergo proximal enterostomy; during the surgery, the anal canal and anal sphincter can be preserved. A second operation is required to remove the colon prolapsing through the anus.
-
(6)
Intersphincteric resection (ISR) is used for ultralow RC with a tumor invasion depth not exceeding the internal sphincter. According to the resection extent of the internal sphincter, this procedure can be divided into partial resection, subtotal resection, and complete resection. Since the patient’s bowel control capability may be compromised after complete ISR, this procedure is not recommended for elderly or weak patients or those with preoperative anal dysfunction.
-
(7)
Natural orifice specimen extraction surgery (NOSES) refers to various conventional operations (resection and reconstruction) to the abdominal and pelvic cavities that are performed by using equipment and platforms such as laparoscopy, “robots”, anal endoscopes, or soft endoscopes. During these procedures, specimens are collected through a natural cavity (rectum, vagina, or oral cavity) of the human body, leaving no auxiliary incision on the abdominal wall. No incision for specimen collection remains on the abdominal wall after the operation, and only a few minor trocar scars remain, thus resulting in an excellent minimally invasive effect. The specimen collection approach for NOSES to treat RC is through the rectum or vagina only. The surgical team should have extensive experience in laparoscopic surgery and be proficient in completing total laparoscopic reconstruction of the digestive tract. NOSES is a highly selective operation with strict indications that is limited to patients with T2 and T3 stage small lesions and patients for whom specimen collection from a natural orifice is feasible. This approach is not recommended for locally advanced tumors [23]; it is not suitable for acute intestinal obstruction and intestinal perforation resulting from tumors.
-
(8)
Transanal total mesorectal excision (taTME) can transanally remove the tumor in lower rectal cancers by using the TAMIS surgical platform to perform upward reverse TME dissection, and this approach is suitable for middle and low RC. This procedure is technically difficult, without sufficient long -term follow-up data; its indications should be strictly adhered to, and this procedure should be carried out with caution by fully trained specialists in regional medical centers.
-
(9)
Extended radical surgery for RC
-
1)
Lateral lymph node dissection (LLND) is used in patients with low RC combined with or highly suspected of having regional lymph node metastasis along the internal and external iliac vessels; in addition, surgery combined with RC resection can achieve the goal of radical treatment. This procedure is technically difficult with a high risk of vascular and neurological injury, and preoperative chemoradiotherapy is required for most patients. This technique should be performed by adequately trained specialists in regional medical centers.
-
2)
Combined organ resection and multiple organ resection. Combined organ resection refers to the complete resection of more than two adjacent organs due to tumor invasion (inflammatory or carcinogenic) into the surrounding organs. This approach is indicated for patients with RC invading adjacent organs (such as the bladder, ureter, uterus, and adnexa) but without distant metastasis. Depending on the extent of tumor involvement, negative margins can be achieved by resection of the adjacent organs. Multiple organ resection refers to the resection of more than two distant organs as required according to the principle of radical treatment in cases where the tumor has metastasized to distant organs (such as RC, which metastasizes to the ovary and liver at the same time). Achieving R0 resection through one multiple-organ surgery is extremely difficult, and cooperation among surgical teams from the appropriate fields is needed. This surgery should be performed in regional medical centers.
-
1)
-
(10)
RC emergency surgery
Emergency surgery is mainly suitable for RC combined with obstruction, major bleeding, or perforation. Appropriate preparations, such as gastrointestinal decompression and correction of water and electrolyte imbalance as well as acid-base imbalance, should be conducted for intestinal obstruction. For obstructive RC patients with the possibility of being cured, surgical treatment should be carried out as the first choice. The procedure is decided according to the intraoperative conditions and includes Dixon stage I anastomosis, Dixon + protective ileostomy, Hartmann’s procedure, Miles’operation, etc. If the mass cannot be resected, a colostomy at the proximal site of the obstruction can be created, followed by postoperative adjuvant therapy and evaluation of the possibility of staged radical surgery. The placement of a colonic self-expanding metal stent (SEMS) or transanal catheter to decompress the intestinal obstruction may be considered in qualified hospitals according to the specific condition of each patient to avoid emergency surgery in critically ill patients. In cases of bleeding, emergency surgery or interventional therapy should be selected according to the amount of bleeding and the impact on vital signs such as blood pressure. Emergency surgery should be performed in cases of perforation.
-
(11)
Hereditary RC
-
1)
In cases of cancerous FAP, the procedure may be selected according to the site of carcinogenesis and includes total coloproctectomy plus ileal pouch-anal anastomosis (IPAA), total colectomy and partial proctectomy with preservation of the ampulla of rectum plus ileoproctostomy, and total coloproctectomy plus end-to-end ileorectal anastomosis or total proctectomy plus ileostomy. Patients without cancerous polyps can undergo panproctocolectomy or intestinal segment resection according to their health status.
-
2)
Lynch syndrome should be followed up with total coloproctectomy or segmental resection in combination with colonoscopy, after full communication with the patient.
-
1)
Intraoperative medications
Antimicrobial drugs and antitumor drugs should be reasonably used during surgery according to the principle of being aseptic and tumor-free. According to the Chinese Guidelines for Clinical Application of Antimicrobials (2015 Edition), if the procedure lasts for more than 3 hours or the blood loss exceeds 1500 mL, a second dose of antimicrobials can be given during the procedure. Intraperitoneal chemotherapy may be considered for RC with a high risk of recurrence, especially in cases of tumor invasion into the serosa, lymph node metastasis, positive or suspected positive free cancer cells in the cytological examination of peritoneal lavage fluid, over compression of the tumor during the procedure, or tumor rupture. During the operation, chemotherapeutic drugs injected into the abdominal cavity directly affect the intraperitoneal implantation and shedding of cancer cells, and maintaining a high concentration of effective drugs in the abdominal cavity can help treat and prevent metastasis from intraperitoneal implantation.
Quality Control of Specimens and Pathological Staging
Surgical resection specimens and their quality as well as pathological staging are potentially essential to guide postoperative treatment and prognostic evaluations. The surgeon should cooperate with the pathologist to ensure the accuracy of the pathological evaluation report, appropriate specimen fixation and preservation, range of material selection, diagnostic criteria, etc. The American Joint Committee on Cancer (AJCC) TNM staging guidelines (eighth edition) are recommended Table 1.
Primary Tumor (T).
Tx: Primary tumor cannot be assessed.
T0: No evidence of primary tumor.
Tis: Carcinoma in situ, intramucosal carcinoma (involving the lamina propria or muscularis mucosae).
T1: Tumor infiltrates the submucosa.
T2: Tumor infiltrates the muscularis propria.
T3: The tumor penetrates the muscularis propria into the tissue around the intestine.
T4a: The tumor penetrates the visceral peritoneum (including tumor-induced intestinal perforation, and the tumor inflammatory region involves the serosa).
T4b: The tumor directly invades or adheres to other organs or structures. Note: T4 lesions include tumors piercing the serosa and invading another intestinal segment or directly invading the adjacent organs or structures at the site without serosal covering (such as tumors at the lower rectal segment invading the prostate); for tumors with macroscopic adhesion to other tissues or structures, T staging should be based on the deepest microscopic invasion.
Regional lymph nodes (N).
Nx: Metastases to the lymph nodes cannot be assessed.
N0: No metastases to the regional lymph nodes.
N1a: Metastasis to 1 regional lymph node.
N1b: Metastasis to 2 ~ 3 regional lymph nodes.
N1c: Tumor deposits in subserous, mesenteric, or nonperitoneally covered pericolonic or perirectal tissue, without regional lymph node metastasis PN2a: Metastasis to 4 to 6 regional lymph nodes PN2b: Metastasis to 7 or more regional lymph nodes.
Distant Metastasis (M).
Mx: Distant metastasis cannot be assessed.
M1: Distant metastasis M1a: Metastasis in one organ or site, without peritoneal metastasis.
M1b: Metastasis in two or more organs or sites, without peritoneal metastasis.
M1c: Peritoneal surface metastases, with or without metastases in other organ sites.
4.2.3 Medical treatment
Preoperative treatment of RC
This section is applicable to middle and low RC with the inferior pole of the tumor less than 10 cm from the anal verge, as assessed by MRI. For high RC with a distance of more than 10 cm, the section for colon cancer treatment principle should be referenced [24, 25]. Stratified treatment may be considered when risk stratification can be accurately performed through MRI (in centers well equipped with MDT to HIM comprehensive treatment; with high-quality MRI images and radiologists for staging) by referring to the European Society for Medical Oncology (ESMO) 2017/American Society for Radiation Oncology (ASTRO) 2020 guidelines for risk stratification:
-
Very low risk: cT1, SM1, cN0. Low risk: cT1 ~ T2, middle/high T3a/b, cN0 (or high cN1); mesorectal fascia (MRF) invasion -; extramural vascular invasion (EMVI)-.
-
Medium risk: very low/middle/high cT3a/b, not involving the levator ani muscle; cN1 ~ N2 (no extranodal invasion); MRF invasion -; EMVI-.
-
High risk: cT3c/d or very low tumor, not involving the levator ani muscle; cN1-N2 (extranodal invasion); MRF invasion -; EMVI +.
-
Very high risk: cT3 with MRF invasion; cT4b, involving the levator ani muscle; lateral lymph nodes +.
-
(1)
Neoadjuvant therapy for RC: The combination of preoperative concomitant chemoradiotherapy + surgery + adjuvant chemotherapy is still the standard treatment strategy for middle and low locally advanced RC [26,27,28,29,30,31,32,33]. Preoperative neoadjuvant therapy concomitant with chemoradiotherapy contributes to organ preservation [34, 35], increasing the complete remission rate (PCR) and decreasing the local recurrence rate, but whether it can prevent distant metastasis or even realize long-term survival is inconclusive. The specific principles are as follows:
-
1)
Direct surgery is recommended in patients with cT1/2N0M0 disease or contraindications to chemoradiotherapy, while neoadjuvant therapy is not recommended [36].
-
2)
For patients staged as cT3 ~ 4 and / or N +, neoadjuvant chemoradiotherapy followed by an evaluation is recommended before surgery.
-
3)
Regarding neoadjuvant chemoradiotherapy before surgery, capecitabine monotherapy or a continuous infusion of 5-FU is recommended as the chemotherapy regimen; in qualified hospitals, irinotecan combined with a capecitabine regimen can be selected for concurrent chemotherapy, with irinotecan dose adjustments guided by UGT1A1 genotyping [37].
-
4)
For patients staged as cT3 ~ 4 and/or N+ who are not suitable for radiotherapy, a decision needs to be made between direct radical surgery according to MDT to HIM principles or further assessments of the possibility of surgery after the patient undergoes neoadjuvant chemotherapy alone [38,39,40,41].
-
5)
For patients in whom anal preservation is difficult but who strongly desire anal preservation, the addition of interval combination chemotherapy [42], including total neoadjuvant therapy (TNT), may be considered [33, 34, 43].
-
1)
-
(2)
Preoperative treatment of cT4b
-
(1)
RC Patients with cT4b RC should be treated under the guidance of an MDT to HIM team. After long-course concomitant chemoradiotherapy or short-course radiotherapy, systemic chemotherapy is recommended according to tumor regression, followed by surgery. The decision to carry out systemic chemotherapy can be made based on previous chemoradiotherapy regimens and efficacy, and the recommended duration of interval chemotherapy is 2 to 6 courses [37, 44].
RC adjuvant therapy
-
(1)
Stage I (T1 ~ 2N0M0) RC: Postoperative adjuvant chemotherapy is not recommended, and observation and follow-up are preferred [36].
-
(2)
Stage II RC: The regimen should be developed based on the presence of clinicopathological factors and microsatellite status. The high-risk factors are as follows [45, 46]: stage T4, poor histological differentiation (grade 3/4, not including patients with MSI-H), lymphovascular invasion, neural invasion, preoperative intestinal obstruction or partial perforation of the tumor, positive or unknown margin status, insufficient resection margin, and fewer than 12 lymph nodes harvested.
-
1)
For MSI-H or dMMR patients without high-risk factors, postoperative adjuvant chemotherapy is not recommended [47,48,49,50], and observation and follow-up are suggested. For patients with microsatellite stability (MSS) or proficient mismatch repair (pMMR) status, monotherapy with a continuous intravenous infusion of 5-FU/LV or oral administration of capecitabine is recommended.
-
2)
For patients with high-risk factors, CapeOx or FOLFOX regimens are recommended for chemotherapy. Monotherapy with a continuous intravenous infusion of 5FU/LV or oral administration of capecitabine may be indicated for patients with MSS or pMMR status who cannot tolerate dual-drug chemotherapy [51].
-
1)
-
(3)
Stage III RC: oxaliplatin-based dual-drug chemotherapy is recommended after surgery. A continuous intravenous infusion of 5-FU / LV or oral administration of capecitabine chemotherapy is recommended for patients who do not tolerate oxaliplatin [52, 53].
The following medications are not recommended for adjuvant chemotherapy: irinotecan, tegafur, trifluridine and tipiracil hydrochloride tablets (TAS-102), bevacizumab, cetuximab, regorafenib, fruquintinib, all immune checkpoint inhibitors, except for clinical trials [54,55,56,57].
Patients who do not receive preoperative radiotherapy or chemotherapy due to contraindications to chemoradiotherapy or other reasons should be evaluated again after surgery. If the results show that chemotherapy and/or radiotherapy are acceptable, postoperative adjuvant therapy is recommended 3 ~ 4 weeks after surgery and no later than 8 weeks [58]. The initiation of postoperative adjuvant radiotherapy can be appropriately delayed according to the patient’s postoperative condition, including wound healing and recovery of intestinal function, but the delay should not exceed 12 weeks after the operation. The total duration of chemoradiotherapy should not exceed 6 months [59].
Regarding clinical complete response (cCR) after neoadjuvant chemoradiotherapy, under the recommendations of watch-and-wait, the patients should be fully informed of the low agreement rate between cCR and pathological clinical complete remission (pCR), and the risk of recurrence is higher than that after standard treatment, but the successful rescue rate after recurrence is high. The risk of recurrence is high within the first 2 years, and follow-up examinations every 1 to 2 months are recommended for 2 years [60, 61].
4.2.4 Radiotherapy
Radiotherapy indications
-
(1)
Radiotherapy for stage I RC: after local resection of stage I RC, radical surgery is recommended for patients with high-risk factors; if further radical surgery cannot be performed for any reason, adjuvant radiotherapy is recommended.
-
(2)
Neoadjuvant chemoradiotherapy for stage II ~ III RC: stratified treatment is recommended according to the tumor site and risk of recurrence suggested by MRI. Neoadjuvant radiotherapy or neoadjuvant concomitant chemoradiotherapy is recommended.
-
(3)
Adjuvant chemoradiotherapy for stage II-III RC: for pathological stage II-III RC after the operation that was not previously treated with neoadjuvant chemoradiotherapy, the decision to perform adjuvant chemoradiotherapy should be determined according to the postoperative pathological examination results, such as the quality of TME, circumferential resection margin status, and distance from the tumor to the anal verge, based on the recurrence risk stratification.
-
(4)
Radical radiotherapy for stage I-III RC: for patients who are not eligible for surgery for any reason, radical radiotherapy combined with concurrent chemotherapy is recommended. Long-course concomitant chemoradiotherapy is mainly used; short course radiotherapy alone is not currently recommended for the radical treatment of RC.
Radiotherapy dose and fractionation mode
-
(1)
Neoadjuvant radiotherapy fractionation mode
For short-course radiotherapy, 5Gy × 5 fractions are recommended for the primary tumor and high-risk areas. The short-course radiotherapy fractionation pattern is not appropriate for patients with mesorectal fascia involvement or stage T4 RC (i. e., locally advanced RC for which initial R0 resection is not available or is considered unresectable).
For long-course chemoradiotherapy, the irradiation dose should be 45.0 ~ 50.4 Gy (1.8 ~ 2.0 Gy/fraction for a total of 25 ~ 28 fractions) to the primary tumor and high-risk areas.
-
(2)
Adjuvant radiotherapy dose: for patients with stage II ~ III disease who do not receive neoadjuvant radiotherapy, the recommended adjuvant therapy dose is 45.0 ~ 50.4 Gy (1.8 ~ 2.0 Gy/fraction for a total of 25 ~ 28 fractions) to the tumor bed and high-risk areas after surgery. For patients with residual tumor tissue or positive resection margin after surgery, a second operation is recommended; if the second operation is not accessible or refused by the patient, shrinking the irradiation field and increasing the dose locally is recommended after whole pelvic irradiation [62].
-
(3)
Radical radiotherapy: for patients with cCR after neoadjuvant chemoradiotherapy, according to the watch-and-wait strategy, boost radiotherapy is not needed; for patients without cCR after neoadjuvant chemoradiotherapy, if surgery is refused, appropriate boost radiotherapy can be performed according to the interval between two courses of radiotherapy and the irradiation exposure dose to normal tissues. For patients who definitely refuse surgery before treatment, conventional fractionated concomitant chemoradiotherapy is recommended with an irradiation dose of 50 ~ 54 Gy in 25 ~ 30 fractions [63].
-
(4)
Palliative radiotherapy: radiotherapy alone can be given to patients who cannot tolerate chemotherapy and surgery due to advanced age or systemic diseases.
Chemoradiotherapy combination principle for RC
-
(1)
Concomitant chemotherapy
-
1)
Fluorouracil monotherapy is recommended for concomitant chemotherapy regimens during long-course radiotherapy.
-
2)
For neoadjuvant concomitant chemoradiotherapy for RC, the CA-PIRI regimen can be carried out in qualified hospitals with the irinotecan dose adjusted based on UGT1A1 genotyping [37].
-
1)
-
(2)
Concomitant chemoradiotherapy or short-course radiotherapy and chemotherapy before surgery. Patients with locally advanced RC, especially those determined to have MRF involvement, stage T4b disease or lateral lymph node metastasis before treatment, can receive chemotherapy after long-course concomitant chemoradiotherapy or short-course radiotherapy according to the MDT to HIM team’s suggestions and tumor response to increase the degree of tumor regression before surgery. FOLFOX, CapeOx, or capecitabine alone can be used for chemotherapy, and 2 to 6 courses of chemotherapy are recommended during this interval.
-
(3)
Postoperative adjuvant chemoradiotherapy and treatment sequence for adjuvant chemotherapy For patients with stage II-III disease requiring additional pelvic radiotherapy after radical surgery, a sandwich treatment approach with concomitant chemoradiotherapy followed by adjuvant chemotherapy or 1 to 2 cycles of adjuvant chemotherapy plus concomitant chemoradiotherapy followed by adjuvant chemotherapy is recommended. For patients with pN2 disease and negative resection margins, adjuvant chemotherapy followed by concomitant chemoradiotherapy is also acceptable.
Timing of surgery after RC chemoradiotherapy With short-course radiotherapy, surgery should be performed 1 week later. With long-course chemoradiotherapy, surgery should be performed 5 to 12 weeks after the completion of chemoradiotherapy so that the patient can recover from the toxicity effects of the preoperative treatment. The overall management process for nonmetastatic RC is shown in Fig. 2.
4.3 Treatment of RC liver metastases
4.3.1 Resectable hepatic metastasis of RC
Treatment principles
Complete surgical resection of primary and hepatic metastases remains the best treatment for liver metastases of RC. The surgical indications are as follows: the primary RC lesion can be or has been radically resected; R0 resection can be achieved for hepatic metastasis with a sufficient functional liver remnant; no unresectable or damaged extrahepatic metastases; and only nodular lesions in the lungs. The contraindications to surgery are as follows: primary RC foci that cannot be radically resected; presence of unresectable extrahepatic metastases; a residual liver volume after surgery predicted to be insufficient; and inability to tolerate the operation. In addition to surgical resection, ablation, radiotherapy, and other treatments can also completely eliminate liver metastases. In the case of a few liver metastases that are difficult to resect surgically, actively combining various methods is recommended to provide more patients with the opportunity to achieve a status of no evidence of disease (NED) and improve the long-term survival rate (Table 1).
Medical treatment
For patients with resectable hepatic metastasis of RC, the risk of local recurrence of the primary tumor in the rectum should be assessed first and stratified according to ESMO 2017 guidelines [64] (see the aforementioned risk stratification for neoadjuvant therapy of nonmetastatic RC). Patients with resectable RC liver metastasis may be assessed to be at very low, low, and moderate risk of recurrence, and their neoadjuvant and adjuvant therapy strategies are as follows:
-
(1)
Neoadjuvant therapy
The objective is to reduce the tumor volume before surgery and minimize the occurrence of micrometastases in the body. This treatment approach can also be used as a basis to evaluate the patient’s sensitivity to chemotherapy regimens and guide the selection of postoperative chemotherapy regimens. First, a clinical risk score (CRS) is recommended for each patient [65, 66], as shown in Table 2.
The specific treatment strategies are as follows:
-
1)
Combined liver metastases found at the time of RC diagnosis that can initially be radically resected: in the absence or resolution of bleeding, obstruction, or perforation at the primary site and if the CRS score is high, preoperative neoadjuvant chemotherapy is recommended [67].
-
2)
Radically resectable liver metastases after radical surgery for RC: If the patient does not undergo chemotherapy after resection of the primary tumor or has completed chemotherapy 12 months prior and has a high CRS score, preoperative neoadjuvant chemotherapy is recommended; if the patient has received chemotherapy within 12 months before the discovery of liver metastases, the effect of neoadjuvant chemotherapy is generally believed to be limited, and the liver metastases can be directly removed, with postoperative adjuvant therapy afterwards.
-
3)
The course of neoadjuvant chemotherapy generally lasts 2 ~ 3 months. The first choice chemotherapy regimen is an oxaliplatin-based regimen (FOLFOX/CapeOx), and those who do not tolerate oxaliplatin can be given an irinotecan-based regimen (FOLFIRI). Generally, the combined use of targeted drugs is not recommended, and the total duration of preoperative and postoperative chemotherapy is 6 months [68].
-
(2)
Adjuvant Therapy
-
(2)
Postoperative adjuvant chemotherapy should be performed after RC resection and local metastasis treatment, regardless of whether the primary site is symptomatic or the CRS score is high. For patients who achieved NED status after removal of the liver metastases, the decision to perform postoperative adjuvant chemotherapy should be made by the MDT to HIM experts according to the preoperative treatment conditions and postoperative pathological findings [33, 69, 70]. Common adjuvant chemotherapy regimens after RC surgery include fluorouracil monotherapy and oxaliplatin-based combination chemotherapy. If a regimen with irinotecan was effective before the operation, it can be continued after the operation.
For patients with resectable liver metastases of RC assessed to have a high or very high risk of recurrence, concomitant chemoradiotherapy (with reference to the treatment regimen for patients with cT3/cT4N + RC) with systemic chemotherapy plus surgery is recommended, where the surgery can be concomitant or a staged resection of the primary rectal tumor and distant metastases [69]. Alternatively, under the guidance of the MDT to HIM team, the integrative treatment regimen of systemic chemotherapy ± concomitant chemoradiotherapy + surgery can be considered according to the specific circumstances of each patient [70].
Local treatment
-
(1)
Surgical Treatment
Procedures for resectable synchronous RC liver metastases: stage I and stage II resection of the primary RC lesion and liver metastases. For patients with liver metastases after radical RC treatment, if the previous primary rectal lesion has been radically resected without any recurrence and the liver metastases are resectable with a liver resection volume less than 70%, the liver metastases should be surgically removed. Surgical resection of liver metastases should comply with the principle of R0 resection. The resection margins should be at least > 1 mm, at least 1 of 3 hepatic veins should be preserved after the surgery, and the residual liver volume should be ≥40% (synchronous hepatectomy) or ≥ 30% (asynchronous hepatectomy). For noncirrhotic patients with a relatively large lesion limited to the left or right half of the liver, standardized hemihepatectomy can be performed. Intraoperative ultrasound is useful for finding metastatic lesions that cannot be diagnosed by preoperative imaging examinations. For patients with hepatic metastases for whom the residual liver volume is estimated to be less than 30% after surgical resection, portal vein embolization (PVE) or portal vein ligation (PVL) may result in a predicted compensatory enlargement of the postoperative residual liver and increase the possibility of surgical resection. Associating liver partition and portal vein ligation (PVL) for staged hepatectomy (ALPPS) can increase the volume of the residual liver in the short term; however, the patients need to be strictly selected, and the surgery should be performed by experienced liver surgeons.
-
(2)
Lesion Destruction
In addition to surgical resection for liver metastases, RFA, microwave ablation (MWA), stereotactic body radiation therapy (SBRT), etc., can also completely eliminate the lesions. Therefore, for some liver metastases that are difficult to resect, the above treatment methods should be actively combined to give more patients an opportunity to achieve an NED status and improve long-term survival. RFA is suitable for liver metastases with a maximum diameter < 3 cm and an ablation margin > 5 mm, and up to 5 metastases can be ablated at a time. MWA can be used for RC liver metastases with a diameter > 3 cm and those adjacent to larger vessels. SBRT can be adopted for patients with ≤5 liver metastases and liver metastases with a maximum diameter < 6 cm.
4.3.2 Potentially Resectable hepatic metastasis of RC
Treatment Principles of Potential resectability
If the primary tumor or liver metastasis is not suitable for radical resection at the initial diagnosis, it may become suitable after treatment. The 5-year survival rate of patients with liver metastases that are resected after conversion therapy is similar to that of patients with initially resectable lesions [71, 72].
Because chemotherapy may increase the complication rate after liver metastasis resection, surgery should be performed as soon as the expected goals of conversion therapy are reached [73, 74]. For patients undergoing radical resection, perioperative treatment for a total of 6 months should be completed to reduce the risk of recurrence [75, 76]. The decision to continue treatment with targeted drugs after surgery should be determined under guidance from the MDT to HIM team. If an obstruction, perforation or medically uncontrollable bleeding is observed at the primary focus before treatment, the primary focus should be treated first, with conversion therapy afterwards. If the primary lesion or liver metastasis cannot be radically resected or the goal of NED cannot be achieved after 6 months of conversion therapy, a switch to low-intensity maintenance therapy is recommended.
Chemotherapy and/or Targeted
Therapy KRAS, NRAS, and BRAF genes, as well as microsatellite status, should be detected to guide the development of conversion therapy regimens.
-
(1)
Chemotherapy regimen
FOLFOX, CapeOx, and FOLFIRI regimens can improve the conversion rate for resection and are all preferentially recommended [77]. The XELIRI regimen is not routinely recommended due to the relatively insufficient evidence supporting its use as conversion therapy. The FOLFIRI triple-drug regimen has a higher response rate and conversion rate than the dual-drug combination regimen [78], and currently, the FOLFIRI combination is more frequently recommended for patients with good physical performance and organ function.
-
(2)
Molecular Targeted Drugs
RAS/BRAF wild-type: as conversion therapy for RC, dual drug therapy in combination with cetuximab is preferred [79].
RAS mutant type: drug chemotherapy combined with bevacizumab is recommended [80]. The triple-drug regimen combined with bevacizumab has a higher response rate but has strict indications, and the patients need to be closely monitored for adverse reactions [81, 82].
The prognosis of patients with BRAF V600E mutations is poor, and there is a little evidence demonstrating the survival benefits of surgical resection for liver metastases [83]. The FOLFOXIRI triple-drug regimen combined with bevacizumab is still recommend-sample studies have shown that surgical resection is beneficial for patients, but there is no high-level evidence supporting the use of immune checkpoint inhibitors for conversion therapy in such patients.
Assessments
-
(1)
Multidisciplinary Assessment of Potential Resectability
Contrast-enhanced CT can be used in the examination of primary RC lesions and distant metastases; contrast-enhanced MRI and contrast-enhanced ultrasonography can be used to assess the number and location of liver lesions. 3D CT and 3D digital imaging techniques are helpful for the assessment of residual liver volume.
-
(2)
Efficacy Assessments
Imaging assessments are recommended once every 6 ~ 8 weeks for conversion therapy. The Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria can be used to assess the efficacy of conversion therapy, and TRG can be used to assess the degree of pathologic tumor regression after conversion therapy. If the treatment is combined with bevacizumab, the interval from the last dose to surgery should be at least 6 weeks, and bevacizumab should be restarted 6 ~ 8 weeks after the surgery.
4.3.3 Unresectable hepatic metastasis of RC
Surgical Treatment of Primary Focus
-
(1)
For primary RC lesions without bleeding, obstruction, or perforation, systemic treatment or resection of the primary lesion followed by further treatment can be considered. However, whether it is necessary to remove the primary lesion in the absence of bleeding, obstruction, or perforation in patients with unresectable liver or lung metastases remains controversial [84,85,86,87].
-
(2)
For primary RC lesions with bleeding, obstruction, or perforation, the primary lesion should be treated first, before systemic chemotherapy. After treatment, an evaluation should be conducted every 6 ~ 8 weeks to decide the next treatment regimen. Management of the primary focus includes resection of the primary lesions, short-circuit surgery, simple ostomy, placement of an intestinal stent to relieve obstruction, and local interventional embolization for bleeding in primary lesions.
Radiotherapy
When there are obvious local symptoms (such as pain, bleeding, and obstruction), palliative radiotherapy to the primary tumor may be considered [88].
Medical Treatment
-
(1)
First-line palliative care Chemotherapy combined with targeted therapy is preferred. The strategy of induction chemotherapy-maintenance therapy is recommended for patients for whom long-term tumor control (PFS 4 ~ 6 months) is promising.
-
1)
Routine detection of KRAS, NRAS, and BRAF genes and microsatellite status in tumor tissue is recommended before treatment.
-
2)
The following regimens are recommended for patients with indications for intensive treatment:
-
a)
Chemotherapy regimen: dual-drug or triple-drug chemotherapy should be selected according to the patient’s age, physical strength, organ function and tumor burden. FOLFOX, CapeOx, and FOLFIRI are similar in efficacy but differ in toxicities [89, 90]. The objective response rate and PFS of the triple-drug combination FOLFOXIRI are better than those of dual-drug chemotherapy, but the adverse reactions, especially myelosuppression, are more obvious [91]. Therefore, the FOLFOXIRI regimen is recommended for patients aged < 70 years with a PS score of 0 ~ 1 points, good organ function, and high tumor burden. For patients suffering from severe underlying cardiac diseases or drug cardiotoxicity, fluorouracil should be replaced with raltitrexed.
-
b)
Targeted drugs: the best targeted therapy should be selected based on gene status. For RAS / BRAF wild / MSS type tumors, FOLFOX / FOLFIRI combined with cetuximab is preferred [92, 93]; for RAS mutant, BRAF wild/MSS type or BRAF mutant /MSS type tumors intolerant to triple-drug chemotherapy, FOLFOX / CapeOx / FOLFIRI combined with bevacizumab is preferred; for young patients with a good performance status, large tumor burden, rapid tumor growth, or BRAF v600E mutation, FOLFOXIRI combined with bevacizumab is optional [81, 94].
-
c)
Immunotherapy: PD-1 monoclonal antibody (pembrolizumab) is preferentially recommended for all patients with MSI-H / dMMR [95]. For those who are not suitable for immunotherapy, the selection principles for palliative first-line therapy may be applied.
-
d)
Maintenance therapy: Patients who achieve CR/PR/SD after a certain duration (usually 6 ~ 8 cycles) of first-line intensive chemotherapy ± targeted therapy (i.e., induction chemotherapy) who are not suitable for local treatment according to the MDT to HIM team may receive maintenance therapy [96]. At present, the maintenance treatment strategy is only recommended after first-line dual-drug or triple-drug chemotherapy. The regimen of capecitabine or 5-FU ± bevacizumab is preferred. If the patient does not want to continue receiving chemotherapy, bevacizumab alone can be used [97,98,99].
-
a)
-
3)
The following regimens are recommended for patients without indications for intensive treatment: For patients aged ≥70 years with poor physical performance or organ function, small tumor burden, and slow tumor growth, e. g., patients with lung metastasis only, capecitabine or 5-FU combined with bevacizumab is recommended [100]; for those who are intolerant to capecitabine-induced hand-foot syndrome or are unwilling to receive a continuous infusion of 5-FU, trifluridine and tipiracil tablets combined with bevacizumab can be considered as an alternative [101], and a combined dual-drug regimen with a 30% ~ 50% dose reduction can also be considered; for patients who are not suitable for bevacizumab, such as those with recent thrombosis or massive hemorrhagic events, capecitabine or 5-FU alone may be considered; for RC patients with RAS and BRAF wild / MSS type tumors, cetuximab alone or in combination with irinotecan can be considered [102].
-
1)
-
(2)
Second-line palliative care
-
1)
The following regimens are recommended for patients with indications for intensive treatment:
-
a)
Chemotherapy regimen: oxaliplatin based and irinotecan based regimens can be used as either first-line or second-line therapies [89]. The mXELIRI regimen is safe and effective in the Chinese population [103], with fewer adverse reactions than the FOLFIRI regimen. For patients with disease progression in the first-line treatment with triple-drug chemotherapy, please refer to the third-line treatment principle for subsequent treatment. The originally induced chemotherapy regimen should be introduced first in case of progression during first-line maintenance therapy. Raltitrexed may be considered in combination with platinum as a second-line treatment [96].
-
b)
Targeted drugs: If targeted drugs are not used during first-line treatment, the second-line treatment should include targeted drugs according to the genotyping results. Bevacizumab cross-line treatment is recommended for patients with RAS or BRAF mutation who show disease progression after first-line bevacizumab [104, 105]. For RAS and BRAF wild-type RC, if the disease progresses after first-line treatment with cetuximab, bevacizumab should be used as the second-line treatment, while cross-line treatment with cetuximab is not recommended; if the disease progresses after first-line treatment with bevacizumab, bevacizumab cross-line treatment should be used for second-line treatment, or the drug should be switched to cetuximab [106]. Chemotherapy combined with targeted therapy is recommended for dMMR / MSI-H patients using immune checkpoint inhibitors as first-line therapy [107]. For patients with BRAF V600E mutations, cetuximab + vemurafenib + irinotecan [108] or dabrafenib + cetuximab ± trametinib may be selected for second-line therapy [109, 110].
-
c)
Immunotherapy: PD-1 monoclonal antibody alone or in combination with CTLA-4 monoclonal antibody is recommended as second-line therapy for patients with dMMR / MSI-H status who do not use immune checkpoint inhibitors as first-line therapy [111, 112]. Patients carrying rare POLE or POLD pathogenic mutations may also be sensitive to immune checkpoint inhibitors [113].
-
a)
-
2)
The following regimens are recommended for patients without indications for intensive treatment: Whether second-line therapy or participation in a clinical study should be selected should be based on the patient’s physical performance, genotype, and previous first-line treatment schemes. For patients with a PS score > 2 points, optimal supportive therapy is recommended; for patients with a PS score of 0 ~ 2 points and RAS and BRAF wild-type tumors who have not previously received anti-EGFR monoclonal antibody, cetuximab monotherapy is recommended, while for patients with RAS or BRAF-mutant tumors who have not previously received targeted drugs, capecitabine, 5-FU, or trifluridine and tipiracil tablets combined with bevacizumab is recommended.
-
1)
-
(3)
Third-line and Later line palliative care
-
1)
Selection guided by nonmolecular markers: the novel combination chemotherapeutic agent trifluridine and tipiracil tablets should be adopted as monotherapy with or without bevacizumab in patients who are intolerant to regorafenib and fruquintinib or fail third-line treatment [114,115,116].
-
2)
Postline treatment options guided by molecular markers:
-
a)
Patients with BRAFV600E mutant / MSS type tumors who were not previously treated with anti-BRAF therapy should receive cetuximab + vemurafenib + irinotecan, or dabrafenib + cetuximab ± trametinib or participate in a clinical study.
-
b)
Patients with HER2 overexpression should receive trastuzumab + lapatinib [117] or trastuzumab + pertuzumab [118] or participate in a clinical study.
-
c)
Patients with dMMR / MSI-H status should receive PD-1 monoclonal antibody therapy. Patients carrying rare POLE or POLD pathogenic mutations may also be sensitive to immune checkpoint inhibitors [119].
-
d)
For patients with RAS and BRAF wildtype tumors who did not previously receive EGFR monoclonal antibody, cetuximab alone or in combination with irinotecan can be considered; for patients who have previously received cetuximab as first-line therapy and achieved an objective response (CR/ PR) with a PFS > 6 months and ctDNA test results showing both wild-type RAS and BRAF, the rechallenge strategy with cetuximab combined with irinotecan can be considered [119].
-
e)
Patients with NTRK gene fusion: NTRK inhibitors may be considered [120].
-
a)
-
1)
-
(4)
Other therapies Local treatment approaches may be selected for advanced patients, such as interventional therapy, intratumoral injection, physical therapy, or traditional Chinese medicine (TCM) treatment, provided that the abovementioned conventional therapies are not applicable. For the overall management process for RC liver metastasis, please refer to Figs. 3 and 4.
4.4 Principles of treatment for RC metastases to other parts
4.4.1 Lung metastasis
At present, high-resolution chest CT is recommended to identify RC lung metastases, and contrast-enhanced chest CT is recommended to identify metastases to the mediastinal and hilar lymph nodes. For indeterminate pulmonary nodules (IPNs) that cannot be identified by chest CT, the nature of the nodules can be comprehensively judged by considering the risk factors, follow-up conditions, and pathological examination findings.
Principles of surgical
Treatment For resectable lung metastases, R0 resection is recommended. Pulmonary metastases should not be excised when there are unresectable lesions outside the lung. The remaining lung must maintain adequate lung function after resection of the lung metastases. Extrapulmonary resectable metastases can be treated simultaneously or in stages [121].
Surgical methods
The most commonly used method is wedge resection, followed by pulmonary segment resection, lobectomy and total pneumonectomy. For patients without suspected hilar or mediastinal lymph node metastases during the preoperative examination, routine lymph node dissection during the operation may be skipped; if lymph node metastases are suspected, lymph node biopsy or dissection may be considered during the operation.
Other local treatments
The treatment techniques include RFA and SBRT.
-
(1)
RFA: For small pulmonary metastases (with a maximum diameter < 3 cm) that are far away from large vessels, RFA shows a good local control rate of approximately 90%.
-
(2)
The indications for SBRT are as follows:
-
1)
The presence of 1 ~ 3 lung metastases and ≤ 5 small metastases and a maximum diameter of ≤5 cm.
-
2)
Relatively limited lung metastases, especially with a unilateral distribution of the lesions and peripheral lung metastases being more suitable for SBRT.
-
3)
A stable primary tumor that has been controlled.
-
4)
Good general condition with normal pulmonary function.
-
5)
Life expectancy ≥6 months.
-
1)
Palliative treatment should be performed for unresectable lung metastases. Whether to implement local lesion treatment should be decided under the guidance of the MDT to HIM team.
4.4.2 Peritoneal metastases
The peritoneum is one of the most common sites of RC metastasis, and peritoneal metastases suggest a worse prognosis [122,123,124]. Peritoneal metastasis was separately determined to be stage M1c by AJCC staging (eighth edition) to distinguish it from metastases to other sites. The clinical diagnosis of peritoneal metastasis is difficult due to the lack of specific clinical manifestations. Imaging examinations, tumor markers, peritoneal effusion cytology or histology, and laparoscopic exploration should be combined if necessary to improve the diagnosis of peritoneal metastasis [125]. The peritoneal carcinomatosis index (PCI) is used to assess the degree of peritoneal metastasis. Treatment strategies for peritoneal metastases of RC should be developed under the guidance of the MDT to HIM team. The treatment techniques include but are not limited to surgery, chemotherapy, targeted drugs, and intraperitoneal therapy.
-
(1)
Limited peritoneal metastases
For some patients with selected peritoneal metastasis, tumor cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) may prolong survival [126]. In centers with HIPEC experience, CRS surgery may be considered for patients with localized peritoneal metastases (PCI < 20) without extensive distant metastases to achieve CC0–1 cytoreduction (i.e., no residual peritoneal tumor or diameter of the residual tumor be < 2.5 mm). Cytoreduction can be achieved by combining HIPEC after complete CRS [127, 128].
-
(2)
Extensive peritoneal metastases or combined extensive distant metastases
Systemic chemotherapy is an important treatment approach for RC peritoneal metastasis and is superior to optimal supportive therapy. Please refer to the treatment recommendations for unresectable advanced RC for the available regimens.
Complete CRS and / or HIPEC may be considered in experienced centers to treat patients with selected localized peritoneal metastasis who may achieve R0 resection. At present, the common drugs used in intraperitoneal chemotherapy for RC in China include fluorouracil implants [129], raltitrexed [130], oxaliplatin, carboplatin, and lobaplatin [131, 132]. In principle, the drug dosage is based on the systematic chemotherapy dosage, which can be appropriately adjusted according to the patient’s age, physical condition, tolerance to chemotherapeutic agents, and bone marrow proliferation capability.
4.4.3 Metastases to ovary, bone, and brain
For patients with definite RC ovarian metastases, bilateral adnexectomy is recommended. If the uterus is involved, hysterectomy should be added. Prophylactic resection of ovaries with a normal appearance is not recommended during RC surgery [133]. Patients who wish to have children should consult a doctor specializing in reproductive medicine for an assessment before initial treatment. Postoperative chemotherapy is recommended for patients with ovarian metastases who have achieved R0 resection. For patients with ovarian metastases who cannot achieve an NED status after treatment, the treatment methods for unresectable advanced RC should be considered.
The diagnosis of bone metastasis mainly depends on emission computed tomography (ECT), X-ray, CT, MRI, or PET-CT. ECT is always used as the main approach for diagnosing bone metastasis [134]. The objectives of comprehensive treatment for RC bone metastases are to improve quality of life, prolong survival time, and prevent or delay skeletal-related events (SREs). In systemic therapy, bisphosphonates are the basic medicine for bone metastasis of RC. When imaging suggests bone destruction or metastasis, bone protective drugs should be used. During treatment with bisphosphonates, continuous administration is recommended for at least 12 months even if SREs occur [134]. Local surgical treatment should be performed with due consideration and care. Local radiotherapy can be performed for bone metastases.
The treatment for brain metastasis of RC is similar to that for brain metastases from other solid tumors and is mainly based on control of the primary lesions and supplementation by local treatment of brain metastasis [135].
4.5 Treatment of locally recurrent RC
4.5.1 Principles of surgical treatments
For RC patients with local recurrence, an MDT to HIM evaluation should be performed. In addition to conventional RC-related disciplines, expert teams from the departments of urology, gynecological oncology, and plastic surgery as well as other related departments should be included. For patients with resectable recurrent lesions, integrative therapy based on surgery combined with perioperative chemoradiotherapy is recommended; for patients with unresectable lesions, radiotherapy and / or systemic therapy are recommended, and resectability should be reassessed after treatment [62].
Patients with relevant surgical contraindications should avoid undergoing surgical treatment. Relative contraindications include the presence of distant metastases, initial treatment for stage IV disease, extensive pelvic sidewall invasion, expected R1 or R2 resection, and invasion into the sacrum above the S2 to S3 junction. Absolute contraindications include external iliac vascular involvement, tumor beyond the ischial notch (i. e., outward invasion through the ischial foramen), lower limb edema due to compression of the lymphatic vessels and veins, and bilateral ureteral obstruction and effusion, as well as poor general condition.
The surgery should be conducted by colorectal surgeons following an appropriate surgical plan tailored to the specific conditions of the patient and lesion. The surgery should be performed following the principle of enbloc resection, and R0 resection should be achieved as much as possible. If invasion into the surrounding organs is present, combined organ resection should be considered if possible. The Leeds classification should be applied [136]. See Table 3.
4.5.2 Radiotherapy principles
For patients who have not previously received pelvic radiotherapy, preoperative concomitant chemoradiotherapy is recommended (every attempt should be made to obtain the pathological diagnosis of recurrent lesions before radiotherapy); for patients with resectable local lesions, surgery followed by postoperative chemoradiotherapy may also be considered; additionally, the decision to undergo chemoradiotherapy before surgery can be made according to the previous chemoradiotherapy regimen. In principle, patients who have received pelvic radiotherapy should no longer receive radiotherapy (repeat radiotherapy and proton and heavy ion therapy can be appropriately carried out at experienced centers). The most reasonable treatment regimen should be developed after an MDT to HIM discussion. For patients with local recurrence who cannot tolerate surgery or external beam radiotherapy (EBRT), radioactive seed implantation (such as I125 seeds) can also exert a palliative effect.
4.5.3 Medical treatment and other treatment principles
An MDT to HIM discussion should be carried out to reassess the recurrent RC as a resectable lesion, potentially resectable lesion, or unresectable lesion based on imaging examination findings and surgical assessments and to clarify whether it is necessary to adopt the anal preservation strategy or medical treatment strategy based on different disease classifications.
-
(1)
For patients with resectable lesions who have not received previous chemoradiotherapy, preoperative concomitant chemoradiotherapy with fluorouracil is the preferred recommendation. In patients with fair physical performance, concomitant chemoradiotherapy with platinum - or irinotecan-based combination chemotherapy may exert a more significant tumor downstaging effect, but the toxicity and side effects may be increased. For patients who cannot tolerate radiotherapy, preoperative dual-drug or triple-drug chemotherapy may be considered. Targeted therapy can be considered for RC patients who demonstrate no increased efficacy after neoadjuvant therapy, but preoperative targeted therapy is not recommended, as it may increase the toxicity and side effects.
-
(2)
For patients with resectable lesions who have previously received concomitant chemoradiotherapy, direct surgery or neoadjuvant chemotherapy is recommended. The selection of the chemotherapy regimen should follow the same guidelines as above.
-
(3)
For patients with unresectable or potentially resectable lesions who have not received previous chemoradiotherapy, intensive chemotherapy (dual-drug chemotherapy) - based concomitant chemoradiotherapy or induced intensive chemotherapy followed by 5-FU concomitant chemoradiotherapy is preferred. For those who have received chemoradiotherapy, the principles for palliative first-line treatment options for metastatic RC should be applied. Efficacy should be assessed every 2 to 3 months, and the possibility of tumor resection should be discussed by the MDT to HIM team. The management process for local RC recurrence is shown in Fig. 5.
4.6 TCM treatment
4.6.1 Treatment principles
Under the guidance of integrative medicine, the selection TCM drugs and treatment with TCM should be carried out according to the principle of syndrome differentiation, in which the basic treatment principles focus on reinforcing healthy qi to eliminate pathogenic factors, treating both the tip and root, and offering integrative treatment according to the variability of individuals, the four seasons, and the local conditions [137,138,139].
4.6.2 Treatment based on syndrome differentiation
Perioperative Syndrome Differentiation of RC Patients with RC mainly preoperatively manifest obstruction of Fu-Qi, and symptoms are specifically described as constipation; intermittent abdominal pain; abdominal fullness and distention; red tongue with a yellow, greasy coating; and a slippery and rapid pulse. The main postoperative manifestations are yuan-primordial qi damage and consumption as well as spleen-stomach deficiency, demonstrated by specific symptoms such as pale or sallow complexion, pale lips and nails, shallow breathing and fatigue, mental fatigue and unwillingness to talk, dull abdominal pain, poor appetite, abdominal distension after eating, pale tongue with thin and white coating, and weak pulse. Therefore, the main principles are to regulate Qi and dredge the bowels, supplement Qi and nourish blood, and strengthen the spleen and stomach to improve the patient’s tolerance to surgery and alleviate postoperative complications.
Syndrome differentiation in the adjuvant treatment period of RC
-
(1)
During RC chemotherapy, patients usually present with spleen-stomach disharmony, qi-blood deficiency, and liver and kidney yin deficiency, with specific symptoms of abdominal fullness and distention, loss of appetite, nausea and vomiting, abdominal distension or diarrhea, enlarged tongue, thin and white or greasy coating, soreness and weakness of the waist and knees, tinnitus, feverish sensation in the chest, palms, and soles, red cheeks and night sweats, red tongue with little coating, and a slippery and rapid pulse. Therefore, the main treatment principles are to strengthen the spleen and harmonize the stomach, direct counterflow downward and stop vomiting, supplement Qi and nourish blood, enrich and nourish the liver and kidney to improve the patient’s tolerance to chemotherapy, reduce the toxicity and side effects of chemotherapy, and improve the completion rate of chemotherapy.
-
(2)
During RC radiotherapy, patients are often characterized by Qi-Yin deficiency and heat toxin accumulation, with specific symptoms of mental fatigue and lassitude, shallow breathing and unwillingness to talk, poor appetite, occasional loose stools, red tongue and little coating, and thready and rapid pulse; or thirst with desire to drink, low - grade fever and night sweats, abdominal distension, abdominal pain refusing to pressure, frequent urination, red and white diarrhea, tenesmus, dark red tongue, yellow and greasy coating, and wiry and slippery or slippery and rapid pulse. Therefore, boosting the kidney and enriching Yin, clearing the intestine and drying dampness, circulating blood and removing toxins are the main therapeutic principles to improve the patient’s tolerance to radiotherapy and reduce the adverse reactions to radiotherapy.
Syndrome differentiation in palliative treatment of advanced RC
During palliative treatment for advanced RC, patients mainly manifest asthenia due to asthenia of vital qi and sthenia of pathogenic factors, the former of which is predominant, mixed with phlegm, stasis, toxin, dampness, and other pathogenic factors. TCM treatment during the palliative treatment phase alleviates the adverse reactions of Western medicine, increases the curative effect, improves quality of life, and prolongs the survival period as long as possible.
5 Whole-course rehabilitation management
5.1 Follow-up
-
(1)
Medical history and physical examination and CEA and CA19–9 monitoring, once every 3 months for the first 2 years, once every 6 months from the 3rd once every year after the 5th to 5th year. Years, and
-
(2)
Chest, abdominal, and pelvic CT or MRI, once every 6 months for the first 2 years and then once a year for 5 years.
-
(3)
Colonoscopy should be performed within 1 year after surgery. If any abnormality develops, reexamination should be performed within 1 year; if no polyps are present, reexamination should be performed within 3 years and then once every 5 years. Resection is recommended for all colonic adenomas found during follow-up. If the preoperative colonoscopy fails to cover the entire colon, colonoscopy should be performed 3 to 6 months after the surgery.
-
(4)
PET-CT is not recommended as a routine examination. For patients with or suspected of having recurrence and distant metastasis, PET-CT may be considered to exclude recurrence and metastasis.
-
(5)
If antitumor therapy is not feasible according to the physical condition of the patient, routine follow-up is not necessary. The management process for persistent postoperative CEA elevation is shown in Fig. 6.
5.2 Whole-course rehabilitation management
5.2.1 Nutrition therapy
Nutrition therapy should be provided throughout the whole process from the initial diagnosis to the completion of comprehensive treatment [140].
-
(1)
Once diagnosed with RC, patients should undergo nutritional risk screening and nutritional status assessment.
-
(2)
For patients with RC, regardless of radical or palliative surgery, perioperative nutritional management should be carried out according to the Enhanced Recovery After Surgery (ERAS) principles and procedures.
-
(3)
For patients with RC who undergo preoperative neoadjuvant therapy or postoperative adjuvant therapy, a nutrition treatment plan needs to be formulated and followed.
5.2.2 TCM rehabilitation therapy of tumors
Rehabilitation therapies based on TCM for cancer can be implemented during the whole process from the initial diagnosis to the completion of integrated treatment. TCM rehabilitation therapies for cancers are guided by syndrome differentiation, and comprehensive rehabilitation methods are adopted, including psychological treatments, acupuncture and Chinese massage (Tui Na) therapy, food therapy, TCM treatments and traditional Chinese health exercise therapy. A reasonable TCM treatment plan should be formulated and implemented according to the different stages and syndromes of disease.
5.2.3 Treatment for delayed or long-term sequelae
Either RC surgery or chemoradiotherapy may lead to late sequelae, affecting the quality of daily life and organ function. The common sequelae and related treatments are as follows:
-
(1)
For sequelae associated with impaired bowel function, such as chronic diarrhea, incontinence, frequent bowel movements, and tenesmus, antidiarrheal drugs, stool hardening drugs, TCM, diet regulation, pelvic floor repair, and adult diapers may be considered [141, 142].
-
(2)
For oxaliplatin-induced neuropathy, only duloxetine is considered for the treatment of painful neuropathy, which is ineffective for numbness, tingling, and cold sensitivity. TCM prescriptions can be tested.
-
(3)
In the case of urogenital dysfunction after pelvic surgery or radiotherapy, screening for sexual dysfunction, erectile dysfunction, dyspareunia and vaginal dryness is recommended. The presence of dysuria, frequent urination and urgent urination should also be assessed. If the symptoms persist, the patient should be referred to a urologist or gynecologist.
-
(4)
Pain management: A comprehensive pain assessment should be performed to determine the etiology of the pain. The differential diagnosis should consider cancer recurrence, disease progression or specific cancer pain syndromes. Opioid therapy may be considered, with the lowest appropriate dose used for the shortest time possible. Other adjuvant therapy could be performed when opioids are used.
-
(5)
Sleep disorders: The course and characteristics of insomnia should be learned in detail, and the patient should receive sleep health education; cognitive behavioral therapy is recommended as the first of choice for insomnia and is superior to interventional therapy with medications. Simultaneously, acupuncture, acupoint massage, intervention with Chinese materia medica and other TCM cancer rehabilitation treatment means can be considered for treatment.
-
(6)
To detect potential pelvic fracture or a decrease in bone mineral density (BMD) following pelvic radiotherapy, BMD should be monitored.
-
(7)
Bone marrow suppression after chemotherapy: For chemotherapy-related neutropenia, rhGCSF or PEG-rhG-CSF can be applied; for chemotherapy-related anemia, erythropoietin (EPO) can be used, while iron, vitamin B12, folic acid, etc., should also be supplemented, and infusions of erythrocyte suspensions may be performed when necessary; for chemotherapy-related thrombocytopenia, nursing care is as important as medication. Patients need to reduce activity, prevent injuries, ensure absolute bed rest when necessary, pay attention to defecation and cough suppression, etc. Thrombopoietin (TPO) and recombinant human interleukin 11 can be used for platelet elevation. If necessary, apheresis platelets may be transfused.
5.2.4 Management of Stoma
-
(1)
Personnel, tasks and structure: stoma therapists (specialty nurses) should be available in medical centers with the appropriate conditions. The responsibilities of these therapists should include preoperative and postoperative care for all stomas (intestinal, gastric, urinary and tracheal stomas, etc.), management of complex incisions and incontinence care. Stoma therapists are also responsible for opening stoma specialist outpatient clinics, liaising with patients, other professionals and stoma product suppliers, organizing social events and carrying out follow ups for patients with stomas.
-
(2)
Psychotherapy: patients should receive full communication of the relevant diagnosis, surgery, and nursing guidelines so that they can accept the onset of disease and have a comprehensive understanding of what is about to happen; in addition, some psychological intervention and guidance should be given before and after surgery.
-
(3)
Stoma positioning: the stoma stie should be selected jointly by the physician, stoma therapist, family members and patient before the operation. The stoma site should be visible to the patient and convenient for care. There should be enough area for adhesion, and there should be no discomfort when the stoma appliance is adhered to the stoma skin.
-
(4)
Nursing of intestinal stoma
-
1)
After surgery, attention should be given to the blood flow of the stoma and whether there is retraction.
-
2)
Ostomy supplies should be lightweight, transparent, odor-resistant, and leak-proof and should be able to protect the surrounding skin and appropriately fit the patient.
-
3)
The skin around the intestinal stoma should be kept clean and dry. Special attention should be given to fungal infections of the intestinal stoma in patients who have been taking antibiotics, immunosuppressants and glucocorticoids for an extended period of time.
-
1)
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Sung H, Ferlay J, Siegel RL, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Cao W, Chen HD, Yu YW, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. 2021;134(7):783–91.
Wu CX, Fu C, He J, et al. Analysis of incidence and mortality of colorectal cancer in China, 2015. China. Oncology. 2020;030(004):241–5.
Sun KX, Zheng RS, Zhang SW, et al. Report of Cancer incidence and mortality in different areas of China, 2015. China Cancer. 2019;28(01):1–11.
Zheng RS, Sun KX, Zhang SW, et al. Report of cancer epidemiology in China, 2015. Chin J Oncol. 2019;41(1):19–28.
Chen XP, Wang JP, Zao JZ. Surgery. 9th ed. Beijing: People’s Medical Publishing House; 2018.
Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–74.
Japanese Society for Cancer of the Colon and Rectum. Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: the 3d English Edition [Secondary Publication]. J Anus Rectum Colon. 2019;3(4):175–95.
Li P, Wang YJ, Chen GY, et al. Consensus on screening, diagnosis, and treatment of early colorectal Cancer and precancerous lesions in China. Chin J Pract Intern Med. 2015;35(3):211–27.
Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal Cancer: US preventive services task force recommendation statement. JAMA. 2021;325(19):1965–77.
Ma QL, Ma XY, Yu LL, et al. Age-specific detection rates of colorectal neoplasms by Colonoscopic screening in high-incidence rural area. Chin J Oncol. 2013;35(002):154–7.
Early diagnosis and treatment group, the oncology Committee of Chinese Medical Association. Expert consensus on early diagnosis and treatment of colorectal Cancer in China. Nat l Med J China. 2020;100(22):1691–8.
National Cancer Center. Expert Group of the Development of China guideline for the screening, early detection and early treatment of colorectal Cancer. China guideline for the screening, early detection and early treatment of colorectal Cancer (2020, Beijing). Chin J Oncol. 2021;43(1):16–38.
Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26(35):5783–8.
Yuan Y, Zhang SZ, Zheng S, et al. Implementation scheme of screening standard for hereditary colorectal Cancer in China. Chin J Oncol. 2004;26(3):191–2.
Committee of Colorectal Cancer, Chinses Society of Clinical Oncology. Genetics Group of the Committee of Colorectal Cancer, China Anti-cancer Association. Genetics Committee of the Committee of colorectal Cancer, Chinese medical doctor association. Consensus on detection of microsatellite instability in colorectal cancer and other related solid tumors in China. Chin J Oncol. 2019;4(10):734–41.
Sieber OM, Segditsas S, Knudsen AL, et al. Disease severity and genetic pathways in attenuated familial adenomatous polyposis vary greatly but depend on the site of the germline mutation. Gut. 2006;55(10):1440–8.
Yang M, Zhu L, Zhu L, et al. Role of a rare variant in APC gene promoter 1B region in classic familial adenomatous polyposis. Digestion. 2021;102(4):527–33.
Tanaka S, Kashida H, Saito Y, et al. Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2020;32(2):219–39.
Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24(2):343–52.
Pathologic Collaborative Croup, Association of Digestive Endoscopy of Chinese Medical Association. Consensus on biopsy and pathologic examination of digestive endoscopy in China (draft). Chin J Dig Endosc. 2014;31(9):481–5.
Wang XS, Li ZF, Su M. An introduction to oncology. 2th ed. Beijing: People’s Medical Publishing House; 2021.
China NOSES Alliance. Professional committee of NOSES, colorectal Cancer Committee of Chinese Medical Doctor Association. Expert consensus of natural orifice specimen extraction surgery in colorectal neoplasm (2019 version). Chin J Colorectal Dis (Electronic Edition). 2019;8(4):336–42.
Beets-Tan RGH, Lambregts DMJ, Maas M, et al. Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28(4):1465–75.
de Jong EA, ten Berge JC, Dwarkasing RS, et al. The accuracy of MRI, endorectal ultrasonography, and computed tomography in predicting the response of locally advanced rectal cancer after preoperative therapy: a metaanalysis. Surgery. 2016;159(3):688–99.
Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40.
Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–33.
Wagman R, Minsky BD, Cohen AM, et al. Sphincter preservation in rectal cancer with preoperative radiation therapy and coloanal anastomosis: long term follow-up. Int J Radiat Oncol Biol Phys. 1998;42(1):51–7.
Gérard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24(28):4620–5.
Bosset JF, Calais G, Mineur L, et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results--EORTC 22921. J Clin Oncol. 2005;23(24):5620–7.
Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–23.
Hofheinz RD, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13(6):579–88.
Guideline Working Committee of China Society of clinical oncology. Chinese Society of Clinical Oncology (CSCO) guidelines for colorectal Cancer 2020. Beijing: People’s Medical Publishing House; 2020.
Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of Total neoadjuvant therapy for locally advanced rectal Cancer. JAMA Oncol. 2018;4(6):e180071.
Petrelli F, Trevisan F, Cabiddu M, et al. Total neoadjuvant therapy in rectal Cancer: a systematic review and Meta-analysis of treatment outcomes. Ann Surg. 2020;271(3):440–8.
Hu X, Li YQ, Li QG, et al. Adjuvant chemotherapy seemed not to have survival benefit in rectal Cancer patients with ypTis-2N0 after preoperative radiotherapy and surgery from a population-based propensity score analysis. Oncologist. 2019;24(6):803–11.
Zhu J, Liu A, Sun X, et al. Multicenter, randomized, phase III trial of neoadjuvant Chemoradiation with Capecitabine and irinotecan guided by UGT1A1 status in patients with locally advanced rectal Cancer. J Clin Oncol. 2020;38(36):4231–9.
Cedermark B, Dahlberg M, Glimelius B, et al. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336(14):980–7.
van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12(6):575–82.
Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93(10):1215–23.
Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: trans-Tasman radiation oncology group trial 01.04. Journal of clinical oncology : official journal of the American society of. Clin Oncol. 2012;30(31):3827–33.
Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16(8):957–66.
Fokas E, Allgäuer M, Polat B, et al. Randomized phase II trial of Chemoradiotherapy plus induction or consolidation chemotherapy as Total neoadjuvant therapy for locally advanced rectal Cancer: CAO/ARO/AIO-12. J Clin Oncol. 2019;37(34):3212–22.
Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):29–42.
Roth AD, Delorenzi M, Tejpar S, et al. Integrated analysis of molecular and clinical prognostic factors in stage II/III colon cancer. J Natl Cancer Inst. 2012;104(21):1635–46.
Wells KO, Hawkins AT, Krishnamurthy DM, et al. Omission of adjuvant chemotherapy is associated with increased mortality in patients with T3N0 Colon Cancer with inadequate lymph node harvest. Dis Colon Rectum. 2017;60(1):15–21.
Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247–57.
Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28(20):3219–26.
Sinicrope FA, Foster NR, Thibodeau SN, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst. 2011;103(11):863–75.
Tejpar S, Saridaki Z, Delorenzi M, et al. Microsatellite instability, prognosis and drug sensitivity of stage II and III colorectal cancer: more complexity to the puzzle. J Natl Cancer Inst. 2011;103(11):841–4.
Quah HM, Chou JF, Gonen M, et al. Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis Colon Rectum. 2008;51(5):503–7.
Schmoll HJ, Tabernero J, Maroun J, et al. Capecitabine plus Oxaliplatin compared with fluorouracil/Folinic acid as adjuvant therapy for stage III Colon Cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol. 2015;33(32):3733–40.
Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109–16.
Van Cutsem E, Labianca R, Bodoky G, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol. 2009;27(19):3117–25.
Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307(13):1383–93.
Allegra CJ, Yothers G, O'Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011;29(1):11–6.
de Gramont A, Van Cutsem E, Schmoll HJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol. 2012;13(12):1225–33.
Biagi JJ, Raphael MJ, Mackillop WJ, et al. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305(22):2335–42.
NCCN Clinical Practice Guideline in Oncology. Rectal Cancer, Version 1, 2021. National Comprehensive Cancer Network [EB/OL]. https://www.nccn.org.
Chinese W. Wait database research cooperation G, Chinese Association of Surgeons CSoCCMDA, et al. [consensus on the watch and wait policy in rectal cancer patients after neoadjuvant treatment (2020 version)]. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23(1):1–9.
Fernandez LM, São Julião GP, Figueiredo NL, et al. Conditional recurrence-free survival of clinical complete responders managed by watch and wait after neoadjuvant chemoradiotherapy for rectal cancer in the International Watch & Wait Database: a retrospective, international, multicentre registry study. Lancet Oncol. 2021;22(1):43–50.
National Health Commission of the People's Republic of China. Chinese guidelines of diagnosis and treatment of colorectal Cancer 2020. Chin J Surg. 2020;8:561–85.
Wo JY, Anker CJ, Ashman JB, et al. Radiation therapy for rectal Cancer: executive summary of an ASTRO clinical practice guideline. Pract Radiat Oncol. 2021;11(1):13–25.
Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European society for. Med Oncol. 2017;28(suppl_4):iv22–40.
Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–18 discussion 18–21.
Ayez N, van der Stok EP, Grünhagen DJ, et al. The use of neo-adjuvant chemotherapy in patients with resectable colorectal liver metastases: clinical risk score as possible discriminator. Eur J Surg Oncol. 2015;41(7):859–67.
Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208–15.
Bridgewater JA, Pugh SA, Maishman T, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (new EPOC): long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(3):398–411.
Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–422.
Chinese College of Surgeons, Chinese Medical Doctor Association. Chinese Society of Gastrointestinal Surgery, Chinese Society of Surgery of Chinese Medical Association. Chinese society of colorectal surgery, Chinese society of surgery of Chinese medical association, etc. China guideline for diagnosis and comprehensive reatment of colorectal liver metastases (2020 Version). Chin J Gastrointestinal Surg. 2021;24(01):1–13.
Adam R. Chemotherapy and surgery: new perspectives on the treatment of unresectable liver metastases. Annals of oncology : official journal of the European society for. Med Oncol. 2003;14 Suppl 2:ii13–6.
Goldberg RM, Sargent DJ, Morton RF, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a north American intergroup trial. J Clin Oncol. 2006;24(21):3347–53.
Aloia T, Sebagh M, Plasse M, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24(31):4983–90.
Fernandez FG, Ritter J, Goodwin JW, et al. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200(6):845–53.
Adam R, Bhangui P, Poston G, et al. Is perioperative chemotherapy useful for solitary, metachronous, colorectal liver metastases? Ann Surg. 2010;252(5):774–87.
Kishi Y, Zorzi D, Contreras CM, et al. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2010;17(11):2870–6.
Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol. 2005;23(22):4866–75.
Souglakos J, Androulakis N, Syrigos K, et al. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic oncology research group (HORG). Br J Cancer. 2006;94(6):798–805.
Ye LC, Liu TS, Ren L, et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol. 2013;31(16):1931–8.
Tang W, Ren L, Liu T, et al. Bevacizumab plus mFOLFOX6 versus mFOLFOX6 alone as first-line treatment for RAS mutant Unresectable colorectal liver-limited metastases: the BECOME randomized controlled trial. J Clin Oncol. 2020;38(27):3175–84.
Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16(13):1306–15.
Stein A, Atanackovic D, Hildebrandt B, et al. Upfront FOLFOXIRI+bevacizumab followed by fluoropyrimidin and bevacizumab maintenance in patients with molecularly unselected metastatic colorectal cancer. Br J Cancer. 2015;113(6):872–7.
Margonis GA, Buettner S, Andreatos N, et al. Association of BRAF mutations with survival and recurrence in surgically treated patients with metastatic colorectal liver Cancer. JAMA Surg. 2018;153(7):e180996.
Faron M, Pignon JP, Malka D, et al. Is primary tumour resection associated with survival improvement in patients with colorectal cancer and unresectable synchronous metastases? A pooled analysis of individual data from four randomised trials. Eur J Cancer (Oxford, England: 1990). 2015;51(2):166–76.
Tarantino I, Warschkow R, Güller U. Palliative primary tumor resection in patients with metastatic colorectal Cancer: for whom and when? Ann Surg. 2017;265(4):e59–60.
Moritani K, Kanemitsu Y, Shida D, et al. A randomized controlled trial comparing primary tumour resection plus chemotherapy with chemotherapy alone in incurable stage IV colorectal cancer: JCOG1007 (iPACS study). Jpn J Clin Oncol. 2020;50(1):89–93.
Hu CY, Bailey CE, You YN, et al. Time trend analysis of primary tumor resection for stage IV colorectal cancer: less surgery, improved survival. JAMA Surg. 2015;150(3):245–51.
Sager O, Dincoglan F, Demiral S, et al. A concise review of pelvic radiation therapy (RT) for rectal Cancer with synchronous liver metastases. Int J Surg Oncol. 2019;2019:5239042.
Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22(2):229–37.
Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–9.
Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25(13):1670–6.
Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–75.
Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first-line chemotherapy combined with Cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal Cancer: a randomized clinical trial. JAMA. 2017;317(23):2392–401.
Cremolini C, Antoniotti C, Rossini D, et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21(4):497–507.
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20.
Xu RH, Shen L, Li J, et al. Expert consensus on maintenance treatment for metastatic colorectal cancer in China. Chin J Cancer. 2016;35:13.
Chibaudel B, Maindrault-Goebel F, Lledo G, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 study. J Clin Oncol. 2009;27(34):5727–33.
Luo HY, Li YH, Wang W, et al. Single-agent capecitabine as maintenance therapy after induction of XELOX (or FOLFOX) in first-line treatment of metastatic colorectal cancer: randomized clinical trial of efficacy and safety. Ann Oncol. 2016;27(6):1074–81.
Quidde J, Hegewisch-Becker S, Graeven U, et al. Quality of life assessment in patients with metastatic colorectal cancer receiving maintenance therapy after first-line induction treatment: a preplanned analysis of the phase III AIO KRK 0207 trial. Ann Oncol. 2016;27(12):2203–10.
Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14(11):1077–85.
Van Cutsem E, Danielewicz I, Saunders MP, et al. Trifluridine/tipiracil plus bevacizumab in patients with untreated metastatic colorectal cancer ineligible for intensive therapy: the randomized TASCO1 study. Ann Oncol. 2020;31(9):1160–8.
Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–45.
Xu RH, Muro K, Morita S, et al. Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): a multicentre, open-label, randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2018;19(5):660–71.
Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14(1):29–37.
Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30(28):3499–506.
Bennouna J, Hiret S, Bertaut A, et al. Continuation of bevacizumab vs Cetuximab plus chemotherapy after first progression in KRAS wild-type metastatic colorectal Cancer: the UNICANCER PRODIGE18 randomized clinical trial. JAMA oncol. 2019;5(1):83–90.
Innocenti F, Ou FS, Qu X, et al. Mutational analysis of patients with colorectal Cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome. J Clin Oncol. 2019;37(14):1217–27.
Kopetz S, Guthrie KA, Morris VK, et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406). J Clin Oncol. 2021;39(4):285–94.
Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK inhibition with Dabrafenib and Trametinib in BRAF V600-mutant colorectal Cancer. J Clin Oncol. 2015;33(34):4023–31.
Corcoran RB, André T, Atreya CE, et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAF(V600E)-mutant colorectal Cancer. Cancer Discov. 2018;8(4):428–43.
Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with Nivolumab plus Ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal Cancer. J Clin Oncol. 2018;36(8):773–9.
Le DT, Kim TW, Van Cutsem E, et al. Phase II open-label study of Pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal Cancer: KEYNOTE-164. J Clin Oncol. 2020;38(1):11–9.
Wang F, Zhao Q, Wang YN, et al. Evaluation of POLE and POLD1 mutations as biomarkers for immunotherapy outcomes across multiple Cancer types. JAMA Oncol. 2019;5(10):1504–6.
Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16(6):619–29.
Li J, Qin S, Xu RH, et al. Effect of Fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal Cancer: the FRESCO randomized clinical trial. JAMA. 2018;319(24):2486–96.
Xu J, Kim TW, Shen L, et al. Results of a randomized, double-blind, placebo-controlled, phase III trial of Trifluridine/Tipiracil (TAS-102) monotherapy in Asian patients with previously treated metastatic colorectal Cancer: the TERRA study. J Clin Oncol. 2018;36(4):350–8.
Sartore-Bianchi A, Trusolino L, Martino C, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(6):738–46.
Meric-Bernstam F, Hurwitz H, Raghav KPS, et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019;20(4):518–30.
Cremolini C, Rossini D, Dell'Aquila E, et al. Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal Cancer with acquired resistance to first-line Cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol. 2019;5(3):343–50.
Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15(12):731–47.
Multidisciplinary Team Committee of Chinese College of Surgeons, Chinese Medical Doctor Association. Committee of Colorectal Cancer, Chinese Anticancer Association. Expert consensus on multidisciplinary therapy of colorectal Cancer with lung metastases (2018 version). Chin J Pract Surg. 2018;38(12):1325–38.
Ceelen WP, Flessner MF. Intraperitoneal therapy for peritoneal tumors: biophysics and clinical evidence. Nat Rev Clin Oncol. 2010;7(2):108–15.
Koppe MJ, Boerman OC, Oyen WJ, et al. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243(2):212–22.
Jayne DG, Fook S, Loi C, et al. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89(12):1545–50.
Passot G, Dumont F, Goéré D, et al. Multicentre study of laparoscopic or open assessment of the peritoneal cancer index (BIG-RENAPE). Br J Surg. 2018;105(6):663–7.
Elias D, Mariani A, Cloutier AS, et al. Modified selection criteria for complete cytoreductive surgery plus HIPEC based on peritoneal cancer index and small bowel involvement for peritoneal carcinomatosis of colorectal origin. Eu J Surg Oncol. 2014;40(11):1467–73.
Ceelen WP, Påhlman L, Mahteme H. Pharmacodynamic aspects of intraperitoneal cytotoxic therapy. Cancer Treat Res. 2007;134:195–214.
Sugarbaker PH. Surgical treatment of peritoneal carcinomatosis: 1988 Du Pont lecture. Can J Surg. 1989;32(3):164–70.
Zhou HY, Yuan M, Min WP, et al. Meta-analysis of implanting sustained-releasing 5-fluorouracil in colorectal Cancer surgery. China Pharm. 2017;28(3):355–9.
Chen JN, Wang Z, Hang AL, et al. Short-term safety evaluation of intraperitoneal chemotherapy with Raltitrexed for colorectal Cancer. Chin J Colorectal Dis (Electronic Edition). 2019;8(3):241–5.
Professional Committee of Peritoneal Neoplasms, Committee of Colorectal Cancer of Chinese Medical Doctor Association. Chinese expert consensus for prophylactic and therapeutic intraperitoneal medication for peritoneal metastases from colorectal Cancer (V 2019) [J/CD]. Chin J Colorectal Dis (Electronic Edition). 2019;8(4):329–35.
Su H, Bao MDL, Zhang YR, et al. The short-term effect analysis of intraoperative intraperitoneal perfusion chemotherapy with Lobaplatin for colorectal Cancer. Chin J Colorectal Dis (Electronic Edition). 2018;7(2):125–9.
Wang XS, Sun L, Cui SZ, et al. Chinese experts consensus on the Management of Ovarian Metastases from colorectal Cancer (2020 version). Chin J Colorectal Dis (Electronic Edition). 2020;9(2):13–9.
Liu Z, Xu SF, Liu ER, et al. Chinese expert consensus on multidisciplinary treatment of bone metastasis from colorectal Cancer (2020 version). Chin J Colorectal Dis (Electronic Edition). 2020;9(3):217–21.
Committee of Colorectal Cancer, Chinese medical doctor association. Chinese expert consensus on multidisciplinary treatment of brain metastases from colorectal Cancer (2020 version). Chin J Colorectal Dis (Electronic Edition). 2020;9(2):109–14.
Boyle KM, Sagar PM, Chalmers AG, et al. Surgery for locally recurrent rectal cancer. Dis Colon Rectum. 2005;48(5):929–37.
Huang LZ. Oncology of integrated traditional Chinese and Western medicine. Beijing: China Press of Traditional Chinese Medicine; 2020.
Wang XM. Practical oncology of integrated traditional Chinese and Western medicine. Beijing: China Press of Traditional Chinese Medicine; 2014.
Zhou DH. Traditional Chinese medicine oncology. Beijing: China Press of Traditional Chinese Medicine; 2011.
Chinese Society of Colorectal Surgery, Chinese Society of Surgery of Chinese Medical Association. Chinese Society of Nutritional Support, Chinese Society of Surgery of Chinese Medical Association. Chinese College of Colorectal Surgeons, Chinese College of Surgeons, Chinese Medical Doctor Association. Chinese Expert Consensus on Perioperative nutrition treatment of colorectal Cancer (2019 Version). Chin J Pract Surg. 2019;39(6):533–7.
Gami B, Harrington K, Blake P, et al. How patients manage gastrointestinal symptoms after pelvic radiotherapy. Aliment Pharmacol Ther. 2003;18(10):987–94.
Downing A, Morris EJ, Richards M, et al. Health-related quality of life after colorectal cancer in England: a patient-reported outcomes study of individuals 12 to 36 months after diagnosis. J Clin Oncol. 2015;33(6):616–24.
Acknowledgments
CACA Guidelines for Holistic Integrative Management of Rectal Cancer revision team members:
Honorary editor: Fan Daiming, National Clinical Research Center for Digestive Diseases and Xijing Hospital of Digestive Diseases, Fourth Military Medical University.
Editor-in-Chief: Wang Xishan, Cancer Hospital Chinese Academy of Medical Science.
Deputy Editor-in-Chief: Gu Jin, Beijing Cancer Hospital. Ding Kefeng, The Second Affiliated Hospital Zhejiang University School of Medicine. Fang Xuedong, China-Japan Union Hospital of Jilin University. Shen Lin, Beijing Cancer Hospital. Xu Zhongfa, The Third Affiliated Hospital of Shandong First Medical University. Xu Jianmin, Zhongshan Hospital Affiliated to Fudan University. Wang Guiyu, The Second Affiliated Hospital of Harbin Medical University.
Editorial Board Member: Cai Jianchun, Zhongshan Hospital Xiamen University. Cai Lianming, Jiangxi Ganzhou Cancer Hospital. Cai Sanjun, Fudan University Shanghai Cancer Center. Chen Gong, Sun Yat-sen University Cancer Center. Chen Jiansi, Guangxi Medical University Cancer Hospital. Cheng Longwei, Jilin Cancer Hospital. Cheng Yong, The First Affiliated Hospital of Chongqing Medical University. Chi Pan, Fujian Medical University Union Hospital. Cui Binbin, Harbin Medical University Cancer Hospital. Dai Guanghai, Chinese PLA General Hospital. Ding Kefeng, The Second Affiliated Hospital Zhejiang University School of Medicine. Fang Xuedong, China-Japan Union Hospital of Jilin University. Fu Chuangang, Shanghai East Hospital. Gu Jin, Beijing Cancer Hospital. Gu Yanhong, Jiangsu Province Hospital. He Guodong, Zhongshan Hospital Affiliated to Fudan University. Hu Junhong, Huaihe Hospital of Henan University. Huang Jing, Cancer Hospital Chinese Academy of Medical Science. Huang Rui, The Second Affiliated Hospital of Harbin Medical University. Huang Zhongcheng, Hunan Provincial People’s Hospital. Jiang Zheng, Cancer Hospital Chinese Academy of Medical Science. Jie Zhigang, The First Affiliated Hospital of Nanchang University. Ju Haixing, Zhejiang Cancer hospital. Li Hai, General Hospital of Ningxia Medical University. Li Jian, Beijing Cancer Hospital. Li Jun, The Second Affiliated Hospital Zhejiang University School of Medicine. Li Ming, Beijing Cancer Hospital. Li Yaoping, Shanxi Provincial People’s Hospital. Li Yunfeng, Yunnan Cancer Hospital. Lin Guole, Chinese Academy of Medical Science, Peking Union Medical College. Liu Haiying, Guangzhou Medical University Cancer Hospital. Liu Ming, The Second Affiliated Hospital of Harbin Medical University. Liu Qian, Cancer Hospital Chinese Academy of Medical Science. Pan Zhizhong, Sun Yat-sen University Cancer Center. Peng Yifan, Gastrointestinal Center of Peking University Cancer Hospital. Qian Niansong, PLA General Hospital Hainan Hospital. Qiu Meng, West China Medical Center Sichuan University. Ren Li, Zhongshan Hospital Affiliated to Fudan University. Shen Lin, Beijing Cancer Hospital. Sun Yueming, Jiangsu Province Hospital. Tang Jianqiang, Cancer Hospital Chinese Academy of Medical Science. Tang Qingchao, The Second Affiliated Hospital of Harbin Medical University. Tang Yuan, Cancer Hospital Chinese Academy of Medical Science. Tao Kaixiong, Union Hospital, Tonji Medical College, Huazhong University of Science and Technology. Tao Min, The First Affiliated Hospital of Soochow University. Wang Guiyu, The Second Affiliated Hospital of Harbin Medical University. Wang Hajjiang, Xinjiang Cancer Hospital. Wang Meng, Zhejiang Cancer hospital. Wang Xishan, Cancer Hospital Chinese Academy of Medical Science. Wang Zejun, Guizhou Cancer Hospital. Wang Zhengguang, The First Affiliated Hospital of Anhui Medical University. Wang Ziqiang, West China Medical Center,Sichuan University. Wang Ziwei, The First Affiliated Hospital of Chongqing Medical University. Wei Shaozhong, Hunan Cancer Hospital. Xing Baocai, Beijing Cancer Hospital. Xiong Bin, Zhongnan Hospital of Wuhan University. Xu Jianmin, Zhongshan Hospital Affiliated to Fudan University. Xu Ye, Fudan University Shanghai Cancer Center. Xu Zhongfa, The Third Affiliated Hospital of Shandong First Medical University. Yan Su, Qinghai University Affiliated Hospital. Yang Bin, Sun Yat-sen Memorial Hospital Sun Yat-sen University. Yang Chunkang, Fujian Cancer Hospital. Yao Qinghua, Zhejiang Cancer hospital. Ye Yingjiang, Peking University people’s Hospital. Yuan Ying, The Second Affiliated Hospital Zhejiang University School of Medicine. Zhang Guozhi, North China University of Science and Technology Affiliated Hospital. Zhang Haizeng. Cancer Hospital Chinese Academy of Medical Science. Zhang Hongmei, Cancer Hospital Chinese Academy of Medical Science. Zhang Zhen Fudan University Shanghai Cancer Center. Zhong Yunshi, Zhongshan Hospital Affiliated to Fudan University. Zhu Ji, Zhejiang Cancer hospital. Zhu Yuan, Zhejiang Cancer hospital. Zhu Yuping, Zhejiang Cancer hospital. Zou Shuangmei, Cancer Hospital Chinese Academy of Medical Science.
Proofreaders: Fan Daiming, National Clinical Research Center for Digestive Diseases and Xijing Hospital of Digestive Diseases, Fourth Military Medical University. Wang Xishan, Cancer Hospital Chinese Academy of Medical Science. Wang Guiyu,The Second Affiliated Hospital of Harbin Medical University. Wang Yuliuming, The Second Affiliated Hospital of Harbin Medical University. Lv Jingfang, Cancer Hospital Chinese Academy of Medical Science. Liu Enrui, The Affiliated Hospital of Qingdao University. Yang Ming, Cancer Hospital Chinese Academy of Medical Science. Zhang Qian, Zhejiang Cancer hospital. Zhang Weiyuan, The Second Affiliated Hospital of Harbin Medical University. Zhang Lin, Cancer Hospital Chinese Academy of Medical Science. Luo Jun, Zhejiang Cancer hospital. Zheng Zhaoxu, Cancer Hospital Chinese Academy of Medical Science. Zhao Zhixun, Cancer Hospital Chinese Academy of Medical Science. Jiang Zheng, Cancer Hospital Chinese Academy of Medical Science. Liu Zheng, Cancer Hospital Chinese Academy of Medical Science. Tao Jinhua, Zhejiang Cancer hospital. Huang Haiyang, Cancer Hospital Chinese Academy of Medical Science. Chen Tianli, Cancer Hospital Chinese Academy of Medical Science.
Funding
No Funding.
Author information
Authors and Affiliations
Consortia
Contributions
All authors read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
There are no ethics issues in the present guideline.
All authors agree to participate in the above research.
Competing interests
No Conflicts of interest. Authors Daiming Fan and Xishan Wang are members of the Editorial Board for Holistic Integrative Oncology. The paper was handled by the other Editor and has undertone rigorous peer review process. Authors Daiming Fan and Xishan Wang were not involved in the journal’s peer review of or decisions related to this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Committee of Colorectal Cancer Society Chinese Anti-Cancer Association., Wang, G., Fan, D. et al. CACA guidelines for holistic integrative management of rectal cancer. Holist Integ Oncol 2, 1 (2023). https://doi.org/10.1007/s44178-023-00023-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44178-023-00023-2